Identification of the DPP-IV Inhibitory Peptides from Donkey Blood and Regulatory Effect on the Gut Microbiota of Type 2 Diabetic Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hemoglobin Peptides from Donkey Blood
2.3. DPP-IV Inhibitory Peptide Activity Assay
2.4. Preparation of Hemoglobin Peptides from Donkey Blood
2.4.1. Ultrafiltration
2.4.2. Sephadex G-25 Medium
2.4.3. UPLC–MS/MS-Based Peptide Identification
2.5. Molecular Docking
2.6. Effects of DPP-IV Inhibitory Peptides on Blood Sugar and Intestinal Microflora of Mice
2.6.1. Modeling of Mice
2.6.2. Blood Glucose Testing
2.6.3. Histological Examination
2.6.4. 16s rRNA Gene Sequencing
2.6.5. GC–MS
2.7. Statistical Analysis
3. Results
3.1. Preparation of Hemoglobin Hydrolysates from Donkey Blood
3.2. The Hemoglobin Hydrolysates from Donkey Blood Inhibit the Activity of DPP-IV
3.3. Identification of DPP-IV Inhibitory Peptides by UPLC–MS/MS
3.4. Molecular Docking Analysis of Identified Peptides with DPP-IV
3.5. DPP-IV Inhibitory Peptides Cause Reductions in the Fasting Glucose Levels of Type 2 Diabetic Mice
3.6. Histopathology
3.7. Effects of DPP-IV Inhibitory Peptides on the Intestinal Microflora of Mice
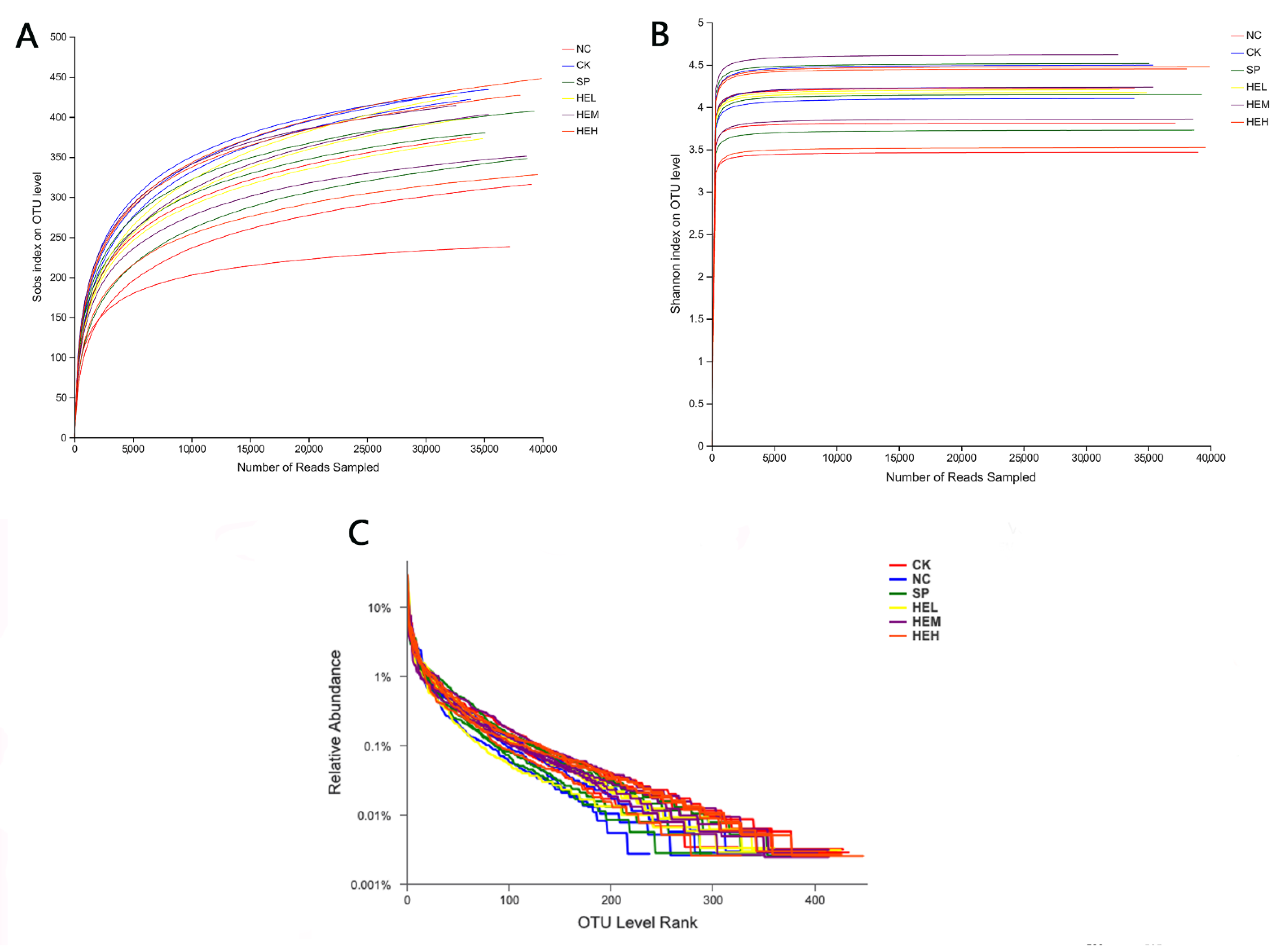
3.7.1. Sample Diversity Analysis
3.7.2. Intestinal Flora
3.7.3. Analysis of Differences between Sample Groups
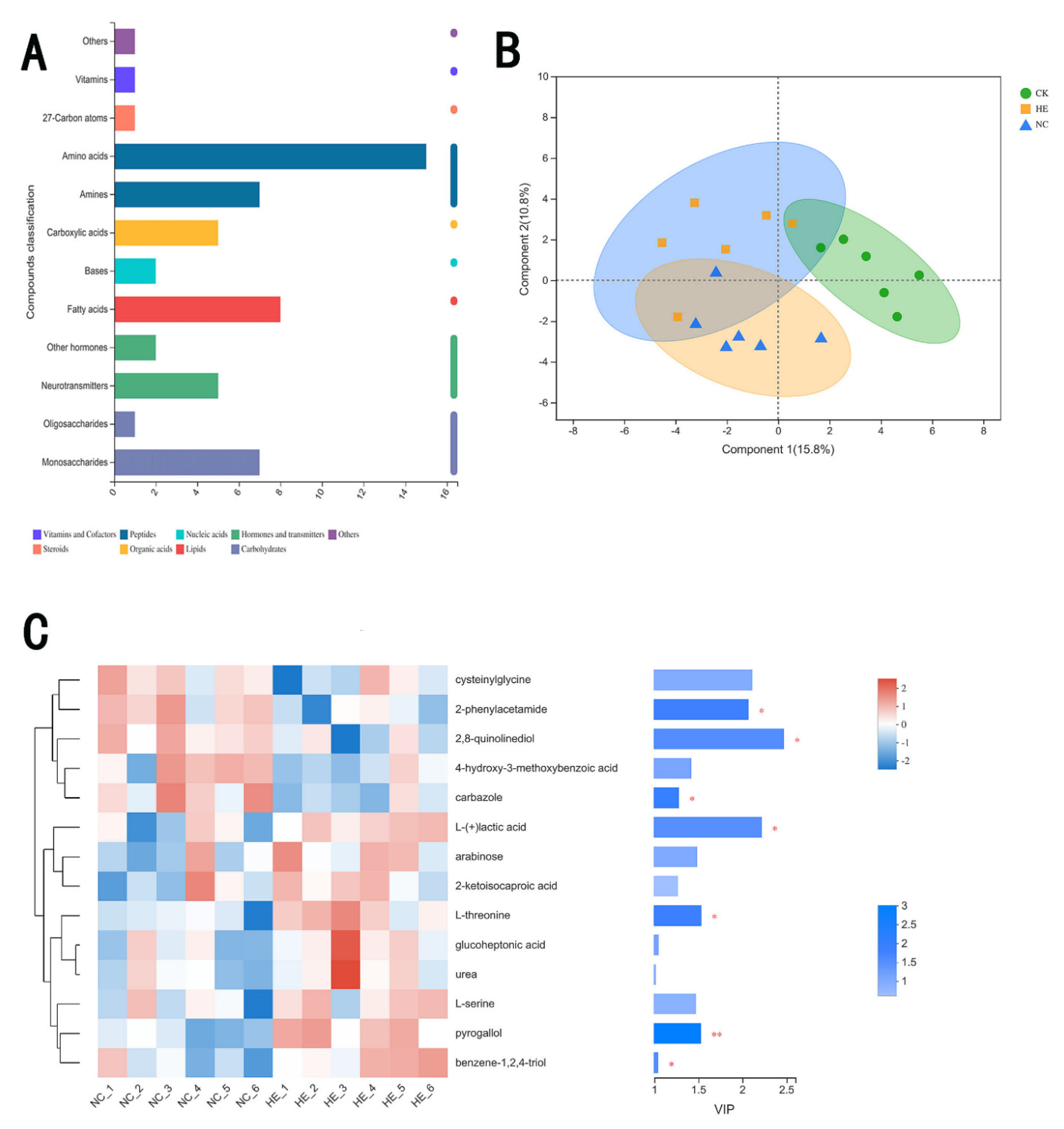
3.7.4. The Effects of Active Peptides on Intestinal Microbial Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kahn, E.S.; Cooper, M.E.; Prato, S.D. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet 2014, 383, 1068–1083. [Google Scholar] [CrossRef] [Green Version]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Thipsawat, S. Early detection of diabetic nephropathy in patient with type 2 diabetes mellitus: A review of the literature. Diabetes Vasc. Dis. Res. 2021, 18, 14791641211058856. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, V.; Gangan, N.; Sheehan, J. Impact of cardiovascular complications among patients with Type 2 diabetes mellitus: A systematic review. Expert Rev. Pharm. Outcomes Res. 2015, 15, 487–497. [Google Scholar] [CrossRef]
- Ofstad, A.P. Myocardial dysfunction and cardiovascular disease in type 2 diabetes. Scand. J. Clin. Lab. Investig. 2016, 76, 271–281. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [Green Version]
- Régnier, M.; van Hul, M.; Knauf, C.; Cani, P.D. Gut microbiome, endocrine control of gut barrier function and metabolic diseases. J. Endocrinol. 2021, 248, R67–R82. [Google Scholar] [CrossRef]
- He, C.; Shan, Y.; Song, W. Targeting gut microbiota as a possible therapy for diabetes. Nutr. Res. 2015, 35, 361–367. [Google Scholar] [CrossRef]
- Padhi, S.; Nayak, A.K.; Behera, A. Type II diabetes mellitus: A review on recent drug based therapeutics. Biomed. Pharmacother. 2020, 131, 110708. [Google Scholar] [CrossRef]
- Seino, S.; Sugawara, K.; Yokoi, N.; Takahashi, H. β-Cell signalling and insulin secretagogues: A path for improved diabetes therapy. Diabetes Obes. Metab. 2017, 19 (Suppl. 1), 22–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sola, D.; Rossi, L.; Schianca, G.P.; Maffioli, P.; Bigliocca, M.; Mella, R.; Corlianò, F.; Fra, G.P.; Bartoli, E.; Derosa, G. Sulfonylureas and their use in clinical practice. Arch. Med. Sci. 2015, 11, 840–848. [Google Scholar] [CrossRef]
- Quillen, D.M.; Samraj, G.; Kuritzky, L. Improving management of type 2 diabetes mellitus: 2. Biguanides. Hosp. Pract. 1999, 34, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.G. Hepatic glucose and lipid metabolism. Diabetologia 2016, 59, 1098–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thangavel, N.; Al Bratty, M.; Javed, S.A.; Ahsan, W.; Alhazmi, H.A. Targeting Peroxisome Proliferator-Activated Receptors Using Thiazolidinediones: Strategy for Design of Novel Antidiabetic Drugs. Int. J. Med. Chem. 2017, 2017, 1069718. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Jin, T. The incretin hormone GLP-1 and mechanisms underlying its secretion. J. Diabetes 2016, 8, 753–765. [Google Scholar] [CrossRef] [Green Version]
- Singla, R.K.; Kumar, R.; Khan, S.; Mohit; Kumari, K.; Garg, A. Natural Products: Potential Source of DPP-IV Inhibitors. Curr. Protein Pept. Sci. 2019, 20, 1218–1225. [Google Scholar] [CrossRef]
- De Meester, I.; Lambeir, A.M.; Proost, P.; Scharpé, S. Dipeptidyl peptidase IV substrates. An update on in vitro peptide hydrolysis by human DPPIV. Adv. Exp. Med. Biol. 2003, 524, 3–18. [Google Scholar]
- Min, Z.; Xiaoming, Y.; Min, W.; Jiechen, M.; Zhihuai, L.; Jun, L.; Jiayun, G.; Hezhi, Z.; Jie, Z. Purification of iron-rich swine hemoglobin and its characteristics. Sci. Technol. Food Ind. 2016, 37, 268–271. [Google Scholar] [CrossRef]
- Drucker, D.J. The biology of incretin hormones. Cell Metab. 2006, 3, 153–165. [Google Scholar] [CrossRef] [Green Version]
- Drucker, D.J. The role of gut hormones in glucose homeostasis. J. Clin. Investig. 2007, 117, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Gorrell, M.D. Dipeptidyl peptidase IV and related enzymes in cell biology and liver disorders. Clin. Sci. 2005, 108, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Andersen, E.S.; Deacon, C.F.; Holst, J.J. Do we know the true mechanism of action of the DPP-4 inhibitors? Diabetes Obes. Metab. 2018, 20, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Chi, T.; Qingchao, L.; Shicheng, S.; Liang, D.; Peng, X.; Guocai, H.; Chunhuan, L. Prospects for Comprehensive Development and Utilization of Donkey Blood Resources. Mod. J. Anim. Husb. Vet. Med. 2019, 2, 16–19. [Google Scholar]
- Lu, Y. Purification, Identification and Characterization of Angiotensin I-Converting Enzyme Inhibitory Peptides Derived from Walnut Protein Hydrolysate; Shaanxi University of Science and Technology: Shaanxi, China, 2015. [Google Scholar]
- Vila, J.; Moreno-Morales, J.; Ballesté-Delpierre, C. Current landscape in the discovery of novel antibacterial agents. Clin. Microbiol. Infect. 2020, 26, 596–603. [Google Scholar] [CrossRef]
- Harnedy-Rothwell, P.A.; McLaughlin, C.M.; O’Keeffe, M.B.; Gouic, A.V.L.; Allsopp, P.J.; McSorley, E.M.; Sharkey, S.; Whooley, J.; McGovern, B.; O’Harte, F.P.M.; et al. Identification and characterisation of peptides from a boarfish (Capros aper) protein hydrolysate displaying in vitro dipeptidyl peptidase-IV (DPP-IV) inhibitory and insulinotropic activity. Food Res. Int. 2020, 131, 108989. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Zhang, G.; Tian, Y.; Kong, F.; Xiong, S.; Zhao, S.; Jia, D.; Manyande, A.; Du, H. Identification of novel antioxidant peptides from snakehead (Channa argus) soup generated during gastrointestinal digestion and insights into the anti-oxidation mechanisms. Food Chem. 2021, 337, 127921. [Google Scholar] [CrossRef]
- Turroni, F.; Milani, C.; Duranti, S.; Mahony, J.; Sinderen, D.V.; Ventura, M. Glycan Utilization and Cross-Feeding Activities by Bifidobacteria. Trends Microbiol. 2018, 26, 339–350. [Google Scholar] [CrossRef]
- Zohreh, K.; Hadi, P.S.; Javad, H.; Behrouz, A.-A.; David, A. Identification and synthesis of multifunctional peptides from wheat germ hydrolysate fractions obtained by proteinase K digestion. J. Food Biochem. 2019, 43, e12800. [Google Scholar] [CrossRef]
- Ahlke, H. An Overview of Murine High Fat Diet as a Model for Type 2 Diabetes Mellitus. Exp. Diabesity Res. 2016, 2016, 2902351. [Google Scholar] [CrossRef] [Green Version]
- Sooyoung, J.; Younjuong, K.; Changjun, L.; Bomi, K.; Jihye, N.; Jee, J.J.; Sun, Y.S.; Hong, K.; Sowon, P. The Effect of Formula-based Nutritional Treatment on Colitis in a Murine Model. J. Korean Med. Sci. 2021, 36, e342. [Google Scholar] [CrossRef]
- Masella, A.P.; Bartram, A.K.; Truszkowski, J.M.; Brown, D.G.; Neufeld, J.D. PANDAseq: Paired-end assembler for illumina sequences. BMC Bioinform. 2012, 13, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregory, C.J.; Justin, K.; Jesse, S.; Kyle, B.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.-Y.; Hsieh, C.-H.; Hung, C.-C.; Jao, C.-L.; Chen, M.-C.; Hsu, K.-C. Fish skin gelatin hydrolysates as dipeptidyl peptidase IV inhibitors and glucagon-like peptide-1 stimulators improve glycaemic control in diabetic rats: A comparison between warm- and cold-water fish. J. Funct. Foods 2015, 19, 330–340. [Google Scholar] [CrossRef]
- Iatcu, C.O.; Steen, A.; Covasa, M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients 2021, 14, 166. [Google Scholar] [CrossRef]
- Shuang, J. Study on the Design, Enzyme-Resistant and Inhibition Mechanismof ACE Inhibitory Peptideshydrolysis Inhibition; Jiangnan University: Jiangnan, China, 2014. [Google Scholar]
- Bautista, C.T.; Wurapa, E.; Sateren, W.B.; Morris, S.; Hollingsworth, B.; Sanchez, J.L. Bacterial vaginosis:a synthesis of the literature on etiology,prevalence,risk factors,and relationship with chlamydia and gonorrhea infections. Mil. Med. Res. 2016, 3, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Wan-Chun, W.; Wei, Z.; Sheng, L. Small intestinal bacteria overgrowth decreases small intestinal motility in the NASH rats. World J. Gastroenterol. 2008, 14, 313. [Google Scholar]
- Hong-Xin, C.; Ya-Nan, H.; Jing-Wan, L.; Ke, Y. Hypoglycemic Mechanism of the Berberine Organic Acid Salt under the Synergistic Effect of Intestinal Flora and Oxidative Stress. Oxidative Med. Cell. Longev. 2018, 2018, 8930374. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Dokter-Fokkens, J.; Lozano, S.F.; Zhang, Q.; de Haan, B.J.; Zhang, H.; Faas, M.M.; de Vos, P. Lactic Acid Bacteria May Impact Intestinal Barrier Function by Modulating Goblet Cells. Mol. Nutr. Food Res. 2018, 62, e1700572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, M.A.; Rama, G.R.; de Souza, C.F.V.; Granada, C.E. Acid lactic lactobacilli as a biotechnological toll to improve food quality and human health. Biotechnol. Prog. 2020, 36, e2937. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.M. Highlighting Diabetes Mellitus: The Epidemic Continues. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e1–e8. [Google Scholar] [CrossRef] [Green Version]
- Bao, P.; Mi, J.; Yu, Z.; Liu, L.; Zhu, Z.; Liu, S.; Nan, Z. Efficacy and safety of acupuncture combined with Chinese herbal medicine in the treatment of type 2 diabetes mellitus: A protocol for a systematic review and meta-analysis. Medicine 2021, 100, e27658. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef]
- Deacon, C.F.; Lebovitz, H.E. Comparative review of dipeptidyl peptidase-4 inhibitors and sulphonylureas. Diabetes Obes. Metab. 2016, 18, 333–347. [Google Scholar] [CrossRef] [Green Version]
- Shirakawa, J.; Terauchi, Y. Potential linkage between dipeptidyl peptidase-4 inhibitor use and the risk of pancreatitis/pancreatic cancer. J. Diabetes Investig. 2020, 11, 789–791. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Yang, X.; Lou, W.; Zhang, X. Discovery of dipeptidyl peptidase 4 inhibitory peptides from Largemouth bass (Micropterus salmoides) by a comprehensive approach. Bioorg. Chem. 2020, 105, 104432. [Google Scholar] [CrossRef]
- Wang, F.; Yu, G.; Zhang, Y.; Zhang, B.; Fan, J. Dipeptidyl Peptidase IV Inhibitory Peptides Derived from Oat (Avena sativa L.), Buckwheat (Fagopyrum esculentum), and Highland Barley (Hordeum vulgare trifurcatum (L.) Trofim) Proteins. J. Agric. Food Chem. 2015, 63, 9543–9549. [Google Scholar] [CrossRef]
- Power, O.; Nongonierma, A.B.; Jakeman, P.; FitzGerald, R.J. Food protein hydrolysates as a source of dipeptidyl peptidase IV inhibitory peptides for the management of type 2 diabetes. Proc. Nutr. Soc. 2014, 73, 34–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, C.; Bittoun, E.; Bhagwat, D.; Valkonen, S.; Saari, A.; Jaakkola, U.; Eerola, E.; Huovinen, P.; Hänninen, A. Effects of a germ-free environment on gut immune regulation and diabetes progression in non-obese diabetic (NOD) mice. Diabetologia 2011, 54, 1398–1406. [Google Scholar] [CrossRef]
- Xu, W.T.; Nie, Y.Z.; Yang, Z.; Lu, N.H. The crosstalk between gut microbiota and obesity and related metabolic disorders. Future Microbiol. 2016, 11, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Wu, J.; Lam, Y.Y.; Kwan, H.Y.; Bian, Z.X.; Wong, H.L.X. Gut-Microbial Metabolites, Probiotics and Their Roles in Type 2 Diabetes. Int. J. Mol. Sci. 2021, 22, 12846. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chaturvedi, J.; Chaudhari, B.P.; Singh, R.L.; Kakkar, P. Probiotic Enterococcus lactis IITRHR1 protects against acetaminophen-induced hepatotoxicity. Nutrition 2012, 28, 173–181. [Google Scholar] [CrossRef]
- Siezen, R.J.; Kleerebezem, M. The human gut microbiome: Are we our enterotypes? Microb. Biotechnol. 2011, 4, 550–553. [Google Scholar] [CrossRef] [Green Version]
- Balakumar, M.; Prabhu, D.; Sathishkumar, C.; Prabu, P.; Rokana, N.; Kumar, R.; Raghavan, S.; Soundarajan, A.; Grover, S.; Batish, V.K.; et al. Improvement in glucose tolerance and insulin sensitivity by probiotic strains of Indian gut origin in high-fat diet-fed C57BL/6J mice. Eur. J. Nutr. 2018, 57, 279–295. [Google Scholar] [CrossRef]
- Dang, F.; Jiang, Y.; Pan, R.; Zhou, Y.; Wu, S.; Wang, R.; Zhuang, K.; Zhang, W.; Li, T.; Man, C. Administration of Lactobacillus paracasei ameliorates type 2 diabetes in mice. Food Funct. 2018, 9, 3630–3639. [Google Scholar] [CrossRef]
- Hu, S.; Wang, J.; Xu, Y.; Yang, H.; Wang, J.; Xue, C.; Yan, X.; Su, L. Anti-inflammation effects of fucosylated chondroitin sulphate from Acaudina molpadioides by altering gut microbiota in obese mice. Food Funct. 2019, 10, 1736–1746. [Google Scholar] [CrossRef]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Zheng, A.; Hu, L.; Zhao, Y.; Zheng, L.; et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 2020, 12, 1832857. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Yu, K.; Fan, X.; Xiao, W.; Cai, Y.; Xu, P.; Yu, M.; Yang, H. 6-Formylindolo(3,2-b)carbazole induced aryl hydrocarbon receptor activation prevents intestinal barrier dysfunction through regulation of claudin-2 expression. Chem. Biol. Interact. 2018, 288, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Verzelloni, E.; Pellacani, C.; Tagliazucchi, D.; Tagliaferri, S.; Calani, L.; Costa, L.G.; Brighenti, F.; Borges, G.; Crozier, A.; Conte, A.; et al. Antiglycative and neuroprotective activity of colon-derived polyphenol catabolites. Mol. Nutr. Food Res. 2011, 55 (Suppl. 1), S35–S43. [Google Scholar] [CrossRef] [PubMed]








| Time (min) | Buffer B | Time (min) |
|---|---|---|
| 0 | 4% | 0 |
| 2 | 8% | 2 |
| 45 | 28% | 45 |
| 55 | 40% | 55 |
| 56 | 95% | 56 |
| Group | Number | Administration Dosage |
|---|---|---|
| Blank control group (CK) | 10 | Saline |
| Model control group (NC) | 10 | Saline |
| Sitagliptin group (PC) | 10 | 15 mg/kg Sitagliptin |
| Active peptide low-dose group (HEL) | 10 | 400 mg/kg polypeptide |
| Active peptide medium-dose group (HEM) | 10 | 800 mg/kg polypeptide |
| Active peptide high-dose group (HEH) | 10 | 1200 mg/kg polypeptide |
| No. | Peptide | m/z | Z | Mass |
|---|---|---|---|---|
| P1 | VDPENFRLL | 551.80 | 2.00 | 1102.59 |
| P2 | HLPNDF | 371.68 | 2.00 | 742.35 |
| P3 | LPGAL | 470.29 | 1.00 | 470.29 |
| P4 | HVDPENFRLL | 620.33 | 2.00 | 1239.65 |
| P5 | VDPENFRL | 495.26 | 2.00 | 989.51 |
| P6 | VDPVNFKLL | 522.81 | 2.00 | 1044.61 |
| P7 | YPWTQ | 694.32 | 1.00 | 694.32 |
| P8 | PVNF | 476.25 | 1.00 | 476.25 |
| P9 | HVDPENFRL | 376.19 | 3.00 | 1126.56 |
| P10 | VDPVNFKL | 466.27 | 2.00 | 931.53 |
| P11 | DLPGAL | 585.33 | 1.00 | 585.33 |
| P12 | FPHFDLSHGS | 572.26 | 2.00 | 1143.52 |
| P13 | HLPNDFTP | 470.73 | 2.00 | 940.45 |
| P14 | DFTPA | 550.25 | 1.00 | 550.25 |
| P15 | PHFDLS | 358.17 | 2.00 | 715.34 |
| P16 | LPNDFTPA | 437.72 | 2.00 | 874.43 |
| No. | Peptide | Peptide Sequence Score | DPP-IV Binding Energy |
|---|---|---|---|
| P1 | VDPENFRLL | 0.74 | −8.70 |
| P2 | HLPNDF | 0.72 | −7.50 |
| P3 | LPGAL | 0.71 | −7.60 |
| P4 | HVDPENFRLL | 0.70 | −8.00 |
| P5 | VDPENFRL | 0.70 | −7.80 |
| P6 | VDPVNFKLL | 0.69 | −8.20 |
| P7 | YPWTQ | 0.66 | −9.10 |
| P8 | PVNF | 0.66 | −7.80 |
| P9 | HVDPENFRL | 0.65 | −8.10 |
| P10 | VDPVNFKL | 0.62 | −8.60 |
| P11 | DLPGAL | 0.62 | −8.20 |
| P12 | FPHFDLSHGS | 0.61 | −8.10 |
| P13 | HLPNDFTP | 0.58 | −8.40 |
| P14 | DFTPA | 0.58 | −7.70 |
| P15 | PHFDLS | 0.54 | −8.50 |
| P16 | LPNDFTPA | 0.53 | −7.30 |
| NO. | Sequence | Interactions with DPP-IV | 3D Polypeptide Structures |
|---|---|---|---|
| P1 | VDPENFRLL | His-748, Tyr-752, Tyr-48, Lys-554, Gln-553, Tyr-547 |  |
| P7 | YPWTQ | Tyr-195, Tyr-211, Trp-124, Asp-709, Ala-707, Tyr-238, Arg-253, Lys-122, Gln-123 |  |
| Group | Blood Glucose (mmol/mL) | |
|---|---|---|
| 0 d | 28 d | |
| CK | 5.95 ± 1.01 | 4.65 ± 0.98 |
| NC | 19.91 ± 1.27 ** | 23.92 ± 3.86 ** |
| SP | 19.97 ± 1.57 ** | 15.98 ± 2.03 **## |
| HEL | 19.88 ± 1.10 ** | 19.64 ± 1.17 **## |
| HEM | 19.92 ± 2.68 ** | 17.89 ± 2.22 **## |
| HEH | 18.97 ± 1.31 ** | 17.16 ± 1.56 **## |
| Group | Sugar Tolerance (mmol·L−1) | AUC | |||
|---|---|---|---|---|---|
| 0 h | 0.5 h | 1.5 h | 2 h | ||
| CK | 4.65 ± 0.98 | 7.20 ± 1.31 | 5.91 ± 1.93 | 5.20 ± 1.38 | 12.26 ± 1.90 |
| NC | 23.92 ± 3.86 ** | 30.24 ± 3.78 ** | 28.19 ± 5.74 **## | 25.64 ± 3.71 ** | 55.45 ± 8.72 **## |
| SP | 15.98 ± 2.03 **# | 24.00 ± 3.47 **## | 14.55 ± 4.77 **## | 13.45 ± 7.19 **## | 38.08 ± 7.95 **## |
| HEL | 19.64 ± 1.17 **# | 26.23 ± 2.88 **## | 18.43 ± 1.63 **## | 17.18 ± 2.71 **## | 45.27 ± 6.70 **## |
| HEM | 17.89 ± 2.22 **# | 24.64 ± 2.98 **## | 16.66 ± 4.24 **## | 15.10 ± 4.70 **## | 40.44 ± 8.73 **## |
| HEH | 17.16 ± 1.56 **# | 26.35 ± 3.92 ** | 16.07 ± 3.56 **## | 12.97 ± 2.59 **## | 40.37 ± 7.92 **## |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Liu, D.; Hao, H.; Wu, X. Identification of the DPP-IV Inhibitory Peptides from Donkey Blood and Regulatory Effect on the Gut Microbiota of Type 2 Diabetic Mice. Foods 2022, 11, 2148. https://doi.org/10.3390/foods11142148
Ma C, Liu D, Hao H, Wu X. Identification of the DPP-IV Inhibitory Peptides from Donkey Blood and Regulatory Effect on the Gut Microbiota of Type 2 Diabetic Mice. Foods. 2022; 11(14):2148. https://doi.org/10.3390/foods11142148
Chicago/Turabian StyleMa, Chaoyue, Dan Liu, Huifang Hao, and Xiaotong Wu. 2022. "Identification of the DPP-IV Inhibitory Peptides from Donkey Blood and Regulatory Effect on the Gut Microbiota of Type 2 Diabetic Mice" Foods 11, no. 14: 2148. https://doi.org/10.3390/foods11142148
APA StyleMa, C., Liu, D., Hao, H., & Wu, X. (2022). Identification of the DPP-IV Inhibitory Peptides from Donkey Blood and Regulatory Effect on the Gut Microbiota of Type 2 Diabetic Mice. Foods, 11(14), 2148. https://doi.org/10.3390/foods11142148






