Brucella spp. Contamination in Artisanal Unpasteurized Dairy Products: An Emerging Foodborne Threat in Tunisia
Abstract
1. Introduction
2. Materials and Methods
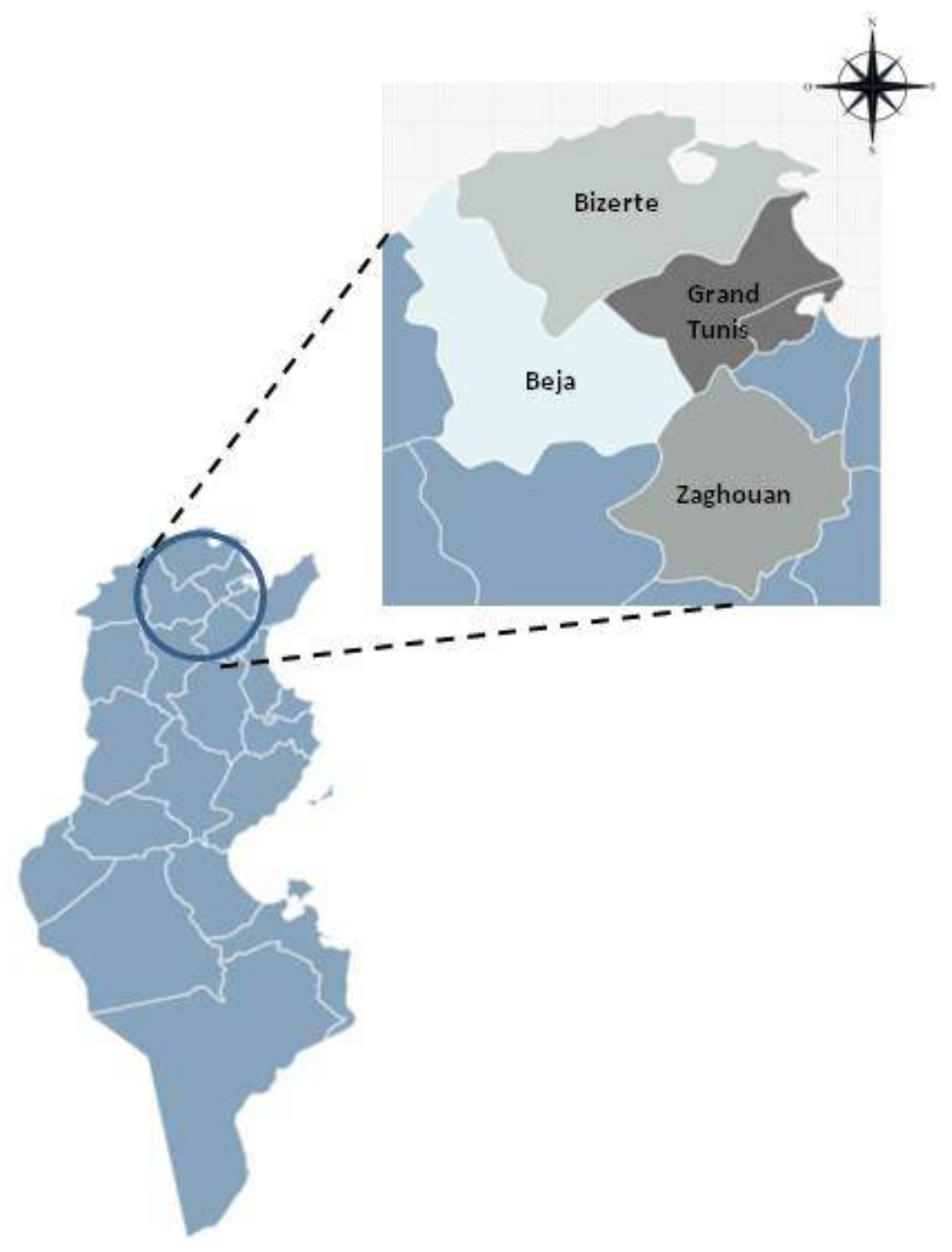
2.1. Samples Collection
2.2. DNA Extraction
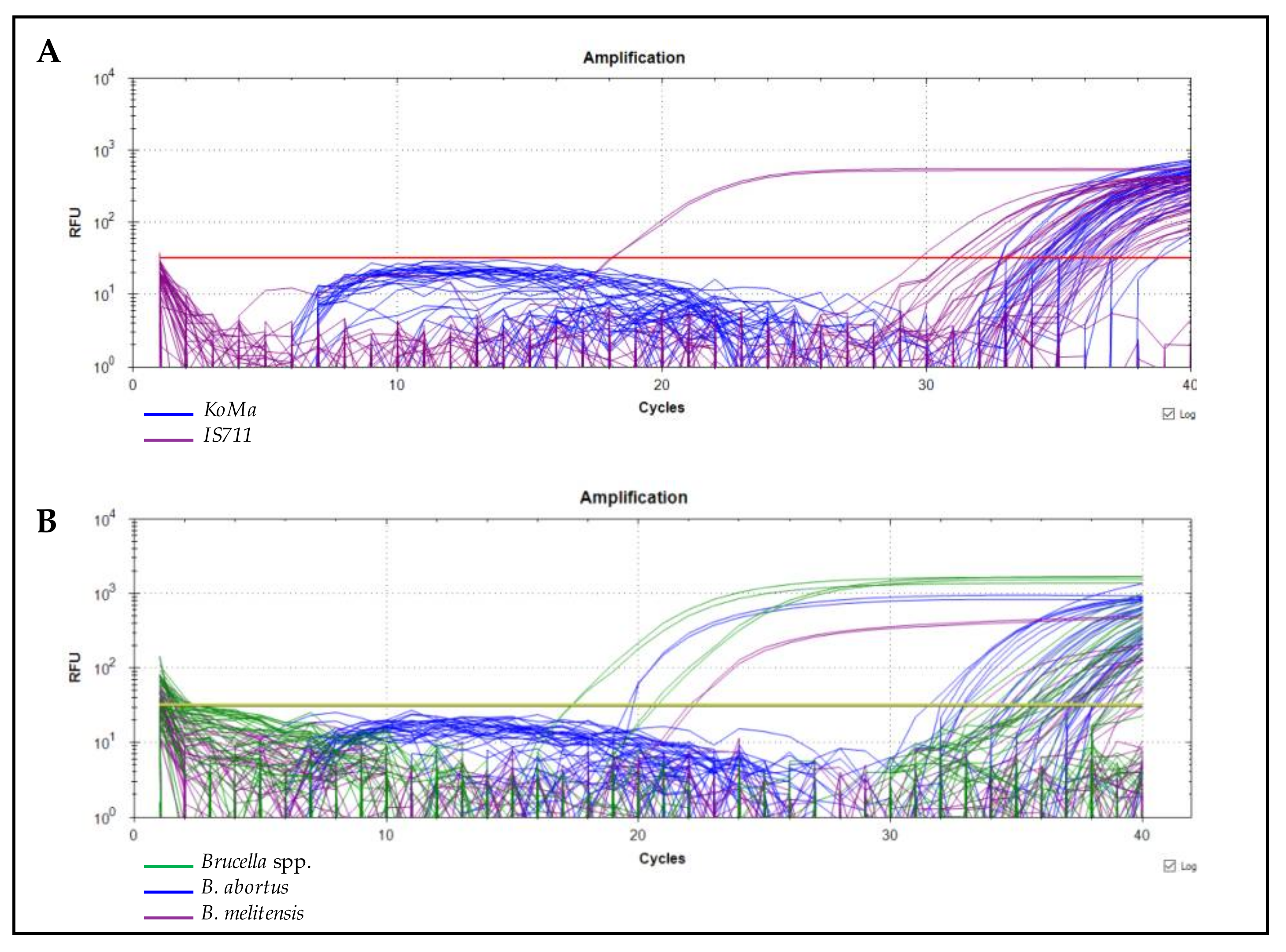
2.3. qPCR Testing
3. Results
3.1. Contamination Rates by Brucella spp.
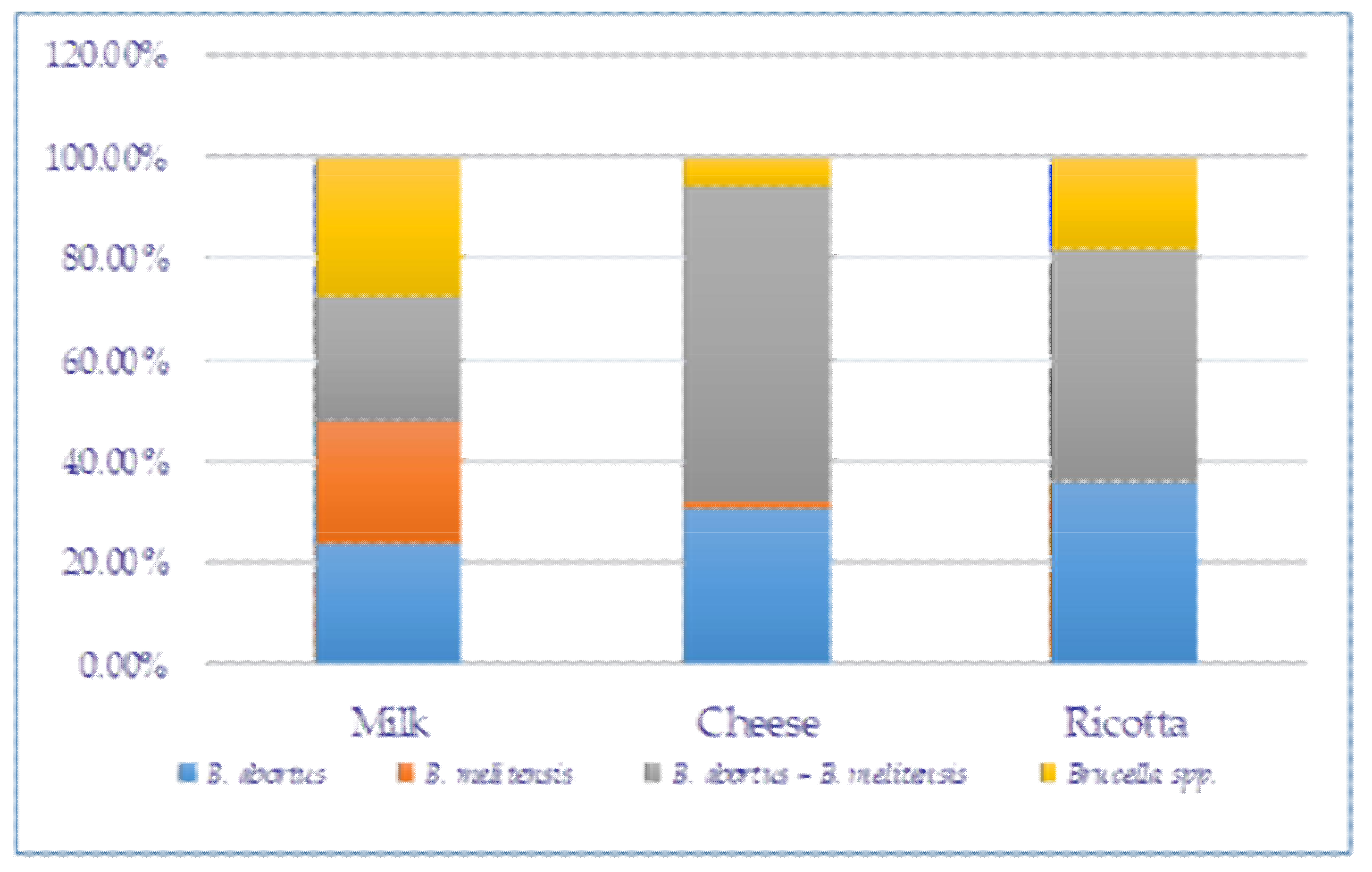
3.2. Frequency of B. melitensis and B. abortus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corbel, M.J.; Food and Agriculture Organization of the United Nations, World Health Organization and World Organization for Animal Health. Brucellosis in Humans and Animals. World Health Organization. 2006. Available online: https:/apps.who.int/iris/handle/10665/43597 (accessed on 7 June 2021).
- Buttigieg, S.C.; Savic, S.; Cauchi, D.; Lautier, E.; Canali, M.; Aragrande, M. Brucellosis Control in Malta and Serbia: A One Health Evaluation. Front. Vet. Sci. 2018, 5, 147. [Google Scholar] [CrossRef] [PubMed]
- Lindahl-Rajala, E.; Hoffman, T.; Fretin, D.; Godfroid, J.; Sattorov, N.; Boqvist, S.; Lundkvist, A.; Magnusson, U. Detection and characterization of Brucella spp. in bovine milk in small-scale urban and peri-urban farming in Tajikistan. PLoS Negl. Trop. Dis. 2017, 11, e0005367. [Google Scholar] [CrossRef] [PubMed]
- Doganay, M.; Aygen, B. Human brucellosis: An overview. Int. J. Infect. Dis. 2003, 7, 173–182. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Brucellosis in Humans and Animals. 2006. Available online: https://www.who.int/publications/i/item/9789241547130 (accessed on 25 January 2020).
- Dadar, M.; Shahali, Y.; Whatmore, A.M. Human brucellosis caused by raw dairy products: A review on the occurrence, major risk factors and prevention. Int. J. Food Microbiol. 2019, 292, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Franc, K.A.; Krecek, R.C.; Häsler, B.N.; Arenas-Gamboa, A.M. Brucellosis remains a neglected disease in the developing world: A call for interdisciplinary action. BMC Public Health. 2018, 18, 125. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Brucellosis. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/brucellosis (accessed on 17 July 2021).
- Hull, N.C.; Schumaker, B.A. Comparisons of brucellosis between human and veterinary medicine. Infect. Ecol. Epidemiol. 2018, 8, 1500846. [Google Scholar] [CrossRef] [PubMed]
- Climate-Data.Org. Climat-Tunisie. Available online: https://fr.climate-data.org/afrique/tunisie (accessed on 16 July 2022).
- Probert, W.S.; Schrader, K.N.; Khuong, N.Y.; Bystrom, S.L.; Graves, M.H. Real-Time Multiplex PCR Assay for Detection of Brucella spp., B. abortus, and B. melitensis. J. Clin. Microbiol. 2004, 42, 1290–1293. [Google Scholar] [CrossRef]
- Kirchner, S.; Kraämer, K.M.; Schulze, M.; Pauly, D.; Jacob, D.; Gessler, F.; Nitsche, A.; Dorner, B.G.; Dorner, M.B. Pentaplexed Quantitative Real-Time PCR Assay for the Simultaneous Detection and Quantification of Botulinum Neurotoxin-Producing Clostridia in Food and Clinical Samples. Appl. Environ. Microbiol. 2010, 76, 4387–4395. [Google Scholar] [CrossRef]
- De Massis, F.; Zilli, K.; Di Donato, G.; Nuvoloni, R.; Pelini, S.; Sacchini, L.; D’Alterio, N.; Di Giannatale, E. Distribution of Brucella field strains isolated from livestock, wildlife populations, and humans in Italy from 2007 to 2015. PLoS ONE 2019, 14, e0213689. [Google Scholar] [CrossRef]
- Colmenero, J.D.; Reguera, J.M.; Martos, F.; Sánchez-De-Mora, D.; Delgado, M.; Causse, M.; Martín-Farfán, A.; Juarez, C. Complications Associated with Brucella melitensis Infection: A Study of 530 Cases. Medicine 1996, 75, 195–211. [Google Scholar] [CrossRef]
- Güler, S.; Kokoglu, O.F.; Uçmak, H.; Gül, M.; Ozden, S.; Özkan, F.; Kökoǧlu, F. Human brucellosis in Turkey: Different clinical presentations. J. Infect. Dev. Ctries. 2014, 8, 581–588. [Google Scholar] [CrossRef]
- Matero, P.; Hemmilä, H.; Tomaso, H.; Piiparinen, H.; Rantakokko-Jalava, K.; Nuotio, L.; Nikkari, S. Rapid field detection assays for Bacillus anthracis, Brucella spp., Francisellatularensis and Yersinia pestis. Clin. Microbiol. Infect. 2011, 17, 34–43. [Google Scholar] [CrossRef]
- Jansen, W.; Linard, C.; Noll, M.; Nöckler, K.; Al Dahouk, S. Brucella-positive raw milk cheese sold on the inner European market: A public health threat due to illegal import? Food Control 2019, 100, 130–137. [Google Scholar] [CrossRef]
- Gupta, V.; Verma, D.K.; Rout, P.; Singh, S.; Vihan, V. Polymerase chain reaction (PCR) for detection of Brucella melitensis in goat milk. Small Rumin. Res. 2006, 65, 79–84. [Google Scholar] [CrossRef]
- Moslemi, E.; Soltandalal, M.M.; Beheshtizadeh, M.R.; Taghavi, A.; Manjili, H.K.; Lamouki, R.M.; Izadi, A. Detection of Brucella spp. in Dairy Products by Real-Time PCR. Arch. Clin. Infect. Dis. 2018, 13, e12673. [Google Scholar] [CrossRef]
- Naveenkumar, V.; Bharathi, M.V.; Porteen, K. Risk Factors Associated with Prevalence of Bovine Brucellosis in Milk from Tamil Nadu, India. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2604–2609. [Google Scholar] [CrossRef][Green Version]
- Abedi, A.-S.; Hashempour-Baltork, F.; Alizadeh, A.M.; Beikzadeh, S.; Hosseini, H.; Bashiry, M.; Taslikh, M.; Javanmardi, F.; Sheidaee, Z.; Sarlak, Z.; et al. The prevalence of Brucella spp. in dairy products in the Middle East region: A systematic review and meta-analysis. Acta Trop. 2019, 202, 105241. [Google Scholar] [CrossRef]
- Kaden, R.; Ferrari, S.; Jinnerot, T.; Lindberg, M.; Wahab, T.; Lavander, M. Brucella abortus: Determination of survival times and evaluation of methods for detection in several matrices. BMC Infect. Dis. 2018, 18, 259. [Google Scholar] [CrossRef]
- Hoffman, T.; Rock, K.; Mugizi, D.R.; Muradrasoli, S.; Lindahl-Rajala, E.; Erume, J.; Magnusson, U.; Lundkvist, A.; Boqvist, S. Molecular detection and characterization of Brucella species in raw informally marketed milk from Uganda. Infect. Ecol. Epidemiol. 2016, 6, 32442. [Google Scholar] [CrossRef]
- Marouf, A.S.; Hanifian, S.; Shayegh, J. Prevalence of Brucella spp. in raw milk and artisanal cheese tested via real-time qPCR and culture assay. Int. J. Food Microbiol. 2021, 347, 109192. [Google Scholar] [CrossRef]
- Zhang, S.-J.; Wang, L.-L.; Lu, S.-Y.; Hu, P.; Li, Y.-S.; Zhang, Y.; Chang, H.-Z.; Zhai, F.-F.; Liu, Z.-S.; Li, Z.-H.; et al. A novel, rapid, and simple PMA-qPCR method for detection and counting of viable Brucella organisms. J. Vet. Res. 2020, 64, 253–261. [Google Scholar] [CrossRef]
- Kaynak-Onurdag, F.; Okten, S.; Sen, B. Screening Brucella spp. in bovine raw milk by real-time quantitative PCR and conventional methods in a pilot region of vaccination, Edirne, Turkey. J. Dairy Sci. 2016, 99, 3351–3357. [Google Scholar] [CrossRef]
- Liu, K.; Yang, Z.; Liang, W.; Guo, T.; Long, Y.; Shao, Z. Effect of climatic factors on the seasonal fluctuation of human brucellosis in Yulin, northern China. BMC Public Health 2020, 20, 506. [Google Scholar] [CrossRef]
- Guesmi, K.; Kalthoum, S.; Belhaj Mohamed, B.; Ben Aicha, I.; Hajlaoui, H.; Hrabech, K. Bilan de la brucellose animale et humaine en Tunisie: 2005–2018. Bull. Zoosanit. 2020, 20, 1–11. [Google Scholar]
- Barkallah, M.; Gharbi, Y.; Zormati, S.; Karkouch, N.; Mallek, Z.; Fendri, I.; Gdoura, R.; Gautier, M. A mixed methods study of ruminant brucellosis in central-eastern Tunisia. Trop. Anim. Health Prod. 2016, 49, 39–45. [Google Scholar] [CrossRef]
- Aliyev, J.; Alakbarova, M.; Garayusifova, A.; Omarov, A.; Aliyeva, S.; Fretin, D.; Godfroid, J. Identification and molecular characterization of Brucella abortus and Brucella melitensis isolated from milk in cattle in Azerbaijan. BMC Vet. Res. 2022, 18, 71. [Google Scholar] [CrossRef]
- Davies, G.; Casey, A. The Survival of Brucella abortus in Milk and Milk Products. Br. Vet. J. 1973, 129, 345–353. [Google Scholar] [CrossRef]
- El-Daher, N.; Na’Was, T.; Al-Qaderi, S. The effect of the pH of various dairy products on the survival and growth of Brucella melitensis. Ann. Trop. Med. Parasitol. 1990, 84, 523–528. [Google Scholar] [CrossRef]
- Falenski, A.; Mayer-Scholl, A.; Filter, M.; Göllner, C.; Appel, B.; Nöckler, K. Survival of Brucella spp. in mineral water, milk and yogurt. Int. J. Food Microbiol. 2011, 145, 326–330. [Google Scholar] [CrossRef]
- European Comission. Corrigendum to Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 Laying Down Specific Hygiene Rules for Food of Animal Origin. Section IX. Available online: https://www.fsai.ie/uploadedFiles/Reg853_2004(1).pdf (accessed on 20 December 2021).
- Journal Officiel de la République Tunisienne. Arrêté du Ministre de la Santé du 1er Décembre 2015, Fixant la Liste des Maladies Transmissibles à Déclaration Obligatoire. Available online: http://www.ordre-medecins.org.tn/pdf/liste-des-Maladies-transmissibles.pdf (accessed on 7 June 2021).
- Khbou, M.K.; Htira, S.; Harabech, K.; Benzarti, M. First case-control study of zoonotic brucellosis in Gafsa district, Southwest Tunisia. One Health 2018, 5, 21–26. [Google Scholar] [CrossRef]
- Bettaieb, J.; Kharroubi, G.; Mallekh, R.; Cherif, I.; Taoufik, A.; Harrabech, K. Epidemiological, clinical, and bacteriological profile of human brucellosis in the district of Tunis. In Proceedings of the World Infectious Disease 2020, World Congress on Clinical Microbiology & Infectious Diseases, Amsterdam, The Netherlands, 19–20 February 2020. [Google Scholar]
- Berger, S. Brucellosis: Global Status; Gideon Informatics, Inc.: Los Angeles, CA, USA, 2016. [Google Scholar]
- Zayoud, R.; Battikh, H.; Mbarki, A.; Ammari, L.; Zribi, M.; Tiouiri, H.; Fendri, C. Recrudescence des cas de brucellose à l’hopital la Rabta: À propos de 109 cas. In Proceedings of the XXIXème Congrès de la Société Tunisienne de Pathologie Infectieuse, Sousse, Tunisia, 4–5 April 2019. [Google Scholar]
- Oueslati, I.; Berriche, A.; Ammari, L.; Abdelmalek, R.; Kanoun, F.; Kilani, B.; Benaissa, H.T. Epidemiological and clinical characteristics of neurobrucellosis case patients in Tunisia. Med. Mal. Infect. 2016, 46, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Koubaa, M.; Maaloul, I.; Marrakchi, C.; Lahiani, D.; Hammami, B.; Mnif, Z.; Ben Mahfoudh, K.; Hammami, A.; Ben Jemaa, M. Spinal brucellosis in South of Tunisia: Review of 32 cases. Spine J. 2014, 14, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
| Target | Primer/Probe | Sequence (5′-3′) |
|---|---|---|
| Brucellaspp. detection | ||
| IS711 | Bru IS-F | GCCATCAGATTGAATGCTTTTTTAAC |
| Bru IS-R | AACCAGATCATAGCGCATGCG | |
| Bru IS-TM | Cy5-CGCTGCGATGCGAGAAAACATTGACC-BHQ-2 | |
| KoMa2 | KoMa-F | GGTGATGCCGCATTATTACTAGG |
| KoMa-R | GGTATTAGCAGTCGCAGGCTT | |
| KoMa-TM | HEX-TTCTTGCTTGAGGATCTGTCGTGGATCG-BHQ-2 | |
| Brucellaspecies differentiation | ||
| Brucella spp. | Bru-F | GCT CGG TTG CCA ATA TCA ATG C |
| Bru-R | GGG TAA AGC GTC GCC AGA AG | |
| Bru-TM | FAM-AAA TCT TCC ACC TTG CCC TTG CCA TCA-BHQ-1 | |
| B. abortus | Bru ab-F | GCG GCT TTT CTA TCA CGG TAT TC |
| Bru ab-R | CAT GCG CTA TCA CGG TAT TC | |
| Bru ab-TM | HEX-CGC TCA TGC TCG CCA GAC TTC AAT G-BHQ-1 | |
| B. melitensis | Bru mel-F | AAC AAG CGG CAC CCC TAA AA |
| Bru mel-R | CAT GCG CTA TGA TCT GGT TAC | |
| Bru mel-TM | Cy5-CAG GAG TGT TTC GGC TCA GAA TAA TCC ACA-BHQ-2 | |
| District | No. of Samples | No. of Positive Samples (%) | Brucella Species Differentiation | |||
|---|---|---|---|---|---|---|
| B. abortus | B. melitensis | Both Species * | Brucella Spp. | |||
| Beja | 50 | 23 (46%) | 4 (17.4%) | 1 (4.3%) | 9 (39.1%) | 9 (39.1%) |
| Bizerte | 50 | 43 (86%) | 22 (51.2%) | - | 21 (48.8) | - |
| Tunis | 50 | 47 (94%) | 8 (17%) | 4 (8.5%) | 28 (59.6%) | 7 (14.9%) |
| Zaghouan | 50 | 37 (74%) | 13 (35.1%) | 3 (8.1%) | 16 (43.2%) | 5 (13.5%) |
| Total | 200 | 150 (75%) | 47 (31.3%) | 8 (5.3%) | 74 (49.3%) | 21 (14%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Béjaoui, A.; Ben Abdallah, I.; Maaroufi, A. Brucella spp. Contamination in Artisanal Unpasteurized Dairy Products: An Emerging Foodborne Threat in Tunisia. Foods 2022, 11, 2269. https://doi.org/10.3390/foods11152269
Béjaoui A, Ben Abdallah I, Maaroufi A. Brucella spp. Contamination in Artisanal Unpasteurized Dairy Products: An Emerging Foodborne Threat in Tunisia. Foods. 2022; 11(15):2269. https://doi.org/10.3390/foods11152269
Chicago/Turabian StyleBéjaoui, Awatef, Ibtihel Ben Abdallah, and Abderrazak Maaroufi. 2022. "Brucella spp. Contamination in Artisanal Unpasteurized Dairy Products: An Emerging Foodborne Threat in Tunisia" Foods 11, no. 15: 2269. https://doi.org/10.3390/foods11152269
APA StyleBéjaoui, A., Ben Abdallah, I., & Maaroufi, A. (2022). Brucella spp. Contamination in Artisanal Unpasteurized Dairy Products: An Emerging Foodborne Threat in Tunisia. Foods, 11(15), 2269. https://doi.org/10.3390/foods11152269





