Characterization and Classification of Direct and Commercial Strawberry Beverages Using Absorbance–Transmission and Fluorescence Excitation–Emission Matrix Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Acquisition of Absorption and EEMs
2.3. Data Analysis
3. Results and Discussion
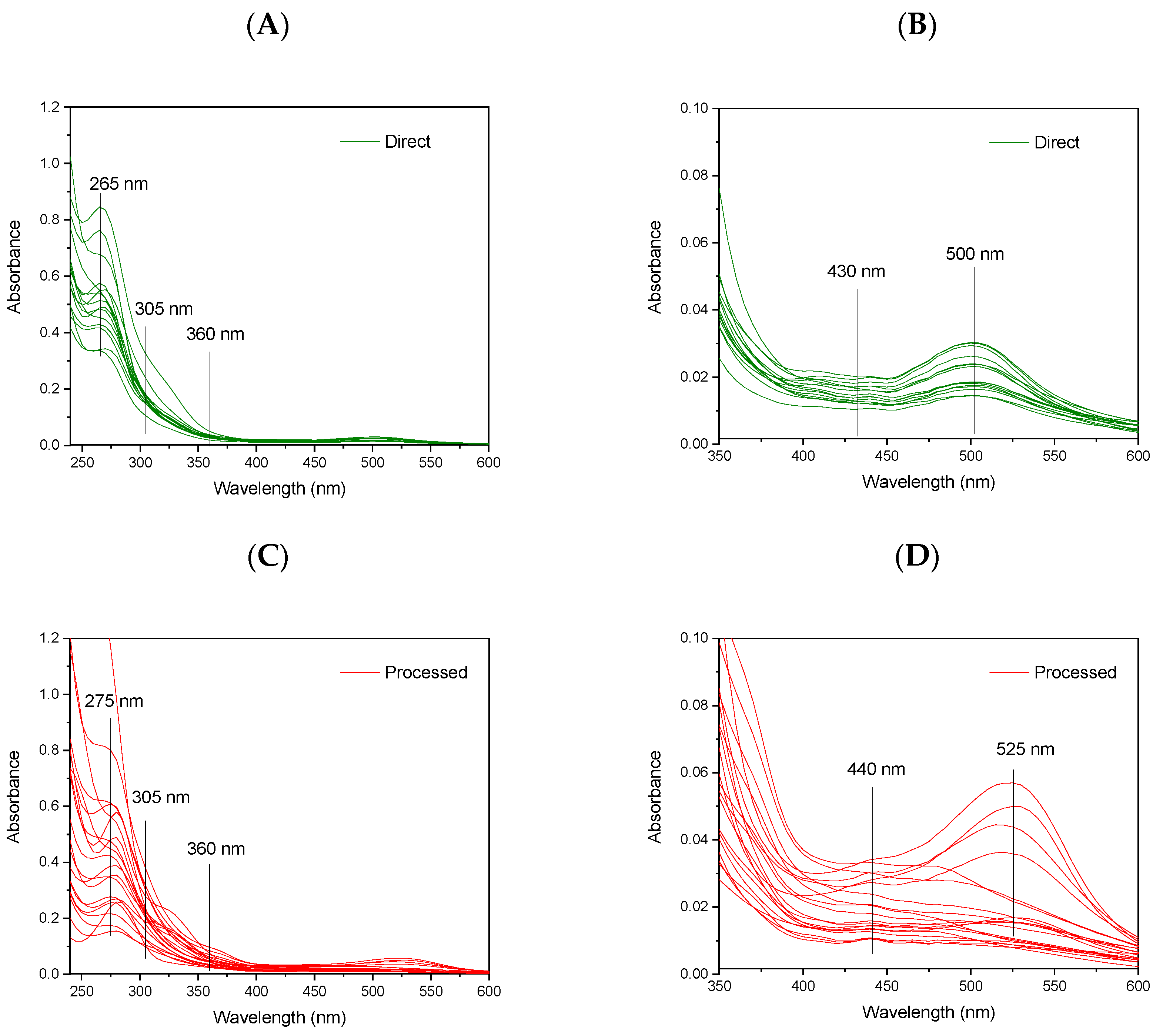
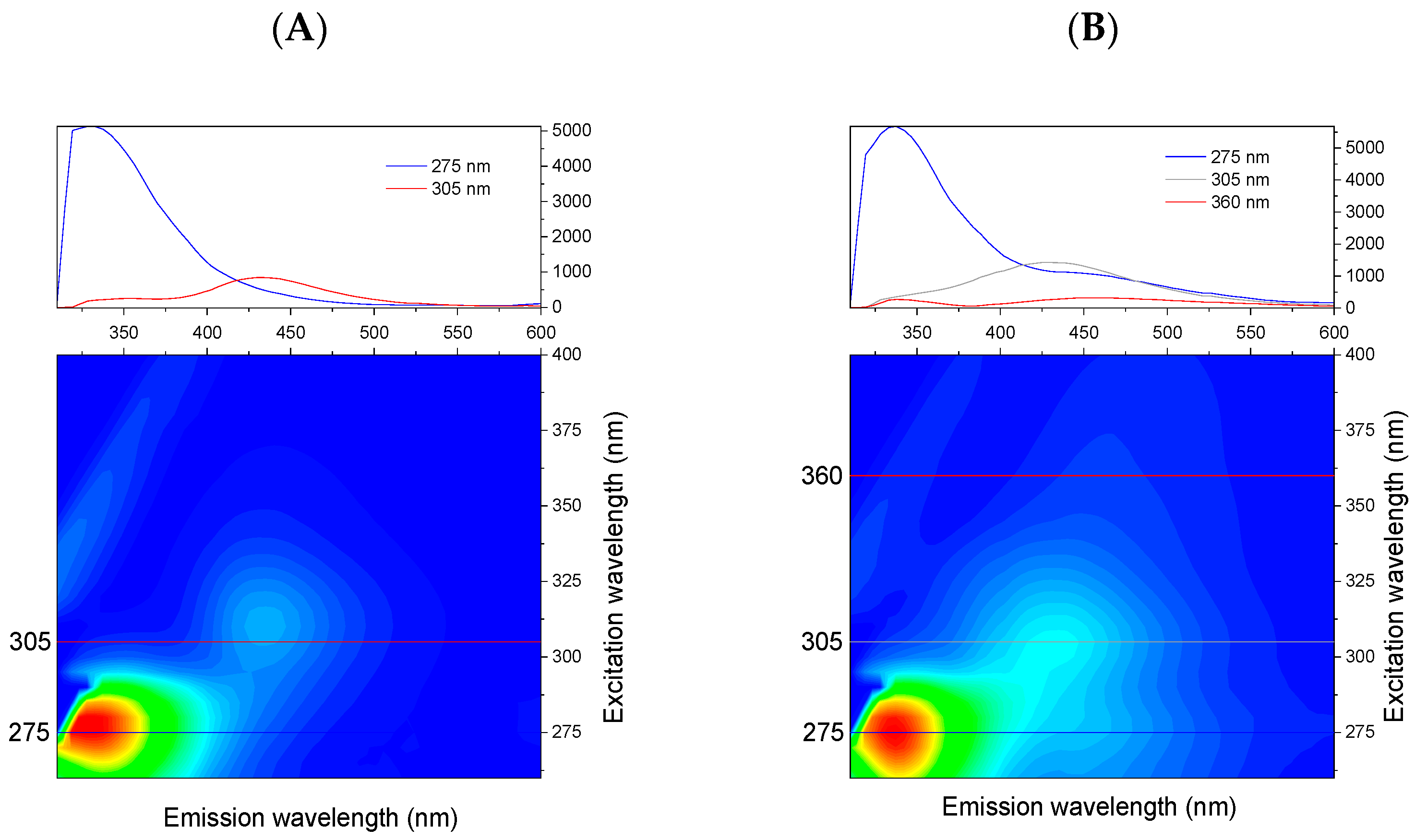
3.1. Absorption Spectra and Excitation–Emission Matrices of Studied Juices
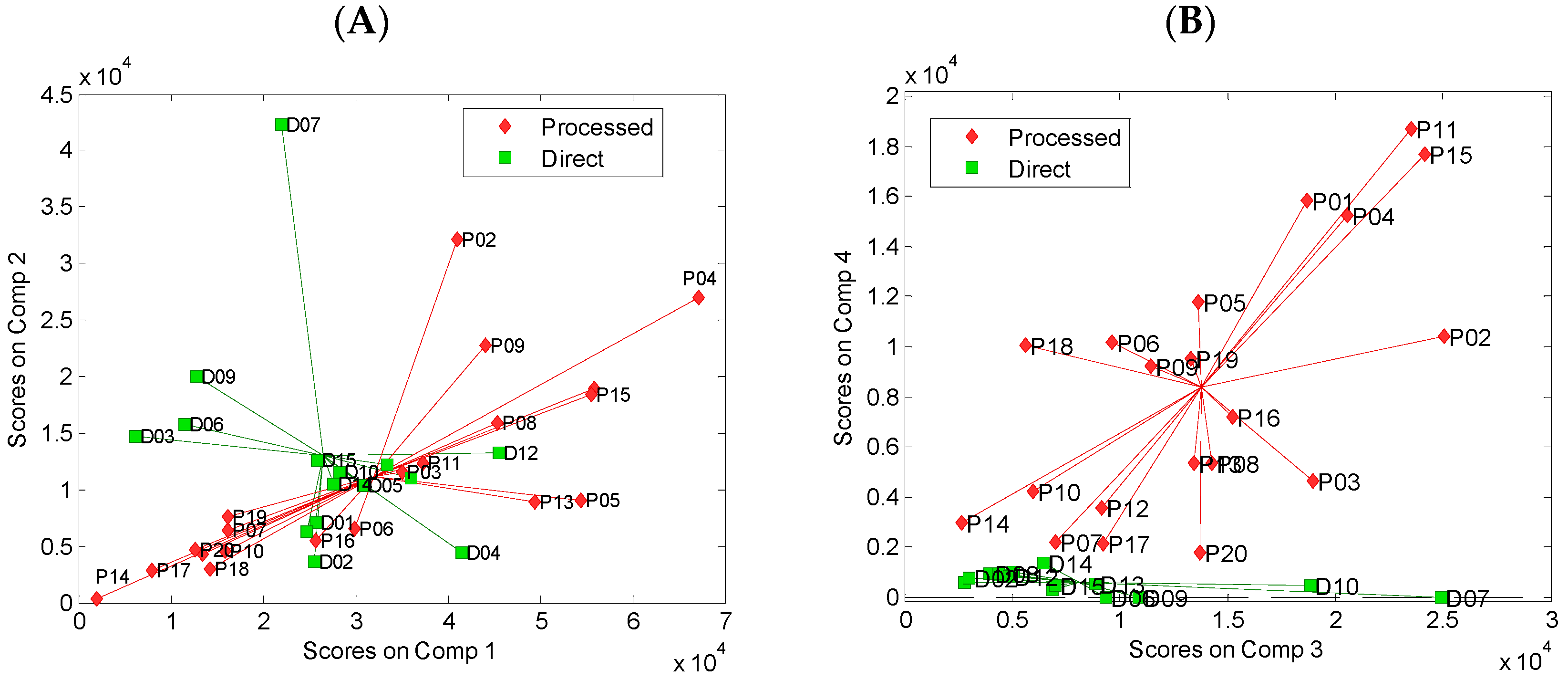
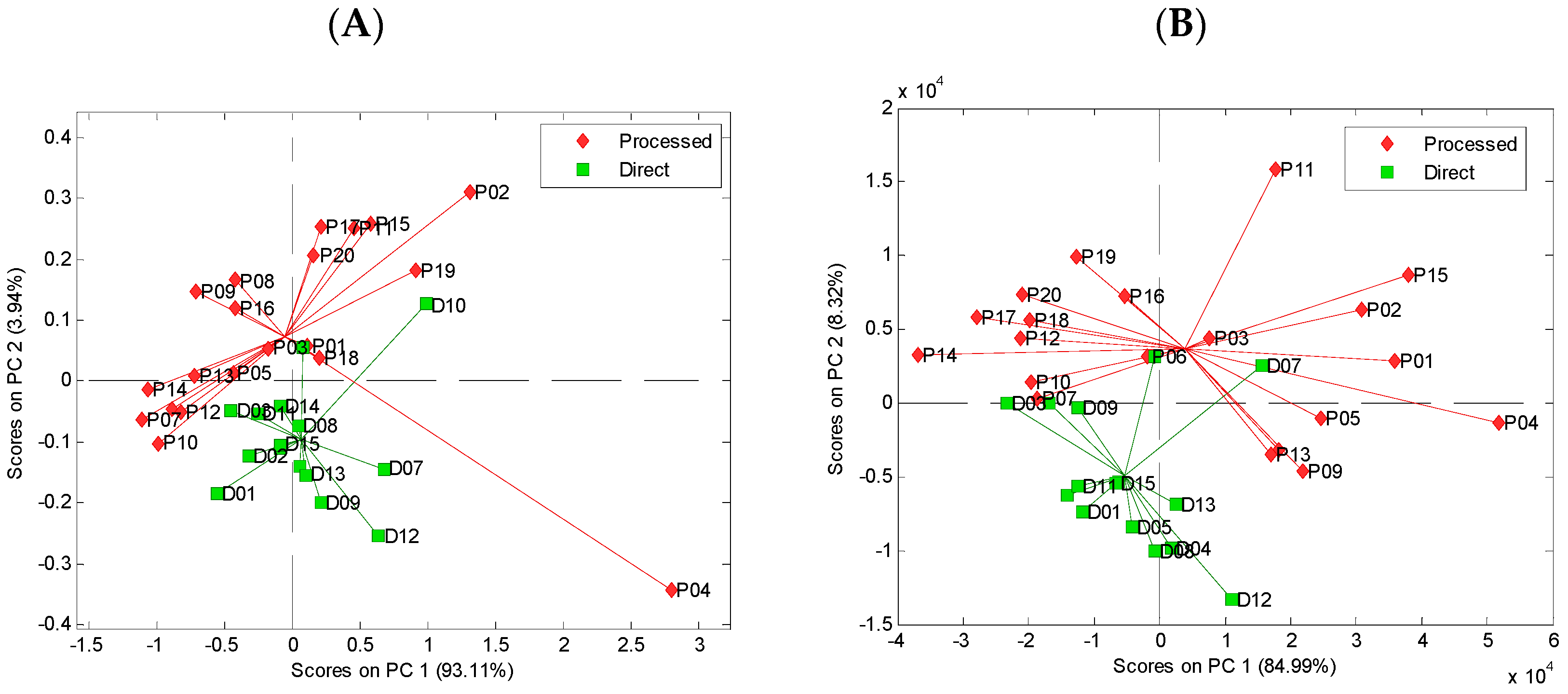
3.2. Principal Component Analysis of Spectral Data
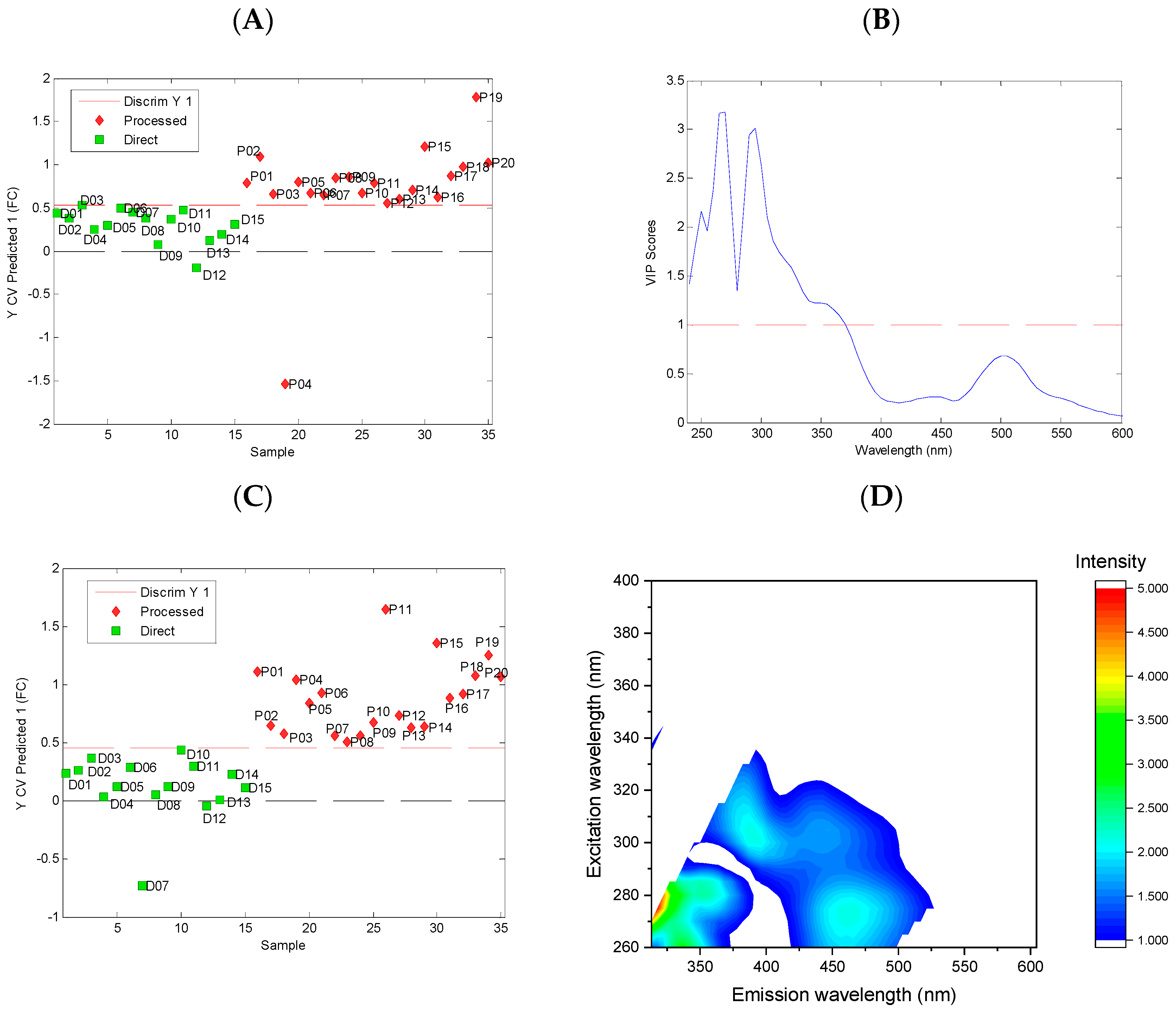
3.3. Classification Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef]
- Pap, N.; Fidelis, M.; Azevedo, L.; do Carmo, M.A.V.; Wang, D.; Mocan, A.; Pereira, E.P.R.; Xavier-Santos, D.; Sant’Ana, A.S.; Yang, B.; et al. Berry polyphenols and human health: Evidence of antioxidant, anti-inflammatory, microbiota modulation, and cell-protecting effects. Curr. Opin. Food Sci. 2021, 42, 167–186. [Google Scholar] [CrossRef]
- Milosavljević, D.M.; Mutavdžić, D.R.; Radotić, K.; Milivojević, J.M.; Maksimović, V.M.; Dragišić Maksimović, J.J. Phenolic profiling of 12 strawberry cultivars using different spectroscopic methods. J. Agric. Food Chem. 2020, 68, 4346–4354. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, Z.; Liao, X. A review of fruit juice authenticity assessments: Targeted and untargeted analyses. Crit. Rev. Food Sci. 2021, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Salo, H.M.; Nguyen, N.; Alakärppä, E.; Klavins, L.; Hykkerud, A.L.; Karppinen, K.; Jaakola, L.; Klavins, M.; Häggman, H. Authentication of berries and berry-based food products. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5197–5225. [Google Scholar] [CrossRef]
- Kamiloglu, S. Authenticity and traceability in beverages. Food Chem. 2019, 277, 12–24. [Google Scholar] [CrossRef]
- Dasenaki, M.E.; Thomaidis, N.S. Quality and authenticity control of fruit juices—A review. Molecules 2019, 24, 1014. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Luo, H.; Hu, M.; Zhang, M.; Feng, J.; Liu, Y.; Dong, Q.; Liu, B. Optical non-destructive techniques for small berry fruits: A review. Artif. Intell. Agric. 2019, 2, 85–98. [Google Scholar] [CrossRef]
- Power, A.C.; Chapman, J.; Chandra, S.; Cozzolino, D. 6—Ultraviolet-visible spectroscopy for food quality analysis. In Evaluation Technologies for Food Quality; Zhong, J., Wang, X., Eds.; Woodhead Publishing: Thorston, UK, 2019; pp. 91–104. [Google Scholar]
- Okino, S.; Kokawa, M.; Islam, M.Z.; Kitamura, Y. Effects of apple juice manufacturing processes on procyanidin concentration and nondestructive analysis by fluorescence fingerprint. Food Bioprocess Technol. 2021, 14, 692–701. [Google Scholar] [CrossRef]
- Bian, H.; Sheng, L.; Yao, H.; Ji, R.; Yu, Y.; Chen, R.; Wei, D.; Han, Y. Application of fluorescence spectroscopy in classifying apple juice according to the variety. Optik 2021, 231, 166361. [Google Scholar] [CrossRef]
- Trivittayasil, V.; Kameya, H.; Shoji, T.; Tsuta, M.; Kokawa, M.; Sugiyama, J. Simultaneous estimation of scavenging capacities of peach extract for multiple reactive oxygen species by fluorescence fingerprint method. Food Chem. 2017, 232, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Bañegil, M.; Lavado Rodas, N.; Prieto Losada, M.H.; Blanco Cipollone, F.; Moñino Espino, M.J.; Muñoz de la Peña, A.; Durán-Merás, I. Evolution of polyphenols content in plum fruits (Prunus salicina) with harvesting time by second-order excitation-emission fluorescence multivariate calibration. Microchem. J. 2020, 158, 105299. [Google Scholar] [CrossRef]
- Monago-Maraña, O.; Cabrera-Bañegil, M.; Rodas, N.L.; Muñoz de la Peña, A.; Durán-Merás, I. First-order discrimination of methanolic extracts from plums according to harvesting date using fluorescence spectra. Quantification of polyphenols. Microchem. J. 2021, 169, 106533. [Google Scholar] [CrossRef]
- Kokawa, M.; Hashimoto, A.; Li, X.; Tsuta, M.; Kitamura, Y. Estimation of ‘Hass’ avocado (Persea americana Mill.) ripeness by fluorescence fingerprint measurement. Food Anal. Methods 2020, 13, 892–901. [Google Scholar] [CrossRef]
- Ammari, F.; Redjdal, L.; Rutledge, D.N. Detection of orange juice frauds using front-face fluorescence spectroscopy and Independent Components Analysis. Food Chem. 2015, 168, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, E.; Włodarska, K.; Khmelinskii, I. Application of multidimensional and conventional fluorescence techniques for classification of beverages originating from various berry fruit. Methods Appl. Fluoresc. 2020, 8, 015006. [Google Scholar] [CrossRef] [PubMed]
- Quatela, A.; Gilmore, A.M.; Gall, K.E.S.; Sandros, M.; Csatorday, K.; Siemiarczuk, A.; Yang, B.; Camenen, L. A-TEEMTM, a new molecular fingerprinting technique: Simultaneous absorbance-transmission and fluorescence excitation-emission matrix method. Methods Appl. Fluoresc. 2018, 6, 027002. [Google Scholar] [CrossRef] [PubMed]
- Kimball, J.; Chavez, J.; Ceresa, L.; Kitchner, E.; Nurekeyev, Z.; Doan, H.; Szabelski, M.; Borejdo, J.; Gryczynski, I.; Gryczynski, Z. On the origin and correction for inner filter effects in fluorescence Part I: Primary inner filter effect-the proper approach for sample absorbance correction. Methods Appl. Fluoresc. 2020, 8, 033002. [Google Scholar] [CrossRef]
- Herbert-Pucheta, J.E.; Padilla-Maya, G.; Milmo-Brittinham, D.; Lojero, D.; Gilmore, A.M.; Raventós-Llopart, L.; Hernández-Pulido, K.E.; Zepeda-Vallejo, L.G. Multivariate spectroscopy for targeting phenolic choreography in wine with A-TEEMTM and NMR crosscheck non-targeted metabolomics. BIO Web Conf. 2019, 15, 02006. [Google Scholar] [CrossRef] [Green Version]
- Ranaweera, R.K.R.; Gilmore, A.M.; Capone, D.L.; Bastian, S.E.P.; Jeffery, D.W. Authentication of the geographical origin of Australian Cabernet Sauvignon wines using spectrofluorometric and multi-element analyses with multivariate statistical modelling. Food Chem. 2021, 335, 127592. [Google Scholar] [CrossRef]
- Ranaweera, R.K.R.; Gilmore, A.M.; Capone, D.L.; Bastian, S.E.P.; Jeffery, D.W. Spectrofluorometric analysis combined with machine learning for geographical and varietal authentication, and prediction of phenolic compound concentrations in red wine. Food Chem. 2021, 361, 130149. [Google Scholar] [CrossRef]
- Kahle, E.-M.; Zarnkow, M.; Jacob, F. Substances in beer that cause fluorescence: Evaluating the qualitative and quantitative determination of these ingredients. Eur. Food Res. Technol. 2019, 245, 2727–2737. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Graeber, D.; Bro, R. Fluorescence spectroscopy and multi-way techniques. PARAFAC. Anal. Methods 2013, 5, 6557–6566. [Google Scholar] [CrossRef] [Green Version]
- Ballabio, D.; Consonni, V. Classification tools in chemistry. Part 1: Linear models. PLS-DA. Anal. Methods 2013, 5, 3790–3798. [Google Scholar] [CrossRef]
- Chong, I.-G.; Jun, C.-H. Performance of some variable selection methods when multicollinearity is present. Chemometr. Intell. Lab. 2005, 78, 103–112. [Google Scholar] [CrossRef]
- Teleszko, M.; Nowicka, P.; Wojdyło, A. Effect of cultivar and storage temperature on identification and stability of polyphenols in strawberry cloudy juices. J. Food Compos. Anal. 2016, 54, 10–19. [Google Scholar] [CrossRef]
- Nowicka, A.; Kucharska, A.Z.; Sokół-Łętowska, A.; Fecka, I. Comparison of polyphenol content and antioxidant capacity of strawberry fruit from 90 cultivars of Fragaria × ananassa Duch. Food Chem. 2019, 270, 32–46. [Google Scholar] [CrossRef]
- Saha, S.; Singh, J.; Paul, A.; Sarkar, R.; Khan, Z.; Banerjee, K. Anthocyanin profiling using UV-vis spectroscopy and liquid chromatography mass spectrometry. J. AOAC Int. 2021, 103, 23–39. [Google Scholar] [CrossRef]
- Da Silva, F.L.; Escribano-Bailón, M.T.; Pérez Alonso, J.J.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanin pigments in strawberry. LWT—Food Sci. Technol. 2007, 40, 374–382. [Google Scholar] [CrossRef]
- Marszałek, K.; Woźniak, Ł.; Kruszewski, B.; Skąpska, S. The effect of high pressure techniques on the stability of anthocyanins in fruit and vegetables. Int. J. Mol. Sci. 2017, 18, 277. [Google Scholar] [CrossRef] [Green Version]
- Aguiló-Aguayo, I.; Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. Changes in quality attributes throughout storage of strawberry juice processed by high-intensity pulsed electric fields or heat treatments. LWT—Food Sci. Technol. 2009, 42, 813–818. [Google Scholar] [CrossRef]
- Agati, G.; Matteini, P.; Oliveira, J.; de Freitas, V.; Mateus, N. Fluorescence approach for measuring anthocyanins and derived pigments in red wine. J. Agric. Food Chem. 2013, 61, 10156–10162. [Google Scholar] [CrossRef] [PubMed]
- Vidot, K.; Devaux, M.-F.; Alvarado, C.; Guyot, S.; Jamme, F.; Gaillard, C.; Siret, R.; Lahaye, M. Phenolic distribution in apple epidermal and outer cortex tissue by multispectral deep-UV autofluorescence cryo-imaging. Plant Sci. 2019, 283, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Obón, J.M.; Díaz-García, M.C.; Castellar, M.R. Red fruit juice quality and authenticity control by HPLC. J. Food Comp. Anal. 2011, 24, 760–771. [Google Scholar] [CrossRef]
- Airado-Rodríguez, D.; Galeano-Díaz, T.; Durán-Merás, I.; Wold, J.P. Usefulness of fluorescence excitation−emission matrices in combination with PARAFAC, as fingerprints of red wines. J. Agric. Food Chem. 2009, 57, 1711–1720. [Google Scholar] [CrossRef]
- Zhu, D.Z.; Ji, B.P.; Eum, H.L.; Zude, M. Evaluation of the non-enzymatic browning in thermally processed apple juice by front-face fluorescence spectroscopy. Food Chem. 2009, 113, 272–279. [Google Scholar] [CrossRef]
- Włodarska, K.; Khmelinskii, I.; Sikorska, E. Authentication of apple juice categories based on multivariate analysis of the synchronous fluorescence spectra. Food Control 2018, 86, 42–49. [Google Scholar] [CrossRef]
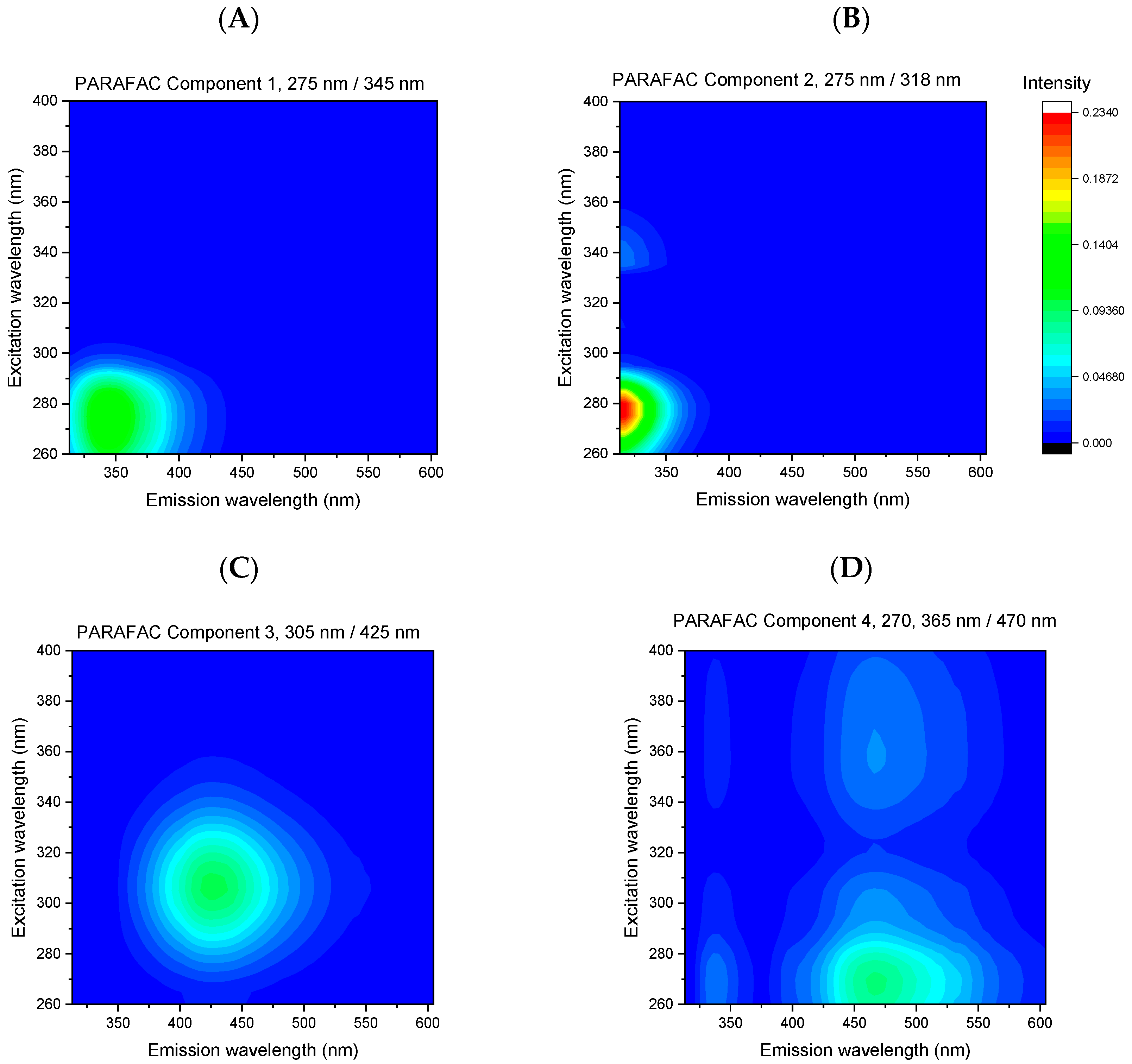
| Component No. | λexc/λem | Mean of Scores Value for Two Classes |
|---|---|---|
| Component 1 | 275/345 nm | Direct: 26,552 ± 10,675; Processed: 31,983 ± 19,104 * |
| Component 2 | 275/318 nm | Direct: 13,051 ± 9164; Processed: 11,129 ± 8727 * |
| Component 3 | 305/425 nm | Direct: 8125 ± 6162; Processed:13,771 ± 6447 ** |
| Component 4 | 270, 365/470 nm | Direct: 615 ± 418; Processed: 8403 ± 5346 ** |
| Model | LV | Misclassification Error | Sensitivity for Class 1 | Specificity for Class 1 |
|---|---|---|---|---|
| Absorption spectra (UV–vis) | 3 | 0.029 | 0.93 | 1.00 |
| Absorption spectra UV (240–350 nm) | 3 | 0.057 | 0.93 | 0.95 |
| Absorption spectra vis (350–600 nm) | 5 | 0 | 1.00 | 1.00 |
| PARAFAC-EEMs | 2 | 0.057 | 0.93 | 0.95 |
| uEEMs | 4 | 0 | 1.00 | 1.00 |
| Emission spectra with excitation at 275, 305, 365 nm | 4 | 0 | 1.00 | 1.00 |
| Emission spectra with excitation EM 305, 365 nm | 6 | 0.086 | 1 | 0.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikorska, E.; Nowak, P.; Pawlak-Lemańska, K.; Sikorski, M. Characterization and Classification of Direct and Commercial Strawberry Beverages Using Absorbance–Transmission and Fluorescence Excitation–Emission Matrix Technique. Foods 2022, 11, 2143. https://doi.org/10.3390/foods11142143
Sikorska E, Nowak P, Pawlak-Lemańska K, Sikorski M. Characterization and Classification of Direct and Commercial Strawberry Beverages Using Absorbance–Transmission and Fluorescence Excitation–Emission Matrix Technique. Foods. 2022; 11(14):2143. https://doi.org/10.3390/foods11142143
Chicago/Turabian StyleSikorska, Ewa, Przemysław Nowak, Katarzyna Pawlak-Lemańska, and Marek Sikorski. 2022. "Characterization and Classification of Direct and Commercial Strawberry Beverages Using Absorbance–Transmission and Fluorescence Excitation–Emission Matrix Technique" Foods 11, no. 14: 2143. https://doi.org/10.3390/foods11142143
APA StyleSikorska, E., Nowak, P., Pawlak-Lemańska, K., & Sikorski, M. (2022). Characterization and Classification of Direct and Commercial Strawberry Beverages Using Absorbance–Transmission and Fluorescence Excitation–Emission Matrix Technique. Foods, 11(14), 2143. https://doi.org/10.3390/foods11142143








