Establishment and Validation of Reference Genes of Brassica napus L. for Digital PCR Detection of Genetically Modified Canola
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples with Different GMO Contents
2.3. DNA Extraction
2.4. Primers and Probes
2.5. Real-Time PCR
2.6. Droplet Digital PCR
2.7. LOD and LOQ of Digital PCR Methods
2.8. Determination of the Dynamic Range
2.9. Method Verification
2.10. Data Analysis
3. Results and Discussion
3.1. Evaluating Intraspecies Inclusiveness and Species Specificity of Canola
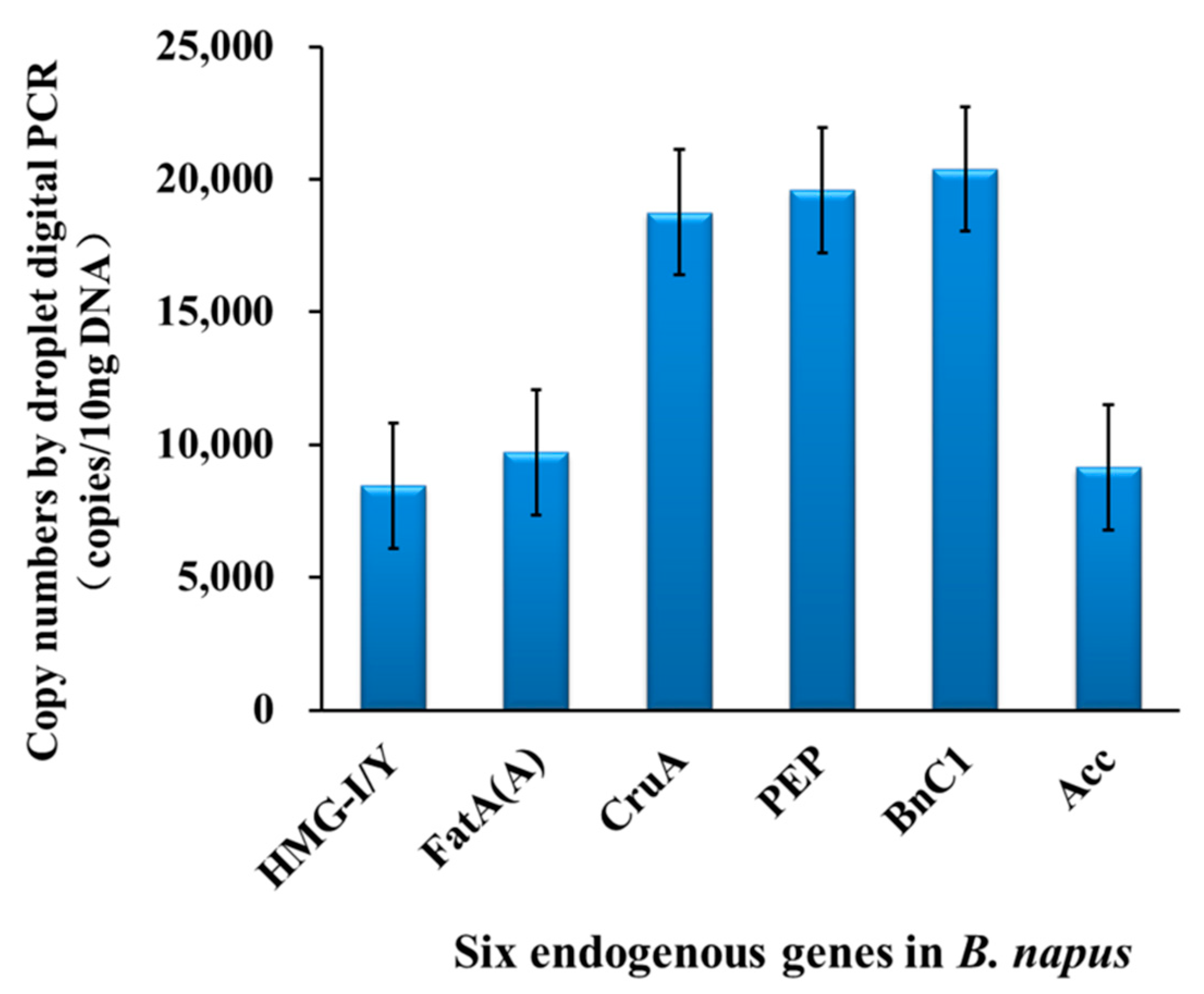
3.2. Verifying the Stability of Copy Numbers of ERGs among B. napus Cultivars by Droplet dPCR
3.3. Assessment of ddPCR for ERGs
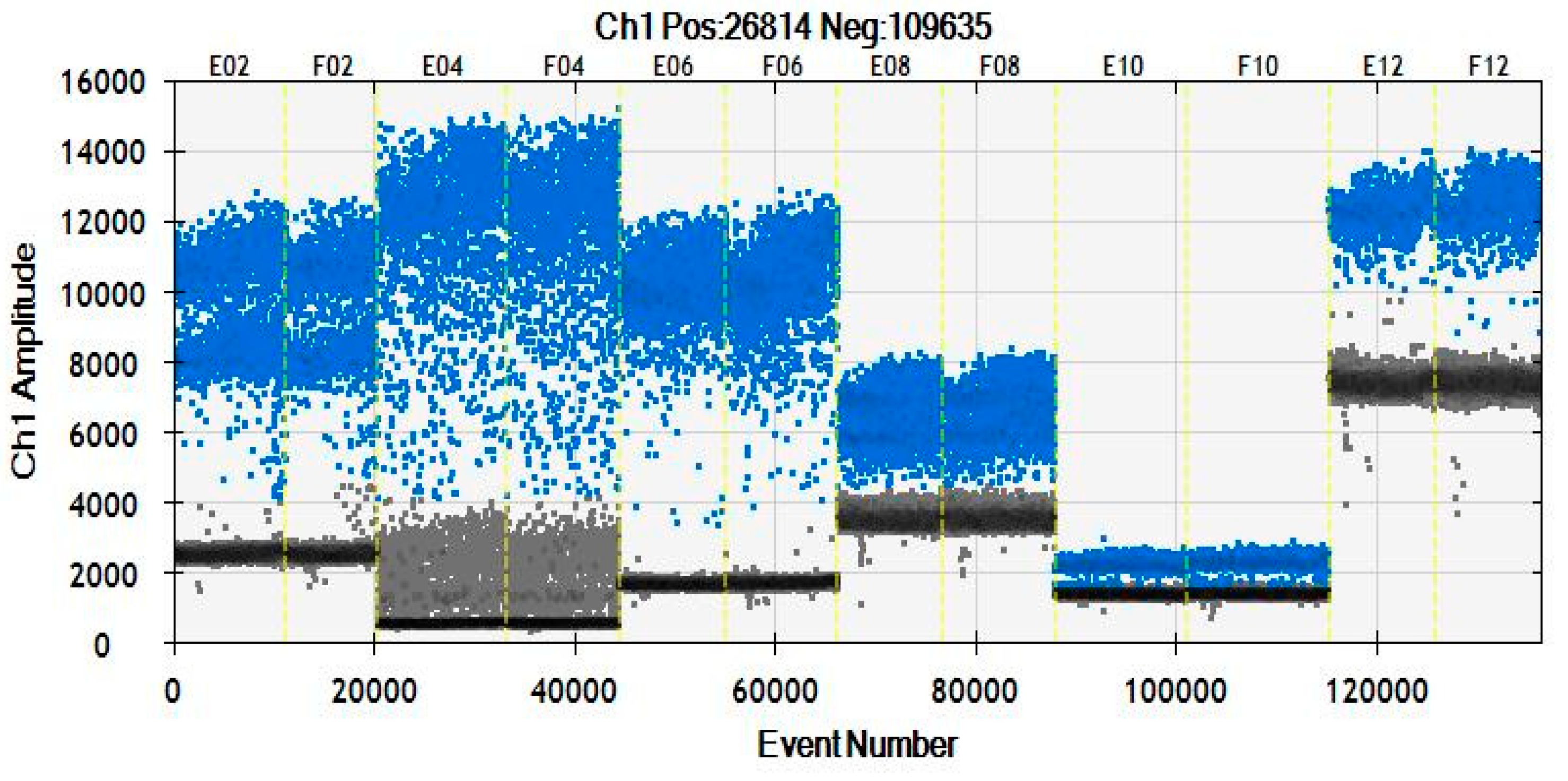
3.3.1. Selection from Observations of ddPCR
3.3.2. aLOQ and aLOD of ddPCR of BnC1 and BnAcc
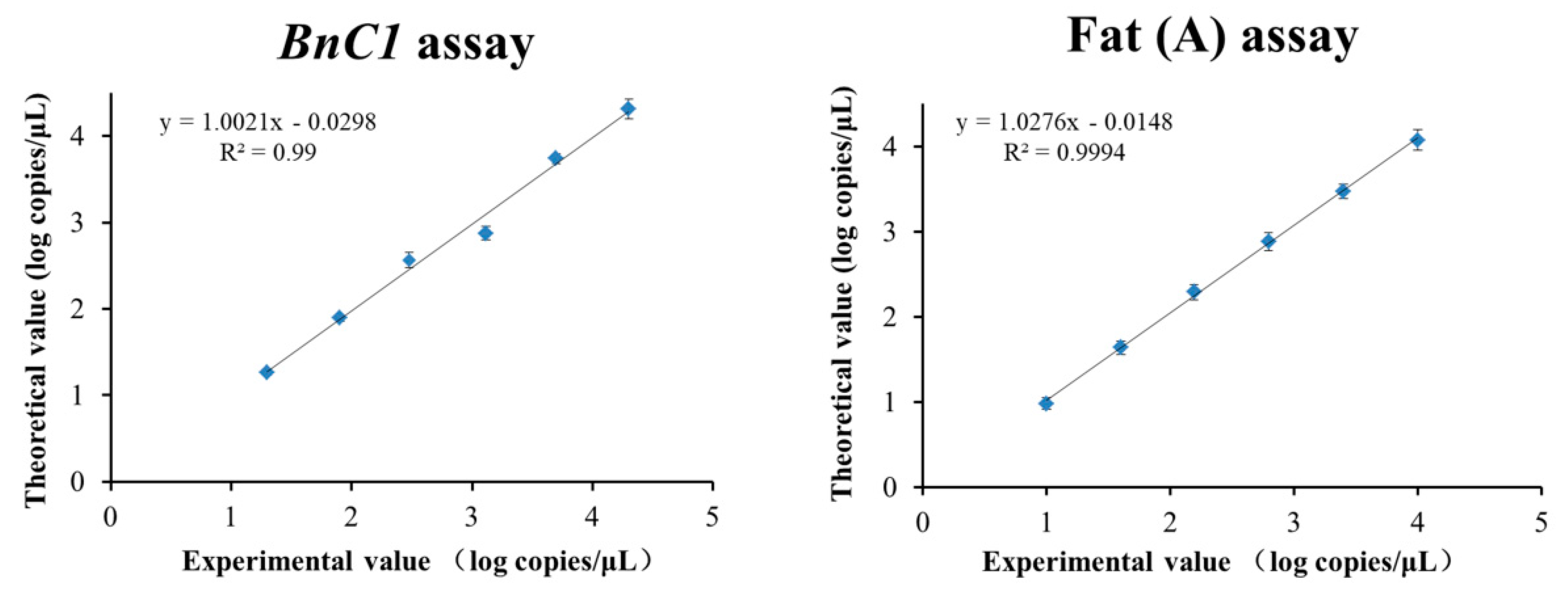
3.3.3. Dynamic Range
3.3.4. Quantitative Detection of GM Canola Events by Real-Time PCR and ddPCR with BnC1 and BnAcc
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ISAAA in 2019: Accomplishment Report. 2019. Available online: https://www.isaaa.org/resources/publications/annualreport/2019/default.asp (accessed on 26 May 2022).
- Kumar, K.; Gambhir, G.; Dass, A.; Tripathi, A.K.; Singh, A.; Jha, A.K.; Yadava, P.; Choudhary, M.; Rakshit, S. Genetically modified crops: Current status and future prospects. Planta 2020, 251, 91. [Google Scholar] [CrossRef] [PubMed]
- Demeke, T.; Lee, S.-J.; Eng, M. Increasing the Efficiency of Canola and Soybean GMO Detection and Quantification Using Multiplex Droplet Digital PCR. Biology 2022, 11, 201. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-G.; Park, J.-E.; Kim, M.-J.; Kim, H.-J.; Kim, H.-Y. Detection of GM Canola MS11, DP-073496-4, and MON88302 events using multiplex PCR coupled with capillary electrophoresis. Food Sci. Biotechnol. 2021, 30, 565–570. [Google Scholar] [CrossRef] [PubMed]
- 32003R1829; Regulation (EC) No 1829/2003 of the European Parliament and of the Council. European Commission: Brussels, Belgium, 2003.
- Verginelli, D.; Paternò, A.; Marchis, M.L.D.; Quarchioni, C.; Vinciguerra, D.; Bonini, P.; Peddis, S.; Fusco, C.; Misto, M.; Marfoglia, C.; et al. Development and comparative study of a pat/bar real-time PCR assay for integrating the screening strategy of a GMO testing laboratory. J. Sci. Food Agric. 2020, 100, 2121–2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Quan, S.; Zhang, D. Endogenous Reference Genes and Their Quantitative Real-Time PCR Assays for Genetically Modified Bread Wheat (Triticum aestivum L.) Detection. Methods Mol. Biol. 2017, 1679, 259–268. [Google Scholar]
- Silva, L.C.C.; Mayrink, D.B.; Bueno, R.D.; Piovesan, N.D.; Ribeiro, C.; Dal-Bianco, M. Reference Genes and Expression Analysis of Seed Desaturases Genes in Soybean Mutant Accessions. Biochem. Genet. 2022, 60, 937–952. [Google Scholar] [CrossRef]
- Demeke, T.; Dobnik, D. Critical assessment of digital PCR for the detection and quantification of genetically modified organisms. Anal. Bioanal. Chem. 2018, 410, 4039–4050. [Google Scholar] [CrossRef] [Green Version]
- Gerdes, L.; Iwobi, A.; Busch, U.; Pecoraro, S. Optimization of digital droplet polymerase chain reaction for quantification of genetically modified organisms. Biomol. Detect. Quantif. 2016, 7, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Zeitler, R.; Pietsch, K.; Waiblinger, H.-U. Validation of real-time PCR methods for the quantification of transgenic contaminations in rape seed. Eur. Food Res. Technol. 2002, 214, 346–351. [Google Scholar] [CrossRef]
- Henderson, N.; Harmon, M.; Zhong, C.X. PCR-Based Detection and Quantification of a Transgenic Glyphosate-Tolerant Canola Using a Novel Reference Gene System. Food Anal. Methods 2016, 9, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Zhang, L.; Wu, Y.; Cao, Y.; Lu, C. Comparison of five endogenous reference genes for specific PCR detection and quantification of Brassica napus. J. Agric. Food Chem. 2010, 58, 2812–2817. [Google Scholar] [CrossRef]
- Demeke, T.; Ratnayaka, I. Multiplex qualitative PCR assay for identification of genetically modified canola events and real-time event-specific PCR assay for quantification of the GT73 canola event. Food Control. 2008, 19, 893–897. [Google Scholar] [CrossRef]
- Weng, H.; Yang, L.; Liu, Z.; Ding, J.; Pan, A.; Zhang, D. Novel Reference Gene, High-mobility-group protein I/Y, Usedin Qualitative and Real-Time Quantitative Polymerase Chain Reaction Detection of Transgenic Rapeseed Cultivars. J. Aoac. Int. 2005, 88, 577–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Li, X.; Wang, C.; Song, G.; Pi, L.; Zheng, L.; Zhang, D.; Yang, L. One Novel Multiple-Target Plasmid Reference Molecule Targeting Eight Genetically Modified Canola Events for Genetically Modified Canola Detection. J. Agric. Food Chem. 2017, 65, 8489–8500. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, T.-W.; Lee, W.-Y.; Park, S.-H.; Kim, H.-Y. Multiplex PCR Detection of the GT73, MS8xRF3, and T45 Varieties of GM Canola. Food Sci. Biotechnol. 2007, 16, 104–109. [Google Scholar]
- Demeke, T.; Eng, M. Effect of endogenous reference genes on digital PCR assessment of genetically engineered canola events. Biomol. Detect. Quantif. 2018, 15, 24–29. [Google Scholar] [CrossRef]
- Akiyama, H.; Makiyama, D.; Nakamura, K.; Sasaki, N.; Minegishi, Y.; Mano, J.; Kitta, K.; Ozeki, Y.; Teshima, R. A novel detection system for the genetically modified canola (Brassica rapa) line RT73. Anal. Chem. 2010, 82, 9909–9916. [Google Scholar] [CrossRef] [PubMed]
- Lliu, J.; Li, Z.-Y.; Gao, D.-W. A universal quantification of transgenic soybean event DAS-68416-4 using duplex digital PCR. J. Sci. Food Agric. 2021, 101, 624–630. [Google Scholar] [CrossRef]
- Corbisier, P.; Buttinger, G.; Savini, C.; Sacco, M.G.; Gatto, F.; Emons, H. Expression of GM content in mass fraction from digital PCR data. Food Control. 2022, 133, 108626. [Google Scholar] [CrossRef]
- Cottenet, G.; Blancpain, C.; Chuah, P.F. Performance assessment of digital PCR for the quantification of GM-maize and GM-soya events. Anal. Bioanal. Chem. 2019, 411, 2461–2469. [Google Scholar] [CrossRef]
- Bogozalec Kosir, A.; Demsar, T.; Stebih, D.; Zel, J.; Milavec, M. Digital PCR as an effective tool for GMO quantification in complex matrices. Food Chem. 2019, 294, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Noma, S.; Kikuchi, Y.; Satou, M.; Tanaka, T.; Takiya, T.; Okusu, H.; Futo, S.; Takabatake, R.; Kitta, K.; Mano, J. Simple, Precise, and Less Biased GMO Quantification by Multiplexed Genetic Element-Specific Digital PCR. J. Aoac. Int. 2022, 105, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Río, A.; Esteve, T.; Prat, S.; Pla, M. A rapeseed-specific gene, acetyl-CoA carboxylase, can be used as a reference for qualitative and real-time quantitative PCR detection of transgenes from mixed food samples. J. Agric. Food Chem. 2001, 49, 3622–3627. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, G.; Xiao, L.; Lu, C. Event-specific qualitative and quantitative PCR detection methods for transgenic rapeseed hybrids MS1xRF1 and MS1xRF2. J. Agric. Food Chem. 2007, 55, 8380–8389. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wu, Y.; Xiao, L.; Lu, C. Event-specific qualitative and quantitative PCR methods for the detection of genetically modified rapeseed Oxy-235. Transgenic Res. 2008, 17, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wu, Y.; Xiao, L.; Lu, C. Event-specific qualitative and quantitative PCR detection of genetically modified rapeseed Topas 19/2. Food Chem. 2009, 112, 232–238. [Google Scholar] [CrossRef]
- Arumuganathan, K.; Earle, E.D. Nuclear DNA Content of Some Important Plant Species. Plant Mol. Biol. Rep. 1991, 9, 208–218. [Google Scholar] [CrossRef]
- Deng, T.; Huang, W.; Ren, J.; Ma, X.; Ge, Y.; Chen, Y. Verification and applicability of endogenous reference genes for quantifying GM rice by digital PCR. Anal. Biochem. 2019, 587, 113442. [Google Scholar] [CrossRef]
- European Network of GMO Laboratories. Definition of Minimum Performance Requirements for Analytical Methods of GMO Testing; European Commission: Brussels, Belgium, 2008. [Google Scholar]
- European Network of GMO Laboratories. Verification of Analytical Methods for GMO Testing When Implementing Interlaboratory Validated Methods; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Paterno, A.; Marchesi, U.; Gatto, F.; Verginelli, D.; Quarchioni, C.; Fusco, C.; Zepparoni, A.; Amaddeo, D.; Ciabatti, I. Finding the joker among the maize endogenous reference genes for genetically modified organism (GMO) detection. J. Agric. Food Chem. 2009, 57, 11086–11091. [Google Scholar] [CrossRef]
- Yang, L.; Pan, A.; Zhang, H.; Guo, J.; Yin, C.; Zhang, D. Event-specific qualitative and quantitative polymerase chain reaction analysis for genetically modified canola T45. J. Agric. Food Chem. 2006, 54, 9735–9740. [Google Scholar] [CrossRef]

| Gene | Accession No. | Primer/Probe Name | Sequences 5′-3′ | Amplicon (bp) | Resource |
|---|---|---|---|---|---|
| CruA | X14555 | CruA-F | GGCCAGGGTTTC CGTGAT | 101 | [13] |
| CruA-R | CCGTCGTTGTAGAACCATTGG | ||||
| CruA-P | FAM-AGTCCTTATGTGCTCCACTTTCTGGTGCA-TAMRA | ||||
| Fat(A) | AJ294419 | Fat(A)-F | ACAGATGAAGTTCGGGACGAGTAC | 126 | [7] |
| Fat(A)-R | CAGGTTGAGATCCACATGCTTAAATAT | ||||
| Fat(A)-P | FAM-AAGAAGAATCATCATGCTTC-TAMRA | ||||
| HMG-I/Y | AF127919 | HMG-F | GGTCGTCCTCCTAAGGCGAAAG | 99 | [15] |
| HMG-R | CTTCTTCGGCGGTCGTCCAC | ||||
| HMG-P | FAM-CGGAGCCACTCGGTGCCGCAACTT-TAMRA | ||||
| BnC1 | X59294.1 | Ccf-F | ATTGGGCTACACCGGGATGTGT | 96 | [18] |
| Ccf-R | GCTTCCGTGATATGCACC AGAAAG | ||||
| Ccf-P | FAM-CGATGGTGTCCCCAGTCCTTATGTGCTC-TAMRA | ||||
| PEP | D13987 | pep-F | CAGTTCTTGGAGCCGCTTGAG | 140 | [12] |
| pep-R | TGACGGATGTCGAGCTTCACA | ||||
| pep-P | FAM-ACAGACCTACAGCCGATGGAAGCCTGC-TAMRA | ||||
| BnACC | X77576 | Acc-F | GGTGAGCTGTATAATCGAGCGA | 104 | [11] |
| Acc-R | GGCGCAGCATCGGCT | ||||
| Acc-P | FAM-AACACCTATTAGACATTCGTTCCATTGGTCGA-TAMRA | ||||
| MS1 | EU090198 | Ms1-F | ACGCTGCGGACATCTACATT | 187 | [25] |
| Ms1-R | CTAGATCGGAAGCTGAAGATGG | ||||
| Ms1-P | FAM-CTCATTGCTGATCCACCTAGCCGACTT-TAMRA | ||||
| OXY235 | KJ608141 | Oxy-F | ATTGACCATCATACTCATTGCTGA | 105 | [26] |
| Oxy-R | AGAGAATCGTGAAATTATCTCTACCG | ||||
| Oxy-P | FAM-CCATGTAGATTTCCCGGACATGAAGCC-TAMRA | ||||
| Topas 19/2 | EU124676 | Topas-F | GTTGCGGTTCTGTCAGTTCC | 95 | [27] |
| Topas-R | CGACCGGCGCTGATATATGA | ||||
| Topas-P | FAM-TCCCGCGTCATCGGCGG-TAMRA |
| Species/Cultivars | Sample Name | CruA | Fat(A) | HMG-I/Y | BnC1 | PEP | BnAcc |
|---|---|---|---|---|---|---|---|
| Brassica juncea | Lu | + | + | + | + | + | + |
| Simian Shanzha | + | + | + | + | + | + | |
| AB1 | + | + | + | + | + | + | |
| Brassica rapa | Shanghai Qing | + | + | + | + | + | + |
| Huangxin Wu | + | + | + | + | + | + | |
| 10 wH008 | + | + | + | + | + | + | |
| Brassica napus | AV-Jade | + | + | + | + | + | + |
| Zhongshuang-11 | + | + | + | + | + | + | |
| Nh No.345 | + | + | + | + | + | + | |
| Zhongshuang-b | + | + | + | + | + | + | |
| Ms1 | + | + | + | + | + | + | |
| Oxy-235 | + | + | + | + | + | + | |
| Topas 19/2 | + | + | + | + | + | + | |
| Brassica oleracea | Chinese kale | + | + | + | - | + | - |
| Brassica nigra | black mustard | + | + | - | - | + | - |
| Brassica carinat | Ethiopian mustard | + | + | + | - | + | - |
| Raphanus sativus L. | radish | + | + | - | - | + | + |
| Nicotiana tabacum | tobacco | - | - | - | - | - | - |
| Solanum tuberosum | potato | - | - | - | - | - | - |
| Lycopericon esculentum | tomato | - | - | - | - | - | - |
| Glycine max | GM Soybean | - | - | - | - | - | - |
| Zea mays | GM corn | - | - | - | - | - | - |
| Oryza sativa | GM rice | - | - | - | - | - | - |
| Gossypium hirsutum | GM cotton | - | - | - | - | - | - |
| Endogenous Genes | Copy Numbers in Cultivars of Brassica napus. (12.5 ng Genome DNA) | Average | RSDr a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MS1 | Oxy-235 | Topas 19/2 | AV-Jade | Zhongshuang-11 | Nh No.345 | Zhongshuang-b | |||
| HMG-I/Y | 9049 | 7956 | 8956 | 7463 | 8856 | 9020 | 7856 | 8451 | 7.90 |
| Fat(A) | 9696 | 9731 | 9719 | 9688 | 10,210 | 8060 | 9860 | 9709 | 7.21 |
| CruA | 20,235 | 17,153 | 19,560 | 15,800 | 18,963 | 18,930 | 20,650 | 18,756 | 9.18 |
| PEP | 19,242 | 19,834 | 20,157 | 20,688 | 20,260 | 16,510 | 19,560 | 19,607 | 7.13 |
| BnC1 | 20,643 | 20,501 | 20,189 | 20,438 | 21,000 | 21,300 | 18,693 | 20,538 | 4.10 |
| BnAcc | 9445 | 8862 | 9216 | 8675 | 8657 | 9560 | 9650 | 9152 | 4.59 |
| Target | Template Copy No. | Signal Ratio | Mean Copy No. | RSD Copy No. (%) |
|---|---|---|---|---|
| HMG-I/Y | 20 | 15/15 | 16 | 14.9 |
| 10 | 15/15 | 8 | 23.5 | |
| 5 | 15/15 | 6 | 41.4 | |
| 2 | 7/15 | / | / | |
| 1 | 5/15 | / | / | |
| Fat(A) | 20 | 15/15 | 19 | 12.2 |
| 10 | 15/15 | 9 | 22.6 | |
| 5 | 12/15 | / | / | |
| 2 | 10/15 | / | / | |
| 1 | 4/15 | / | / | |
| CruA | 20 | 15/15 | 22 | 19.8 |
| 10 | 15/15 | 13 | 24.7 | |
| 5 | 15/15 | 8 | 45.8 | |
| 2 | 10/15 | / | / | |
| 1 | 5/15 | / | / | |
| PEP | 20 | 15/15 | 23 | 13.5 |
| 10 | 15/15 | 13 | 20.9 | |
| 5 | 13/15 | / | / | |
| 2 | 7/15 | / | / | |
| 1 | 5/15 | / | / | |
| BnC1 | 20 | 15/15 | 21 | 12.1 |
| 10 | 15/15 | 11 | 20.3 | |
| 5 | 15/15 | 8 | 24.8 | |
| 2 | 11/15 | / | / | |
| 1 | 8/18 | / | / | |
| BnAcc | 20 | 15/15 | 22 | 14.7 |
| 10 | 15/15 | 10 | 18.9 | |
| 5 | 15/15 | 6 | 22.5 | |
| 2 | 8/15 | / | / | |
| 1 | 6/15 | / | / |
| GM Rapeseed Event | Method | Exogenous Gene | BnAcc | BnC1 | |||||
|---|---|---|---|---|---|---|---|---|---|
| GM Content by Mass Ratio (%) | Average Copy Numbers | Average Copy Numbers | Experimental GM Concentration (%) | Bias % | Average Copy Numbers | Experimental GM Concentration (%) | Bias % | ||
| MS1 | Real-timePCR | 10 | 1056 | 9830 | 10.74 | 7.43 | 20,715 | 10.20 | 1.96 |
| 5 | 551 | 10,840 | 5.08 | 1.66 | 21,385 | 5.15 | 3.06 | ||
| 1 | 110 | 9687 | 1.14 | 13.55 | 20,380 | 1.08 | 7.95 | ||
| 0.1 | 13 | 9937 | 0.12 | 20.76 | 20,150 | 0.11 | 19.11 | ||
| ddPCR | 10 | 890 | 9600 | 9.27 | −7.29 | 20,925 | 8.51 | −14.93 | |
| 5 | 517 | 9490 | 5.45 | 8.96 | 20,824 | 4.97 | −0.69 | ||
| 1 | 102 | 9138 | 1.12 | 11.62 | 20,610 | 0.99 | −1.02 | ||
| 0.1 | 9 | 9088 | 0.10 | −0.97 | 20,700 | 0.09 | −13.04 | ||
| Topas 19/2 | Real-timePCR | 10 | 1100 | 10,863 | 10.13 | 1.26 | 20,438 | 10.76 | 7.64 |
| 5 | 540 | 9445 | 5.72 | 14.35 | 20,697 | 5.22 | 4.36 | ||
| 1 | 96 | 9160 | 1.05 | 4.80 | 21,104 | 0.91 | −9.02 | ||
| 0.1 | 11 | 9100 | 0.12 | 20.88 | 18,008 | 0.12 | 22.17 | ||
| ddPCR | 10 | 952 | 10,800 | 8.81 | −11.85 | 19,938 | 8.15 | −18.55 | |
| 5 | 406 | 9507 | 4.27 | −14.59 | 20,680 | 5.67 | 13.35 | ||
| 1 | 85 | 10,563 | 0.80 | −19.53 | 21,104 | 0.81 | −19.45 | ||
| 0.1 | 8 | 10,288 | 0.08 | −22.24 | 19,038 | 0.08 | −15.96 | ||
| Oxy235 | Real-timePCR | 10 | 1101 | 10,925 | 10.08 | 0.78 | 19,938 | 11.04 | 10.44 |
| 5 | 499 | 9407 | 5.30 | 6.09 | 18,963 | 5.26 | 5.26 | ||
| 1 | 110 | 10,563 | 1.04 | 4.14 | 20,650 | 1.07 | 6.54 | ||
| 0.1 | 11 | 9289 | 0.12 | 18.42 | 18,130 | 0.12 | 21.35 | ||
| ddPCR | 10 | 966 | 10,920 | 8.85 | −11.54 | 17,360 | 11.13 | 11.29 | |
| 5 | 478 | 8657 | 5.52 | 10.43 | 19,070 | 5.01 | 0.26 | ||
| 1 | 100 | 9240 | 1.08 | 8.23 | 19,830 | 1.01 | 0.86 | ||
| 0.1 | 9 | 9650 | 0.09 | −6.74 | 21,038 | 0.09 | −14.44 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, L.; Xing, Z.; He, Y.; Yan, W.; Li, C.; Xia, W.; Dong, L.; Zhao, N.; Ma, Y.; Xie, Y.; et al. Establishment and Validation of Reference Genes of Brassica napus L. for Digital PCR Detection of Genetically Modified Canola. Foods 2022, 11, 2535. https://doi.org/10.3390/foods11162535
Long L, Xing Z, He Y, Yan W, Li C, Xia W, Dong L, Zhao N, Ma Y, Xie Y, et al. Establishment and Validation of Reference Genes of Brassica napus L. for Digital PCR Detection of Genetically Modified Canola. Foods. 2022; 11(16):2535. https://doi.org/10.3390/foods11162535
Chicago/Turabian StyleLong, Likun, Zhenjuan Xing, Yuxuan He, Wei Yan, Congcong Li, Wei Xia, Liming Dong, Ning Zhao, Yue Ma, Yanbo Xie, and et al. 2022. "Establishment and Validation of Reference Genes of Brassica napus L. for Digital PCR Detection of Genetically Modified Canola" Foods 11, no. 16: 2535. https://doi.org/10.3390/foods11162535
APA StyleLong, L., Xing, Z., He, Y., Yan, W., Li, C., Xia, W., Dong, L., Zhao, N., Ma, Y., Xie, Y., Liu, N., & Li, F. (2022). Establishment and Validation of Reference Genes of Brassica napus L. for Digital PCR Detection of Genetically Modified Canola. Foods, 11(16), 2535. https://doi.org/10.3390/foods11162535





