Pomegranate (Punica granatum L.) Attenuates Neuroinflammation Involved in Neurodegenerative Diseases
Abstract
:1. Introduction
2. Microglia and Neuroinflammation in the Context of Neurodegenerative Diseases
3. Polyphenols in Clinical Research of Alzheimer’s Disease
4. Nutritional Characterization and Metabolites Compounds of Punica granatum L.
| Anthocyanins | ||
|---|---|---|
| [40] | [40] | [40] |
Pelargonidin R1 = R2 = H | Cyanidin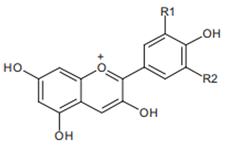 R1 = H, R2 = OH | Delphinidin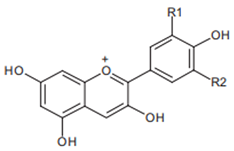 R1 = R2 = OH |
| Ellagitannins | ||
| [41] | [42] | [42] |
Punicalin | Ellagic acid | Punicalagin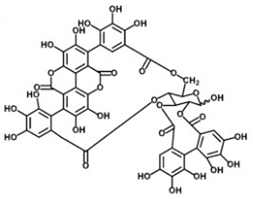 |
| Flavonoids | ||
| [41] | [41] | [41] |
| Luteolin (flavones) 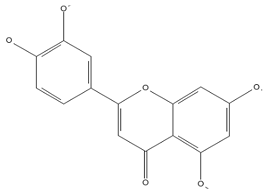 | Kaempferol (flavonols) 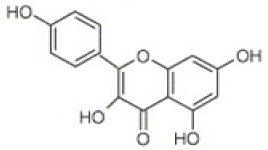 | Catechin (flavan-3-ols)  |
| PG Part or Product | Solvent | Variety | Country | TPC | TAC | TFC | TPA | TT | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Data is presented in mg/L | |||||||||
| Juice | Aqueous | Malase Ashkzar | Iran | 8130 ± 0.1 | 654,830 ± 0.1 | ni | ni | ni | [43] |
| Sweet Aalak | 2380 ± 0.0 | 815 ± 0.0 | ni | ni | ni | ||||
| Sooleghan | 7440 ± 0.1 | 5980 ± 0.1 | ni | ni | ni | ||||
| Malase Ardestan | 7920 ± 0.1 | 6430 ± 0.2 | ni | ni | ni | ||||
| Saveh Black Leather | 4200 ± 0.2 | 2750 ± 0.1 | ni | ni | ni | ||||
| Ardestan Black Leather | 5820 ± 0.1 | 4330 ± 0.0 | ni | ni | ni | ||||
| Saveh Sweet White Leather | 5490 ± 0.2 | 4070 ± 0.2 | ni | ni | ni | ||||
| Ostokhani Tabas | 9300 ± 0.1 | 7760 ± 0.1 | ni | ni | ni | ||||
| Juice | Aqueous | RPS | 1018 ± 22.1 | 0.87 ± 0.1 | 104.2 ± 12.5 | ni | 3.05 ± 0.34 | [44] | |
| RPGH | 1082 ± 12.8 | 0.18 ± 0.04 | 259.6 ± 9 | ni | 2.22 ± 0.9 | ||||
| ZZ | 960 ± 17. 5 | 0.44 ± 0.1 | 282.1 ± 11.0 | ni | 6.33 ± 0.1 | ||||
| SZ | 1026 ± 21.3 | 8.22 ± 1.9 | 342 ± 12.1 | ni | 4.12 ± 0.8 | ||||
| MY | 706 ± 23.9 | 0.63 ± 0.2 | 320.6 ± 9.9 | ni | 5.21 ± 1.1 | ||||
| MS | 1062 ± 14.1 | 0.48 ± 0.1 | 14.3 ± 4.8 | ni | 6.55 ± 0.5 | ||||
| B | 896 ± 12.1 | 1.13 ± 0.2 | 100 ± 10.1 | ni | 1.01 ± 0.4 | ||||
| Skin | Methanol | RPS | 785 ± 11.1 | 6.40 ± 1.3 | 749.5 ± 15.8 | ni | 3.23 ± 0.2 | ||
| RPGH | 645 ± 31.1 | 0.26 ± 0.03 | 328.2 ± 13.9 | ni | 4.46 ± 1 | ||||
| ZZ | 671 ± 20.02 | 1.57 ± 0.5 | 587.2 ± 10.2 | ni | 1.09 ± 0.7 | ||||
| SZ | 705 ± 23.3 | 1.69 ± 0.6 | 930 ± 16 | ni | 2.43 ± 1 | ||||
| MY | 553 ± 12.5 | 0.05 ± 0.02 | 502.4 ± 9.1 | ni | 4.43 ± 2.4 | ||||
| MS | 597 ± 18.8 | 0.16 ± 0.01 | 382.1 ± 17.5 | ni | 4.22 ± 2.2 | ||||
| B | 783 ± 10.02 | 11.20 ± 2.4 | 712.7 ± 9 | ni | 4.1 ± 0.8 | ||||
| Juice | Aqueous | unknown cultivar | Germany | 2015.2 ± 21.6 | 198.3 ± 0.8 | ni | 6.9 ± 0.0 a 21.4 ± 0.9 b | 423.8 ± 15.3 | [45] |
| 5186.0 ± 172.5 | 124.2 ± 2.2 | ni | 1.1 ± 0.0 a b: nd | 2074.4 ± 47.3 | |||||
| 2122.0 ± 0.0 | 557.7 ± 48.3 | ni | 10.6 ± 0.3 a 28.3 ± 1.4 b | 93.2 ± 3.6 | |||||
| Juice | Aqueous | Ermioni variety | Greece | 1271 ± 40 | 382.8 ± 0.1 | ni | ni | ni | [46] |
| Data is presented in mg/kg | |||||||||
| Peel | Ethanol, formic acid, MeOH, water, and acetonitrile | Italian cultivar | Italy | - | not detected | 16,156.4 ± 7450.9 | 5285.6 ± 9316.1 | ni | [47] |
| Pulp | - | 264.3 ± 184.7 | 344.4 ± 281.4 | 77.4 ± 33.3 | ni | ||||
| Peel | Aqueous | unknown cultivar | Germany | 44,261.5 ± 414.0 | 447.1 ± 11.3 | ni | 270.4 ± 18.5 a b: nd | 43,991.2 ± 395.5 c | [45] |
| Mesocarp | 40,625.1 ± 4434.7 | nd | ni | a: nd b: nd | 40,625.1 ± 4434.7 c | ||||
| Data is presented in mg/g | |||||||||
| Peel | Ethanol, formic acid, methanol, water, and acetonitrile | Gaeta 1 | Italy | 179.92 ± 1.31 | - | - | - | ni | [47] |
| Gaeta 3 | 244.61 ± 1.41 | - | - | - | ni | ||||
| Gaeta 4 | 182.15 ± 1.57 | - | - | - | ni | ||||
| Tordimonte A | 89.68 ± 0.61 | - | - | - | ni | ||||
| Itri A | 141.14 ± 0.45 | - | - | - | ni | ||||
| Wonderful | 137.28 ± 1.19 | - | - | - | ni | ||||
| Formia | 191.59 ± 3.38 | - | - | - | ni | ||||
| Pulp | Gaeta 1 | 8.89 ± 0.08 | - | - | - | ni | |||
| Gaeta 3 | 5.40 ± 0.09 | - | - | - | ni | ||||
| Gaeta 4 | 4.95 ± 0.06 | - | - | - | ni | ||||
| Tordimonte A | 3.19 ± 0.07 | - | - | - | ni | ||||
| Itri A | 6.11 ± 0.07 | - | - | - | ni | ||||
| Wonderful | 6.14 ± 0.03 | - | - | - | ni | ||||
| Formia | 5.32 ± 0.05 | - | - | - | ni | ||||
| Aril | Aqueous | Unknown cultivar | 0.040 ± 0.0072 | ni | ni | ni | ni | [48] | |
| Ethyl acetate | 0.007 ± 0.0012 | ni | ni | ni | ni | ||||
| Juice | Aqueous | 0.052 ± 0.0001 | ni | ni | ni | ni | |||
| Ethyl acetate | 0.001 ± 0.0003 | ni | ni | ni | ni | ||||
| Rind | Aqueous | 0.907 ± 0.0757 | ni | ni | ni | ni | |||
| Ethyl acetate | 0.031 ± 0.0003 | ni | ni | ni | ni | ||||
| Seed | aqueous | Gabsi variety | Tunisia | 7.94 ± 1.25 | 19.62 ± 3.12 | 3.30 ± 0.52 | ni | 32.86 ± 4.24 c | [49] |
| Leave | 9.85 ± 0.82 | 40.91 ± 3.43 | 12.77 ± 0.23 | ni | 64.40 ± 4.85 c | ||||
| Flower | 42.70 ± 2.17 | 80.20 ± 7.02 | 21.45 ± 0.58 | ni | 57.04 ± 3.41 c | ||||
| Peel | 53.65 ± 4.13 | 51.02 ± 10.33 | 21.03 ± 1.62 | ni | 62.71 ± 11.32 c | ||||
| Seed | Methanol | 11.84 ± 1.92 | 40.84 ± 7.77 c | 6.79 ± 0.57 | ni | 29.57 ± 4.54 c | |||
| Leave | 14.78 ± 2.10 | 89.81 ± 7.50 | 26.08 ± 1.24 | ni | 128.02 ± 4.49 c | ||||
| Flower | 66.29 ± 3.06 | 168.91 ± 3.1 | 72.52 ± 5.59 | ni | 148.24 ± 10.29 c | ||||
| Peel | 85.60 ± 4.87 | 102.20 ± 16.42 | 51.52 ± 8.14 | ni | 139.63 ± 4.25 c | ||||
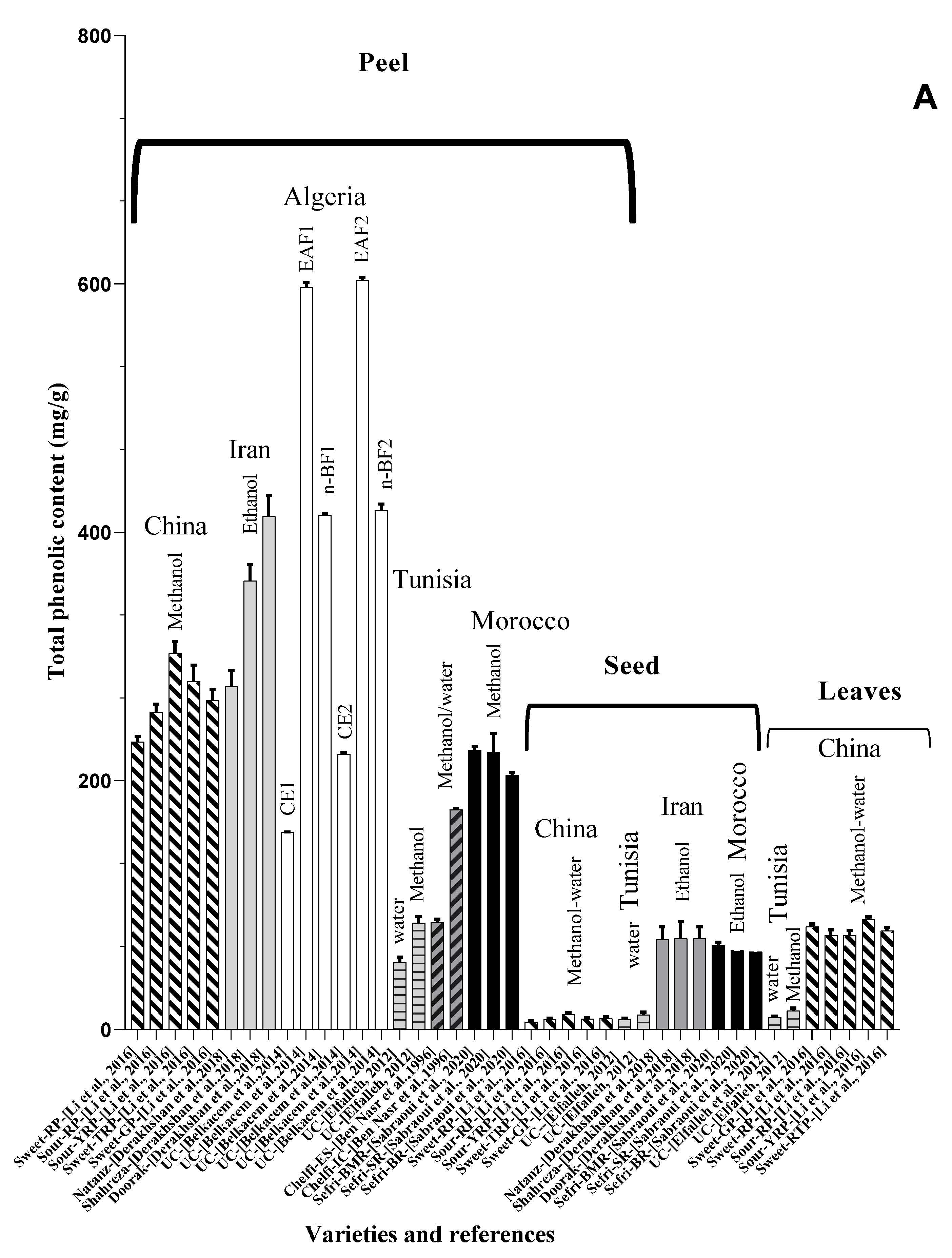
| Peel | Aqueous | Chelfi variety | 216.9 ± 7.3 | ni | ni | ni | ni | [50] | |
| Fruit | Methanol-water | Unknown cultivar | Algeria | 15.39 ± 0.08 | ni | 12.95 ± 0.07 | ni | ni | [51] |
| Seed | Methanol | Sefri | Morocco (BMR) | 67. 85 ± 1.98 | ni | 1.76 ± 0.02 | ni | ni | [52] |
| Morocco (SR) | 63.34 ± 0.7 | ni | 2.11 ± 0.28 | ni | ni | ||||
| Morocco (BR) | 62.17 ± 3.26 | ni | 1.94 ± 0.00 | ni | ni | ||||
| Peel | Morocco (BMR) | 224.39 ± 3 | ni | 62.63 ± 3.23 | ni | ni | |||
| Morocco (SR) | 223.21 ± 15 | ni | 52.12 ± 1.36 | ni | ni | ||||
| Morocco (BR) | 204.58 ± 1.96 | ni | 46.17 ± 2.18 | ni | ni | ||||
| Flowers | Hydromethanolic | Unknown cultivar | Iran | 348.81 ± 5.58 | ni | 225.776 ± 2.93 | ni | ni | [53] |
| Aqueous | 509.83 ± 11.61 | ni | 98.399 ± 1.15 | ni | ni | ||||
| n-Butanol | 866.47 ± 26.61 | ni | 258.127 ± 16.19 | ni | ni | ||||
| Ethyl acetate | 944.75 ± 8.27 | ni | 359.6 ± 13.91 | ni | ni | ||||
| Peel | Ethanol | Natanz | 276 ± 12.69 | ni | 36 ±3.56 | ni | ni | [54] | |
| Shahreza | 361 ± 12.87 | ni | 45 ± 6.25 | ni | ni | ||||
| Doorak | 413 ± 16.84 | ni | 54 ± 8.96 | ni | ni | ||||
| Seed | Natanz | 72.4 ± 10.02 | ni | 30.5 ± 6.38 | ni | ni | |||
| Shahreza | 73 ± 13.35 | ni | 7.55 ± 2.12 | ni | ni | ||||
| Doorak | 73 ± 9.45 | ni | 38 ± 6.38 | ni | ni | ||||
| Juice | Ethanol | Natanz | 23.8 ± 6.74 | ni | 1.8 ± 1.03 | ni | ni | ||
| Shahreza | 15.8 ± 5.81 | ni | 2.14 ± 0.92 | ni | ni | ||||
| Doorak | 12.4 ± 5.21 | ni | 8.7 ± 2.47 | ni | ni | ||||
| Peel | Methanol-water | Sweet-GP | China | 264.58 ± 8.74 | ni | ni | ni | ni | [55] |
| Flesh | 217.14 ± 16.80 | ni | ni | ni | ni | ||||
| Seeds | 9.04 ± 1.23 | ni | ni | ni | ni | ||||
| Juices | 8.62 ± 1.05 | ni | ni | ni | ni | ||||
| Leaves | 82.31 ± 2.26 | ni | ni | ni | ni | ||||
| Peel | Sweet-RP (Chinese cultivar) | 231.36 ± 4.40 | ni | ni | ni | ni | |||
| Flesh | 203.20 ± 12.68 | ni | ni | ni | ni | ||||
| Seeds | 6.17 ± 0.60 | ni | ni | ni | ni | ||||
| Juices | 6.12 ± 0.91 | ni | ni | ni | ni | ||||
| Leaves | 75.56 ± 4.39 | ni | ni | ni | ni | ||||
| Peel | Sour-RP (Chinese cultivar) | 255.31 ± 6.42 | ni | ni | ni | ni | |||
| Flesh | 208.39 ± 6.31 | ni | ni | ni | ni | ||||
| Seeds | 8.31 ± 0.83 | ni | ni | ni | ni | ||||
| Juices | 10.36 ± 1.12 | ni | ni | ni | ni | ||||
| Leaves | 75.61 ± 3.62 | ni | ni | ni | ni | ||||
| Peel | Sour-YRP | 302.43 ± 9.54 | ni | ni | ni | ni | |||
| Flesh | 272.10 ± 9.98 | ni | ni | ni | ni | ||||
| Seeds | 12.44 ± 0.96 | ni | ni | ni | ni | ||||
| Juices | 13.31 ± 0.87 | ni | ni | ni | ni | ||||
| Leaves | 88.33 ± 1.92 | ni | ni | ni | ni | ||||
| Peel | Sweet-TRP | 279.76 ± 13.32 | ni | ni | ni | ni | |||
| Flesh | 222.62 ± 7.72 | ni | ni | ni | ni | ||||
| Seeds | 8.59 ± 1.04 | ni | ni | ni | ni | ||||
| Juices | 12.91 ± 0.87 | ni | ni | ni | ni | ||||
| Leaves | 79.10 ± 2.74 | ni | ni | ni | ni | ||||
| Peel | CE1 | Unknown cultivar | Algeria | 158.18 ± 0.66 | ni | 12.8 ± 2.2 | ni | - | [56] |
| CE2 | 221.54 ± 1.08 | ni | 30.9 ± 1.6 | ni | - | ||||
| EAF1 | 597.08 ± 3.9 | ni | 135.5 ± 2.0 | ni | - | ||||
| n-BF1 | 413.6 ± 1.48 | ni | 91.1 ± 0.88 | ni | - | ||||
| EAF2 | 602.8 ± 2.41 | ni | 149.4 ± 1.27 | ni | - | ||||
| n-BF2 | 417.56 ± 5.3 | ni | 115.56 ± 1.81 | ni | - | ||||
| Data is presented in µg/mg | |||||||||
| Fruit | Water | Unknown cultivar | India | 11.99 | ni | ni | ni | ni | [57] |
| Ethanol | 28.13 | ni | ni | ni | ni | ||||
| Acetone | 6.44 | ni | ni | ni | ni | ||||
| Ether | 24.13 | ni | ni | ni | ni | ||||
| Ethanol-Ether | 24.2 | ni | ni | ni | ni | ||||
| Ethanol-water | 15.12 | ni | ni | ni | ni | ||||
| Ethanol-Ether-water | 24.5 | ni | ni | ni | ni | ||||
| Data is presented in mg/mL | |||||||||
| Juice | Aqueous | Gaeta 1 | Italy | 1.93 ± 0.05 | - | - | - | ni | [47] |
| Gaeta 3 | 1.34 ± 0.07 | - | - | - | ni | ||||
| Gaeta 4 | 0.87 ± 0.03 | - | - | - | ni | ||||
| Tordimonte A | 1.22 ± 0.03 | - | - | - | ni | ||||
| Itri A | 1.20 ± 0.05 | - | - | - | ni | ||||
| Wonderful | 1.58 ± 0.02 | - | - | - | ni | ||||
| Formia | 1.08 ± 0.06 | - | - | - | ni | ||||

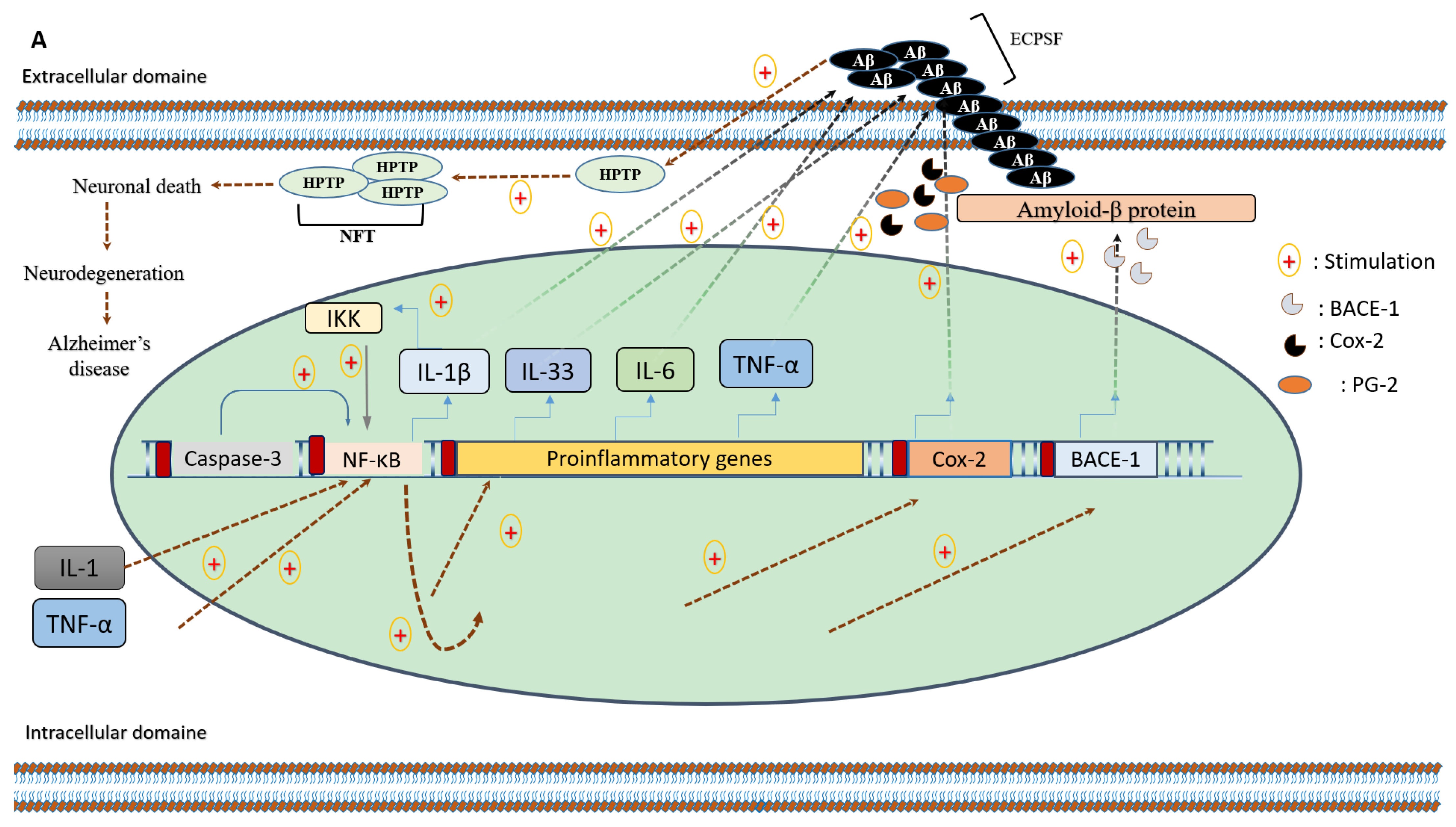
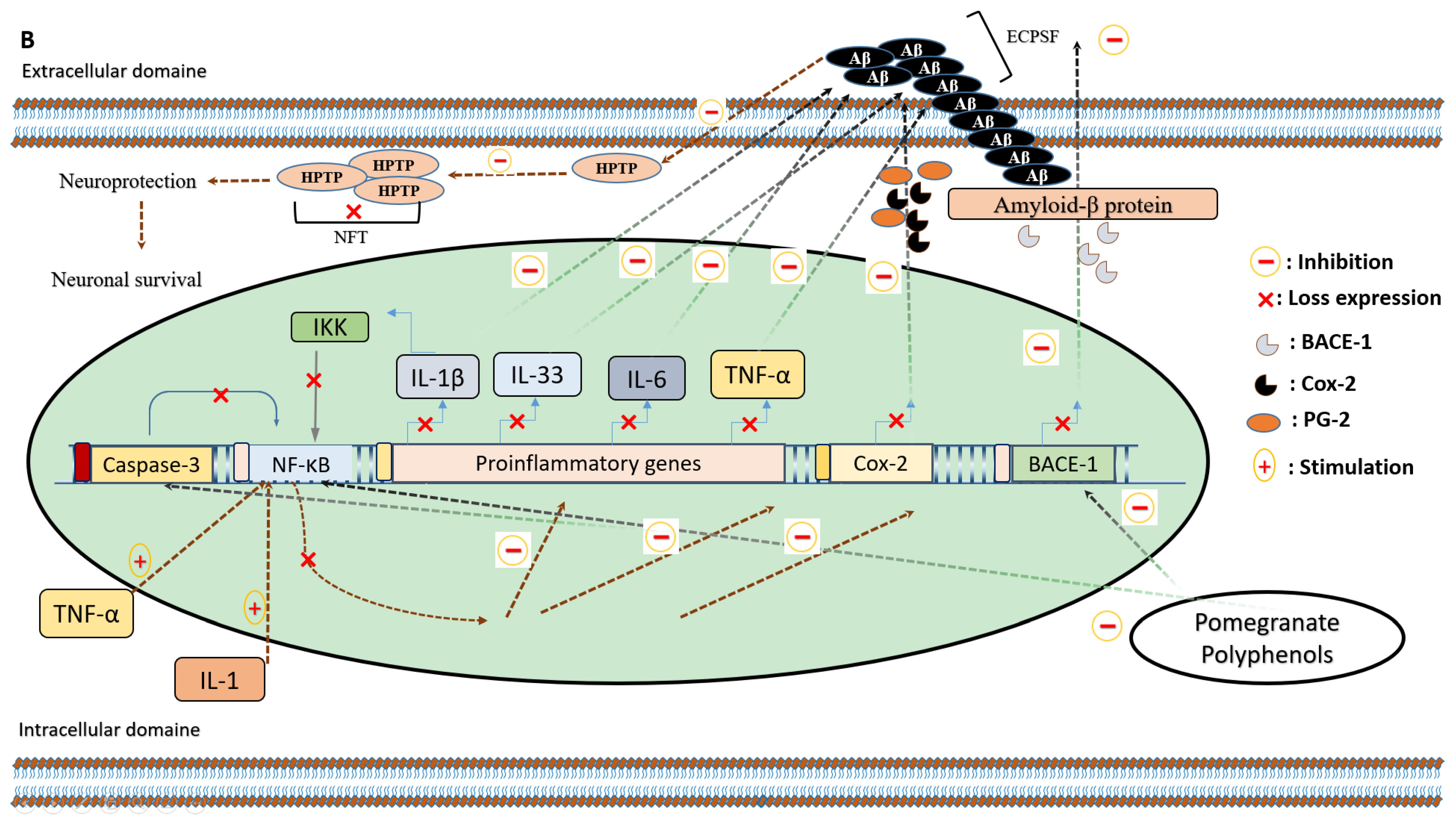
5. Pomegranate Consumption and Neurodegenerative Disorders
5.1. Anti-Inflammatory Effects of Pomegranate Intake on Alzheimer’s Disease
5.2. Anti-Inflammatory Effects of Pomegranate Intake against Parkinson’s Disease
5.3. The Preventive Role of Pomegranate against Multiple Sclerosis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swardfager, W.; Lanctôt, K.; Rothenburg, L.; Wong, A.; Cappell, J.; Herrmann, N. A meta-analysis of cytokines in Alzheimer’s disease. Biol. Psychiatry 2010, 68, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Forlenza, O.V.; Diniz, B.S.; Talib, L.L.; Mendonça, V.A.; Ojopi, E.B.; Gattaz, W.F.; Teixeira, A.L. Increased serum IL-1beta level in Alzheimer’s disease and mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2009, 28, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Essa, M.M.; Subash, S.; Akbar, M.; Al-Adawi, S.; Guillemin, G.J. Guillemin Long-Term dietary supplementation of pomegranates, figs and dates alleviate neuroinflammation in a transgenic mouse model of alzheimer’s disease. PLoS ONE 2015, 10, e120964. [Google Scholar] [CrossRef] [PubMed]
- Flex, A.; Giovannini, S.; Biscetti, F.; Liperoti, R.; Spalletta, G.; Straface, G.; Landi, F.; Angelini, F.; Caltagirone, C.; Ghirlanda, G.; et al. Effect of proinflammatory gene polymorphisms on the risk of Alzheimer’s disease. Neurodegener. Dis. 2014, 13, 230–236. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Effect of non-steroidal anti-inflammatory drugs on risk of Alzheimer’s disease. BMJ 2003, 327, 128. [Google Scholar]
- Kim, Y.E.; Hwang, C.J.; Lee, H.P.; Kim, C.S.; Son, D.J.; Ham, Y.W.; Hellström, M.; Han, S.B.; Kim, H.S.; Park, E.K.; et al. Inhibitory effect of punicalagin on lipopolysaccharide-induced neuroinflammation, oxidative stress and memory impairment via inhibition of nuclear factor-kappaB. Neuropharmacology 2017, 117, 21–32. [Google Scholar] [CrossRef]
- Sun, S.C.; Chang, J.H.; Jin, J. Regulation of nuclear factor-kB in autoimmunity. Trends Immunol. 2013, 34, 282–289. [Google Scholar] [CrossRef]
- Velagapudi, R.; Baco, G.; Khela, S.; Okorji, U.; Olajide, O. Pomegranate inhibits neuroinflammation and amyloidogenesis in IL-1 β-stimulated SK-N-SH cells. Eur. J. Nutr. 2015, 55, 1653–1660. [Google Scholar] [CrossRef]
- Loren, D.J.; Seeram, N.P.; Schulman, R.N.; Holtzman, D.M. Maternal dietary supplementation with pomegranate juice is neuroprotective in an animal model of neonatal hypoxic-ischemic brain injury. Pediatric Res. 2005, 57, 858–864. [Google Scholar] [CrossRef]
- Yoo, H.J.; Kwon, M. Aged Microglia in Neurodegenerative Diseases: Microglia Lifespan and Culture Methods. Front. Aging Neurosci. 2022, 13, 766267. [Google Scholar] [CrossRef]
- Cuní-López, C.; Stewart, R.; Quek, H.; White, A.R. Recent Advances in Microglia Modelling to Address Translational Outcomes in Neurodegenerative Diseases. Cells 2022, 11, 1662. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, S.; Basilico, B.; Marrone, M.C.; Ragozzino, D. Microglia-neuron crosstalk: Singh naling mechanism and control of synaptic transmission. Semin. Cell Dev. Biol. 2019, 94, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Correa, F.C. Miriam Hernangómez, Carmen Guaza. Understanding Microglia–Neuron Cross Talk: Relevance of the Microglia–Neuron Cocultures; Microglia: Totowa, NJ, USA, 2013. [Google Scholar]
- Damulewicz, M.; Szypulski, K.; Pyza, E. Glia-Neurons Cross-Talk Regulated Through Autophagy. Front. Physiol. 2022, 13, 886273. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, G.G.; Flores Alvarado, L.J.; Pacheco Moisés, F.P.; Mireles Ramírez, M.A.; González Renovato, E.D.; Sánchez López, A.L.; Sánchez Romero, L.; Santoscoy, J.F.; Velázquez Brizuela, I.E.; Sánchez González, V.J. Cross-talk between glial cells and neurons: Relationship in Multiple Sclerosis. Clin. Case Rep. Rev. 2016, 2, 565–571. [Google Scholar]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Neumann, H.; Kotter, M.R.; Franklin, R.J.M. Debris clearance by microglia: An essential link between degeneration and regeneration. Brain 2009, 132, 288–295. [Google Scholar] [CrossRef]
- Lull, M.E.; Block, M.L. Microglial Activation and Chronic Neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef]
- Pires, B.R.; Silva, R.C.; Ferreira, G.M.; Abdelhay, E. NF-kappaB: Two Sides of the Same Coin. Genes 2018, 9, 24. [Google Scholar] [CrossRef]
- Tsatsanis, C.; Androulidaki, A.; Venihaki, M.; Margioris, A.N. Signalling networks regulating cyclooxygenase-2. Int. J. Biochem. Cell Biol. 2006, 38, 1654–1661. [Google Scholar] [CrossRef]
- Chen, C.H.; Zhou, W.; Liu, S.; Deng, Y.; Cai, F.; Tone, M.; Tone, Y.; Tong, Y.; Song, W. Increased NF-kB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int. J. Neuropsychopharmacol. 2012, 15, 77–90. [Google Scholar] [CrossRef]
- Perlmutter, L.S.; Scott, S.A.; Barron, E.; Chui, H.C. MHC class II-positive microglia in human brain: Association with alzheimer lesions. J. Neurosci. Res. 1992, 33, 549–558. [Google Scholar] [CrossRef] [PubMed]
- YOuchi, Y.; Yoshikawa, E.; Sekine, Y.; Futatsubashi, M.; Kanno, T.; Ogusu, T.; Torizuka, T. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann. Neurol. 2005, 57, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, Y.; Yagi, S.; Yokokura, M.; Sakamoto, M. Neuroinflammation in the living brain of Parkinson’s disease. Park. Relat. Disord. 2009, 15, S200–S204. [Google Scholar] [CrossRef]
- Brites, D.; Vaz, A.R. Microglia centered pathogenesis in ALS: Insights in cell interconnectivity. Front. Cell. Neurosci. 2014, 8, 117. [Google Scholar] [CrossRef]
- Tai, Y.F.; Pavese, N.; Gerhard, A.; Tabrizi, S.J.; Barker, R.A.; Brooks, D.J.; Piccini, P. Microglial activation in presymptomatic Huntington’s disease gene carriers. Brain 2007, 130, 1759–1766. [Google Scholar] [CrossRef]
- Singhrao, S.K.; Neal, J.W.; Morgan, B.P.; Gasque, P. Increased complement biosynthesis by microglia and complement activation on neurons in Huntington’s disease. Exp. Neurol. 1999, 159, 362–376. [Google Scholar] [CrossRef]
- Kaltschmidt, B.; Uherek, M.; Volk, B.; Baeuerle, P.A.; Kaltschmidt, C. Transcription factor NF-kB is activated in primary neurons by amyloid b peptides and in neurons surrounding early plaques from patients with Alzheimer disease. Proc. Natl. Acad. Sci. USA 1997, 94, 2642–2647. [Google Scholar] [CrossRef]
- Patrick, A. Baeuerle and Thomas Henkel. Function and activation of NF-kB in the immune system. Annu. Rev. Immunol. 1994, 12, 141–179. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Noroozian, M.; Mohammadi, M.; Ohadinia, S.; Jamshidi, A.H.; Khani, M. Melissa officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: A double blind, randomised, placebo-controlled trial. J. Neurol. Neurosurg. Psychiatry 2003, 74, 863–866. [Google Scholar] [CrossRef]
- Hishikawa, N.; Takahashi, Y.; Amakusa, Y.; Tanno, Y.; Tuji, Y.; Niwa, H.; Murakami, N.; Krishna, U.K. Effects of turmeric on Alzheimer′s disease with behavioral and psychological symptoms of dementia. AYU Int. Q. J. Res. Ayurveda 2012, 33, 499. [Google Scholar] [CrossRef]
- Morillas-Ruiz, J.M.; Rubio-Perez, J.M.; Albaladejo, M.D.; Zafrilla, P.; Parra, S.; Vidal-Guevara, M.L. Effect of an antioxidant drink on homocysteine levels in Alzheimer’s patients. J. Neurol. Sci. 2010, 299, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.; Bhattacharyya, S.; Bose, S. Efficacy and Safety of Ashwagandha (Withania somnifera (L.) Dunal) Root Extract in Improving Memory and Cognitive Functions. J. Diet. Suppl. 2017, 14, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Tsolaki, M.; Lazarou, E.; Kozori, M.; Petridou, N.; Tabakis, I.; Lazarou, I.; Karakota, M.; Saoulidis, I.; Melliou, E.; Magiatis, P. A Randomized Clinical Trial of Greek High Phenolic Early Harvest Extra Virgin Olive Oil in Mild Cognitive Impairment: The MICOIL Pilot Study. J. Alzheimer’s Dis. 2020, 78, 801–817. [Google Scholar] [CrossRef]
- Gul, A.; Bakht, J.; Mehmood, F. Huperzine-A response to cognitive impairment and task switching deficits in patients with Alzheimer’s disease. J. Chin. Med. Assoc. 2019, 82, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yan, H.; Tang, X.C. Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol. Sin. 2006, 27, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.S.; Cai, Z.Y.; Qu, Z.W.; Yang, R.M.; Cai, Y.L.; Wang, G.Q.; Su, X.Q.; Zhong, X.S.; Cheng, R.Y.; Xu, W.A.; et al. Huperzine-A in capsules and tablets for treating patients with Alzheimer disease. Zhongguo Yao Li Xue Bao 1999, 20, 486–490. [Google Scholar] [PubMed]
- Turner, R.S.; Thomas, R.G.; Craft, S.; Van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. Placebo-Controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflamm. 2017, 14, 1–10. [Google Scholar] [CrossRef]
- Hasnaoui, N.; Jbir, R.; Mars, M.; Trifi, M.; Kamal-Eldin, A.; Melgarejo, P.; Hernandez, F. Organic acids, sugars, and anthocyanins contents in juices of Tunisian pomegranate fruits. Int. J. Food Prop. 2011, 14, 741–757. [Google Scholar] [CrossRef]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef]
- Du, L.; Li, J.; Zhang, X.; Wang, L.; Zhang, W. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of MAPKs activation. J. Funct. Foods 2018, 43, 62–69. [Google Scholar] [CrossRef]
- Mousavinejad, G.; Emam-Djomeh, Z.; Rezaei, K.; Khodaparast, M.H.H. Identification and quantification of phenolic compounds and their effects on antioxidant activity in pomegranate juices of eight Iranian cultivars. Food Chem. 2009, 115, 1274–1278. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Ayanmanesh, M.; Babadayei-Samani, R.; Javid, A.; Sanaeifard, M.; Vitalini, S.; Iriti, M. Total anthocyanin, flavonoid, polyphenol and tannin contents of seven pomegranate cultivars grown in Iran [pdf]. Acta Sci. Pol. Technol. Aliment. 2018, 17, 211–217. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD-ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef]
- Gardeli, C.; Varela, K.; Krokida, E.; Mallouchos, A. Investigation of Anthocyanins Stability from. Medicines 2019, 6, 90. [Google Scholar] [CrossRef]
- Russo, M.; Fanali, C.; Tripodo, G.; Dugo, P.; Muleo, R.; Dugo, L.; De Gara, L.; Mondello, L. Analysis of phenolic compounds in different parts of pomegranate (Punica granatum) fruit by HPLC-PDA-ESI/MS and evaluation of their antioxidant activity: Application to different Italian varieties. Anal. Bioanal. Chem. 2018, 410, 3507–3520. [Google Scholar] [CrossRef]
- Ricci, D.; Giamperi, L.; Bucchini, A.; Fraternale, D. Antioxidant activity of Punica granatum fruits. Fitoterapia 2006, 77, 310–312. [Google Scholar] [CrossRef]
- Elfalleh, W. Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. J. Med. Plants Res. 2012, 6, 4724–4730. [Google Scholar] [CrossRef]
- Ben Nasr, C.; Ayed, N.; Metche, M. Quantitative determination of the polyphenolic content of pomegranate peel. Z. Fur Leb. Unters. Und Forsch. 1996, 203, 374–378. [Google Scholar] [CrossRef]
- Zeghad, N.; Ahmed, E.; Belkhiri, A.; Vander Heyden, Y.; Demeyer, K. Antioxidant activity of Vitis vinifera, Punica granatum, Citrus aurantium and Opuntia ficus indica fruits cultivated in Algeria. Heliyon 2019, 5, e01575. [Google Scholar] [CrossRef]
- Sabraoui, T.; Khider, T.; Nasser, B.; Eddoha, R.; Moujahid, A.; Benbachir, M.; Essamadi, A. Determination of Punicalagins Content, Metal Chelating, and Antioxidant Properties of Edible Pomegranate (Punica granatum L.) Peels and Seeds Grown in Morocco. Int. J. Food Sci. 2020, 2020, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Amjad, L.; Shafighi, M. Evaluation of Antioxidant Activity, Phenolic and Flavonoid Content in Punica granatum var. Isfahan Malas Flowers. Int. J. Agric. Crop Sci. 2013, 5, 1133–1139. [Google Scholar]
- Derakhshan, Z.; Ferrante, M.; Tadi, M.; Ansari, F.; Heydari, A.; Hosseini, M.S.; Conti, G.O.; Sadrabad, E.K. Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem. Toxicol. 2018, 114, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, X.G.; Jia, K.; Liu, Z.P.; Peng, H.Y. A systematic determination of polyphenols constituents and cytotoxic ability in fruit parts of pomegranates derived from five Chinese cultivars. Springerplus 2016, 5, 914. [Google Scholar] [CrossRef] [PubMed]
- Belkacem, N.; Djaziri, R.; Lahfa, F.; El-Haci, I.A.; Boucherit, Z. Screening phytochimique et activité antioxidante in vitro de différents extraits de l’épicarpe de Punica granatum L. d’Algérie: Étude comparative. Phytotherapie 2014, 12, 372–379. [Google Scholar] [CrossRef]
- Singh, M.; Jha, A.; Kumar, A.; Hettiarachchy, N.; Rai, A.K.; Sharma, D. Influence of the solvents on the extraction of major phenolic compounds (Punicalagin, ellagic acid and gallic acid) and their antioxidant activities in pomegranate aril. J. Food Sci. Technol. 2014, 51, 2070–2077. [Google Scholar] [CrossRef]
- Kim, H.; Banerjee, N.; Ivanov, I.; Pfent, C.M.; Prudhomme, K.R.; Bisson, W.H.; Dashwood, R.H.; Talcott, S.T.; Mertens-Talcott, S.U. Comparison of anti-inflammatory mechanisms of mango (Mangifera Indica L.) and pomegranate (Punica Granatum L.) in a preclinical model of colitis. Mol. Nutr. Food Res. 2016, 60, 1912–1923. [Google Scholar] [CrossRef]
- Türkyilmaz, M. Anthocyanin and organic acid profiles of pomegranate (Punica granatum L.) juices from registered varieties in Turkey. Int. J. Food Sci. Technol. 2013, 48, 2086–2095. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Changes in physical properties, chemical and elemental composition and antioxidant capacity of pomegranate (cv. Ruby) fruit at five maturity stages. Sci. Hortic. Amst. 2013, 150, 37–46. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Effects of maturity status on biochemical content, polyphenol composition and antioxidant capacity of pomegranate fruit arils (cv. ’Bhagwa’). South Afr. J. Bot. 2013, 85, 23–31. [Google Scholar] [CrossRef]
- de Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Quantitative analysis of flavan-3-ols in Spanish foodstuffs and beverages. J. Agric. Food Chem. 2000, 48, 5331–5337. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Caravaca, A.M.; Verardo, V.; Toselli, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Caboni, M.F. Determination of the major phenolic compounds in pomegranate juices by HPLC-DAD-ESI-MS. J. Agric. Food Chem. 2013, 61, 5328–5337. [Google Scholar] [CrossRef]
- Van Elswijk, D.A.; Schobel, U.P.; Lansky, E.P.; Irth, H.; Van Der Greef, J. Rapid dereplication of estrogenic compounds in pomegranate (Punica granatum) using on-line biochemical detection coupled to mass spectrometry. Phytochemistry 2004, 65, 233–241. [Google Scholar] [CrossRef]
- Noda, Y.; Kaneyuki, T.; Mori, A.; Packer, L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: Delphinidin, cyanidin, and pelargonidin. J. Agric. Food Chem. 2002, 50, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, F.; Melgarejo, P.; Tomas-Barberan, F.A.; Artes, F. Evolution of juice anthocyanins during ripening of new selected pomegranate (Punica granatum) clones. Eur. Food Res. Technol. 1999, 210, 39–42. [Google Scholar] [CrossRef]
- Ali, M.; Sharma, N. Phytochemical investigation of the flowers of Punica granatum. Indian J. Chem. Sect. B Org. Med. Chem. 2006, 45, 1681–1685. [Google Scholar] [CrossRef]
- Negi, P.S.; Jayaprakasha, G.K.; Jena, B.S. Antioxidant and antimutagenic activities of pomegranate peel extracts. Food Chem. 2003, 80, 393–397. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Aarabi, A.; Barzegar, M.; Azizi, M.H. Effect of cultivar and cold storage of pomegranate (Punica granatum L.) Juices on organic acid composition. Int. Food Res. J. 2008, 15, 45–55. [Google Scholar]
- Garima, P.; Akoh, C.C. Antioxidant capacity and lipid characterization of six georgia-grown pomegranate cultivars. J. Agric. Food Chem. 2009, 57, 9427–9436. [Google Scholar] [CrossRef]
- Poyrazoğlu, E.; Gökmen, V.; Artιk, N. Organic Acids and Phenolic Compounds in Pomegranates (Punica granatum L.) Grown in Turkey. J. Food Compos. Anal. 2002, 15, 567–575. [Google Scholar] [CrossRef]
- Wang, R.; Wang, W.; Wang, L.; Liu, R.; Ding, Y.; Du, L. Constituents of the flowers of Punica granatum. Fitoterapia 2006, 77, 534–537. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef]
- El-Toumy, S.A.A.; Rauwald, H.W. Two new ellagic acid rhamnosides from Punica granatum heartwood. Planta Med. 2003, 69, 682–684. [Google Scholar] [CrossRef]
- Nawwar, M.A.; Hussein, S.A.; Merfort, I. NMR spectral analysis of polyphenols from Punica granatum. Phytochemistry 1994, 36, 793–798. [Google Scholar] [CrossRef]
- Tanaka, T.; Nonaka, G.I.; Nishioka, I. Tannins and related compounds.XL1. Revision of the structures of Punicalin and Punicalagin and isolation and charcterization of 2-O-Galloylpunicalin from the Bark of Punica granatum L. Chem. Pharm. Bull. 1986, 34, 650–655. [Google Scholar] [CrossRef]
- Huang, T.H.W.; Peng, G.; Kota, B.P.; Li, G.Q.; Yamahara, J.; Roufogalis, B.D.; Li, Y. Anti-diabetic action of Punica granatum flower extract: Activation of PPAR-γ and identification of an active component. Toxicol. Appl. Pharmacol. 2005, 207, 160–169. [Google Scholar] [CrossRef]
- Wang, R.F.; Xie, W.D.; Zhang, Z.; Xing, D.M.; Ding, Y.; Wang, W.; Ma, C.; Du, L.J. Bioactive compounds from the seeds of Punica granatum (pomegranate). J. Nat. Prod. 2004, 67, 2096–2098. [Google Scholar] [CrossRef]
- Kulkarni, A.P.; Aradhya, S.M.; Divakar, S. Isolation and identification of a radical scavenging antioxidant-Punicalagin from pith and carpellary membrane of pomegranate fruit. Food Chem. 2004, 87, 551–557. [Google Scholar] [CrossRef]
- Ishimoto, H.; Shibata, M.; Myojin, Y.; Ito, H.; Sugimoto, Y.; Tai, A.; Hatano, T. In vivo anti-inflammatory and antioxidant properties of ellagitannin metabolite urolithin A. Bioorg. Med. Chem. Lett. 2011, 21, 5901–5904. [Google Scholar] [CrossRef]
- Yuan, T.; Ma, H.; Liu, W.; Niesen, D.B.; Shah, N.; Crews, R.; Rose, K.N.; Vattem, D.A.; Seeram, N.P. Pomegranate’s Neuroprotective Effects against Alzheimer’s Disease Are Mediated by Urolithins, Its Ellagitannin-Gut Microbial Derived Metabolites. ACS Chem. Neurosci. 2016, 7, 26–33. [Google Scholar] [CrossRef]
- Rogers, J.; Luber-Narod, J.; Styren, S.D.; Civin, W.H. Expression of immune system-associated antigens by cells of the human central nervous system: Relationship to the pathology of Alzheimer’s disease. Neurobiol. Aging 1988, 9, 339–349. [Google Scholar] [CrossRef]
- Shukla, M.; Gupta, K.; Rasheed, Z.; Khan, K.A.; Haqqi, T.M. Consumption of hydrolyzable tannins-rich pomegranate extract suppresses inflammation and joint damage in rheumatoid arthritis. Nutrition 2008, 24, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Husari, A.; Hashem, Y.; Bitar, H.; Dbaibo, G.; Zaatari, G.; El Sabban, M. Antioxidant activity of pomegranate juice reduces emphysematous changes and injury secondary to cigarette smoke in an animal model and human alveolar cells. Int. J. COPD 2016, 11, 227–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachoual, R.; Talmoudi, W.; Boussetta, T.; Braut, F.; El-Benna, J. An aqueous pomegranate peel extract inhibits neutrophil myeloperoxidase in vitro and attenuates lung inflammation in mice. Food Chem. Toxicol. 2011, 49, 1224–1228. [Google Scholar] [CrossRef]
- Asghari, G.; Sheikholeslami, S.; Mirmiran, P.; Chary, A.; Hedayati, M.; Shafiee, A.; Azizi, F. Effect of pomegranate seed oil on serum TNF-α level in dyslipidemic patients. Int. J. Food Sci. Nutr. 2012, 63, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, Z.; Akhtar, N.; Anbazhagan, A.N.; Ramamurthy, S.; Shukla, M.; Haqqi, T.M. Polyphenol-rich pomegranate fruit extract (POMx) suppresses PMACI-induced expression of pro-inflammatory cytokines by inhibiting the activation of MAP kinases and NF-κB in human KU812 cells. J. Inflamm. 2009, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Morzelle, M.C.; Salgado, J.M.; Telles, M.; Mourelle, D.; Bachiega, P.; Buck, H.S.; Viel, T.A. Neuroprotective effects of pomegranate peel extract after chronic infusion with amyloid-β peptide in mice. PLoS ONE 2016, 11, e166123. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Essa, M.M.; Poljak, A.; Selvaraju, S.; Al-Adawi, S.; Manivasagm, T.; Thenmozhi, A.J.; Ooi, L.; Sachdev, P.; Guillemin, G.J. Consumption of pomegranates improves synaptic function in a transgenic mice model of Alzheimer’s disease. Oncotarget 2016, 7, 64589–64604. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Levine, H. Alzheimer’s disease and the amyloid-β peptide. J. Alzheimer’s Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Račková, L.; Ergin, V.; Burcu Bali, E.; Kuniakova, M.; Karasu, Ç. Pomegranate seed oil modulates functions and survival of BV-2 microglial cells in vitro. Int. J. Vitam. Nutr. Res. 2014, 84, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Siddarth, P.; Li, Z.; Miller, K.J.; Ercoli, L.M.; Merril, D.A.; Henning, S.M.; Heber, D.; Small, G.W. Randomized placebo-controlled study of the memory effects of pomegranate juice in middle-aged and older adults. Am. J. Clin. Nutr. 2020, 111, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Huang, J.; Xu, B.; Ou, Z.; Zhang, L.; Lin, X.; Ye, X.; Kong, X.; Long, D.; Sun, X.; et al. Urolithin A attenuates memory impairment and neuroinflammation in APP/PS1 mice. J. Neuroinflamm. 2019, 16, 62. [Google Scholar] [CrossRef]
- Tapias, V.; Cannon, J.R.; Greenamyre, J.T. Pomegranate juice exacerbates oxidative stress and nigrostriatal degeneration in Parkinson’s disease. Neurobiol. Aging 2014, 35, 1162–1176. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.A.; Kamal, M.M.; Khalil, M.G.; Ali, S.A.; Elariny, H.A.; Bekhit, A.; Wahid, A. Behavioral, Biochemical and Histopathological effects of Standardised Pomegranate extract with Vinpocetine, Propolis or Cocoa in a rat model of Parkinson’s disease. BioRxiv 2020. [Google Scholar] [CrossRef]
- Amer, O.S.; Dkhil, M.A.; Hikal, W.M.; Al-Quraishy, S. Antioxidant and anti-inflammatory activities of pomegranate (punica granatum) on eimeria papillata -induced infection in mice. Biomed Res. Int. 2015, 2015, 219670. [Google Scholar] [CrossRef] [PubMed]
- Bekir, J.; Mars, M.; Vicendo, P.; Fterrich, A.; Bouajila, J. Chemical composition and antioxidant, anti-inflammatory, and antiproliferation activities of pomegranate (Punica granatum) flowers. J. Med. Food. 2013, 16, 544–550. [Google Scholar] [CrossRef]
- Bekir, J.; Mars, M.; Souchard, J.P.; Bouajila, J. Assessment of antioxidant, anti-inflammatory, anti-cholinesterase and cytotoxic activities of pomegranate (Punica granatum) leaves. Food Chem. Toxicol. 2013, 55, 470–475. [Google Scholar] [CrossRef]
- Kujawska, M.; Jourdes, M.; Kurpik, M.; Szulc, M.; Szaefer, H.; Chmielarz, P.; Kreiner, G.; Krajka-Kuźniak, V. Neuroprotective Effects of Pomegranate Juice against Parkinson’s Disease and Presence of Ellagitannins- Derived Metabolite—Urolithin A—In the Brain. Int. J. Mol. Sci. 2020, 21, 202. [Google Scholar] [CrossRef]
- Stojanović, I.; Šavikin, K.; Đedović, N.; Živković, J.; Saksida, T.; Momčilović, M.; Koprivica, I.; Vujičić, M.; Stanisavljević, S.; Miljković, Đ.; et al. Pomegranate peel extract ameliorates autoimmunity in animal models of multiple sclerosis and type 1 diabetes. J. Funct. Foods 2017, 35, 522–530. [Google Scholar] [CrossRef]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Binyamin, O.; Larush, L.; Frid, K.; Keller, G.; Friedman-Levi, Y.; Ovadia, H.; Abramsky, O.; Magdassi, S.; Gabizon, R. Treatment of a multiple sclerosis animal model by a novel nanodrop formulation of a natural antioxidant. Int. J. Nanomed. 2015, 10, 7165–7174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Animal Model/ Cell Line/CTI | Induction of Neuro-Inflammation | Pomegranate Part or Product | Dose and Period of Treatment | Findings | Ref |
|---|---|---|---|---|---|
| Animal studies | |||||
| Male C57BI/6 mice | Chronic injection of Aβ peptide | Pomegranate peel extract | 800 mg/kg/day for 35 days | Improves spatial memory, and the expression of neurotrophin BDNF; reduce amyloid plaque density, AchE activity, lipid peroxidation and TNF-α level; no hepatic lesions. | [89] |
| Male ICR mice | LPS injection (250 µg/kg) | Punicalagin | 1.5 mg/kg for 4 weeks | Memory improvement; no effect on swimming speed; reduction of BACE1 expression and Aβ 1-42 generation; no effect on APP expression; decrease the Il-1-β, IL-6 and TNF-α, H2O2 and MDA level; increase GSH/GSSG ratio; inhibition of iNOS and Co-2 activities; decrease NF-kB DNA binding activity; suppress IkB-α phosphorylation and P50 and P65 subunits translocation. | [6] |
| APPsw/Tg2576 mice | Experimental animal model overexpressing APP | Pomegranate fruit | 4% w/w, for 15 months | Decrease serum concentration of IL-2, IL-3, IL-4, IL-5, IL-9, IL-10, and Eotaxin; decrease the level of IL-1β, TNF-α, and IL-6, Aβ1–42 and 40, both in the cortex and hippocampus areas. | [3] |
| APPsw/Tg2576 mice | Transgenic female | Pomegranate extract | 4% w/w, for 15 months | Significant inhibition of TNF-α, Il-1-β, iNOS, ccl2, and Il-10 genes expression. | [90] |
| APP/PS1 transgenic mice | Transgenic female | Urolithin A | 300 mg/kg, for 14 days | Neuronal death attenuation; hippocampal neurogenesis improvement; diminished astrocytes and microglia activation; reduction in the level of IL-1β, IL-6, and TNF-α. | [94] |
| In vitro studies | |||||
| The human neuroblastoma (SK-N-SH) cells | IL-1 β (10 U/mL) | Standardized Pomegranate fruit juice (skins) | 25–200 μg/mL, for 24 h | Inhibition of Co-2 activity, and PGE-2 production; significant inhibition of IκB and IKK phosphorylation; reduction of BACE1 gene expression and Aβ production. | [8] |
| HEK293 cells | TNFα (1 ng/mL) | 25–200 μg/mL, for 6 h | Significant inhibition of NF-κB transactivation. | ||
| Primary astrocyte and microglial BV-2 cells | LPS-(10, 20 and 50 µM), for 1 h | Punicalagin | (1 µg/mL), for 24h | Suppress the activation of NF-kB; inhibit IkB degradation and p50 and p65 translocation; inhibit APP and BACE1 transcription. | [6] |
| Microglial BV-2 cell | LPS | Pomegranate seed oil | 25 μg/mL, for 24h | Slight inhibition of TNF-α and iNOS gene expression; prevent microglia cells apoptosis. | [92] |
| Clinical trials | |||||
| NCT02093130 | _ | Pomegranate juice | 236.5 mL, for 12 months | Pomegranate juice supplementation stabilizes human capacities in terms of the acquisition of new visual information during advancement with age. | [93] |
| Animal Model | Induction of Parkinson’s Disease | Pomegranate Part or Product | Dose And Period Of Treatment | Findings | Ref |
|---|---|---|---|---|---|
| Male Lewis rats | intraperitoneal administration of Rotenone (3 mg/kg) | Pomegranate juice | 6.5–7.5 mL/day, for 2 weeks | ↑ iNOS expression and activation; ↔ IL-1β; ↔ TNF-α level; ↔ Cox2 expression; ↑ p65 catalytic subunit of the NF-κB; ↑caspase-3 activity. | [95] |
| Male albino rats | Rotenone (2.5 mg/kg), for 30 days | Pomegranate extract | 150 mg/kg/day, for 30 days | ↑ locomotor activity; ↓ AchE activities; ↑ BDNF and glutamate; ↓ lipid peroxidation; ↑ SOD; ↑ TAA; ↓ IL-1β; ↓ TNF-α; ↓ iNOS. | [96] |
| Male albino Wistar rats | subcutaneous injection of Rotenone (1.3 mg/kg), for 35 days | Pomegranate juice | 500 mg/kg/day, for 45 days | ↑ postural stability; ↑ neuronal survival; ↓ MDA; ↓ α-synuclein; ↑ mitochondrial ALDH activity; ↑ antiapoptotic Bcl-xL protein. | [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehdi, A.; Lamiae, B.; Samira, B.; Ramchoun, M.; Abdelouahed, K.; Tamas, F.; Hicham, B. Pomegranate (Punica granatum L.) Attenuates Neuroinflammation Involved in Neurodegenerative Diseases. Foods 2022, 11, 2570. https://doi.org/10.3390/foods11172570
Mehdi A, Lamiae B, Samira B, Ramchoun M, Abdelouahed K, Tamas F, Hicham B. Pomegranate (Punica granatum L.) Attenuates Neuroinflammation Involved in Neurodegenerative Diseases. Foods. 2022; 11(17):2570. https://doi.org/10.3390/foods11172570
Chicago/Turabian StyleMehdi, Alami, Benchagra Lamiae, Boulbaroud Samira, Mhamed Ramchoun, Khalil Abdelouahed, Fulop Tamas, and Berrougui Hicham. 2022. "Pomegranate (Punica granatum L.) Attenuates Neuroinflammation Involved in Neurodegenerative Diseases" Foods 11, no. 17: 2570. https://doi.org/10.3390/foods11172570
APA StyleMehdi, A., Lamiae, B., Samira, B., Ramchoun, M., Abdelouahed, K., Tamas, F., & Hicham, B. (2022). Pomegranate (Punica granatum L.) Attenuates Neuroinflammation Involved in Neurodegenerative Diseases. Foods, 11(17), 2570. https://doi.org/10.3390/foods11172570









