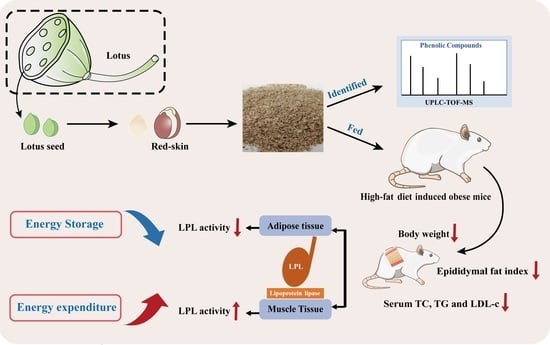
Red-Skin Extracts of Lotus Seeds Alleviate High-Fat-Diet Induced Obesity via Regulating Lipoprotein Lipase Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Lotus Seed Red-Skin Extracts
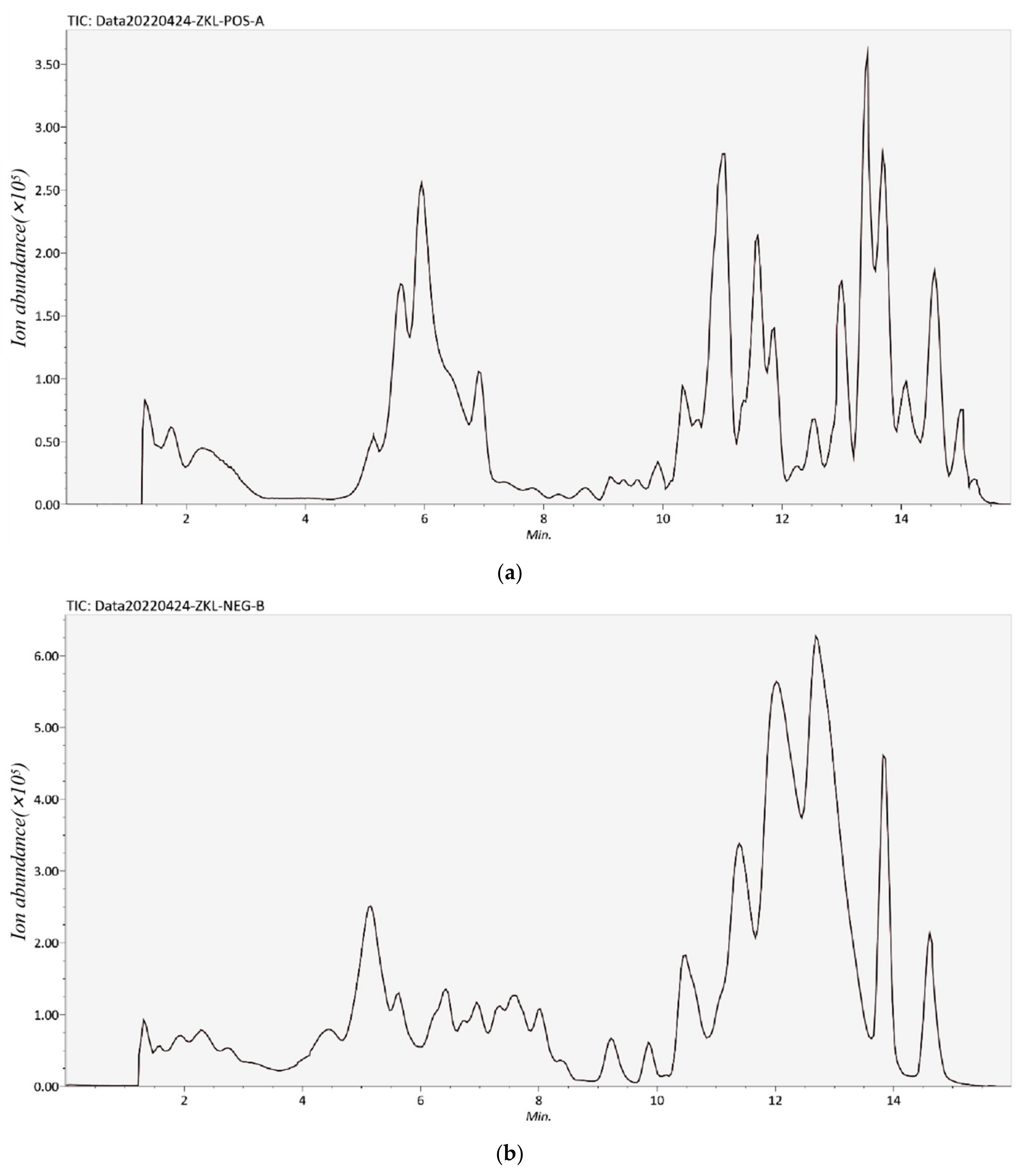
2.3. Identification of Phenolic Compounds by UPLC-TOF-MS
2.4. Animals Study
2.5. Serum Biochemical Analyses
2.6. LPL Activity Measurements
2.7. Western Blot Analysis
2.8. RNA Extraction and RT-PCR Analysis
2.9. Statistical Analysis
3. Results and Discussions
3.1. Identification or Tentatively Characterization of Phenolic Compounds in RSE
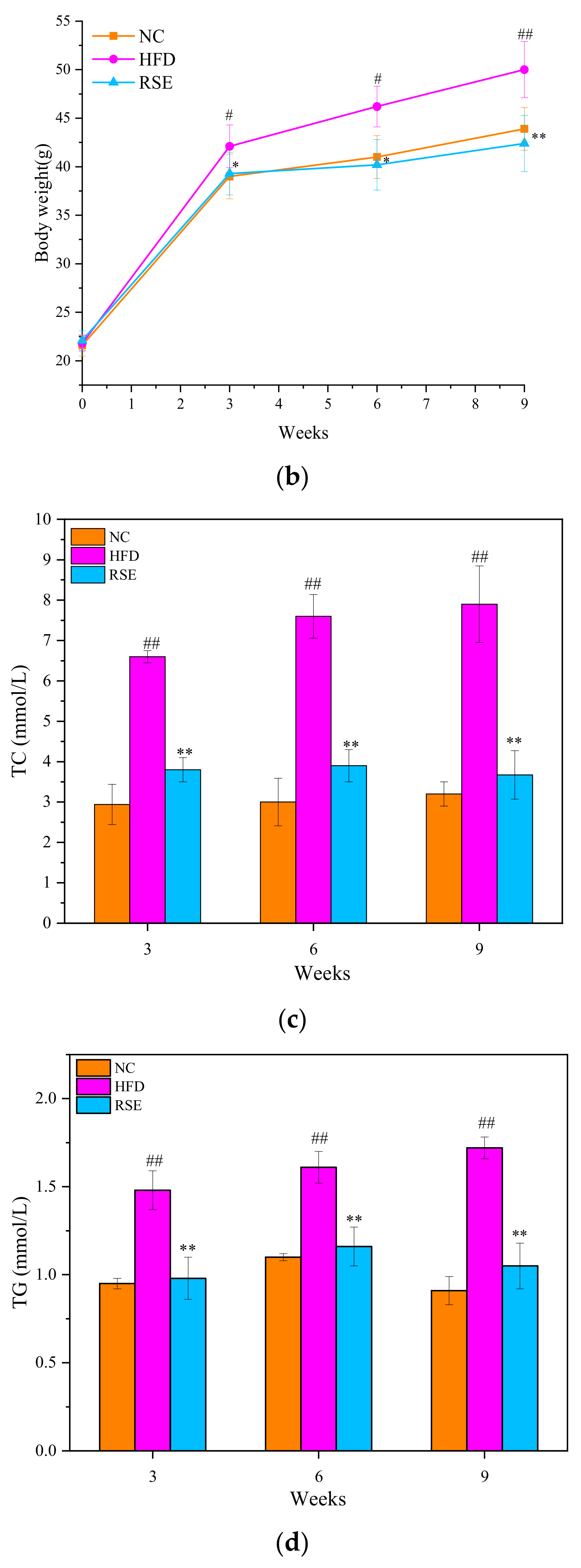
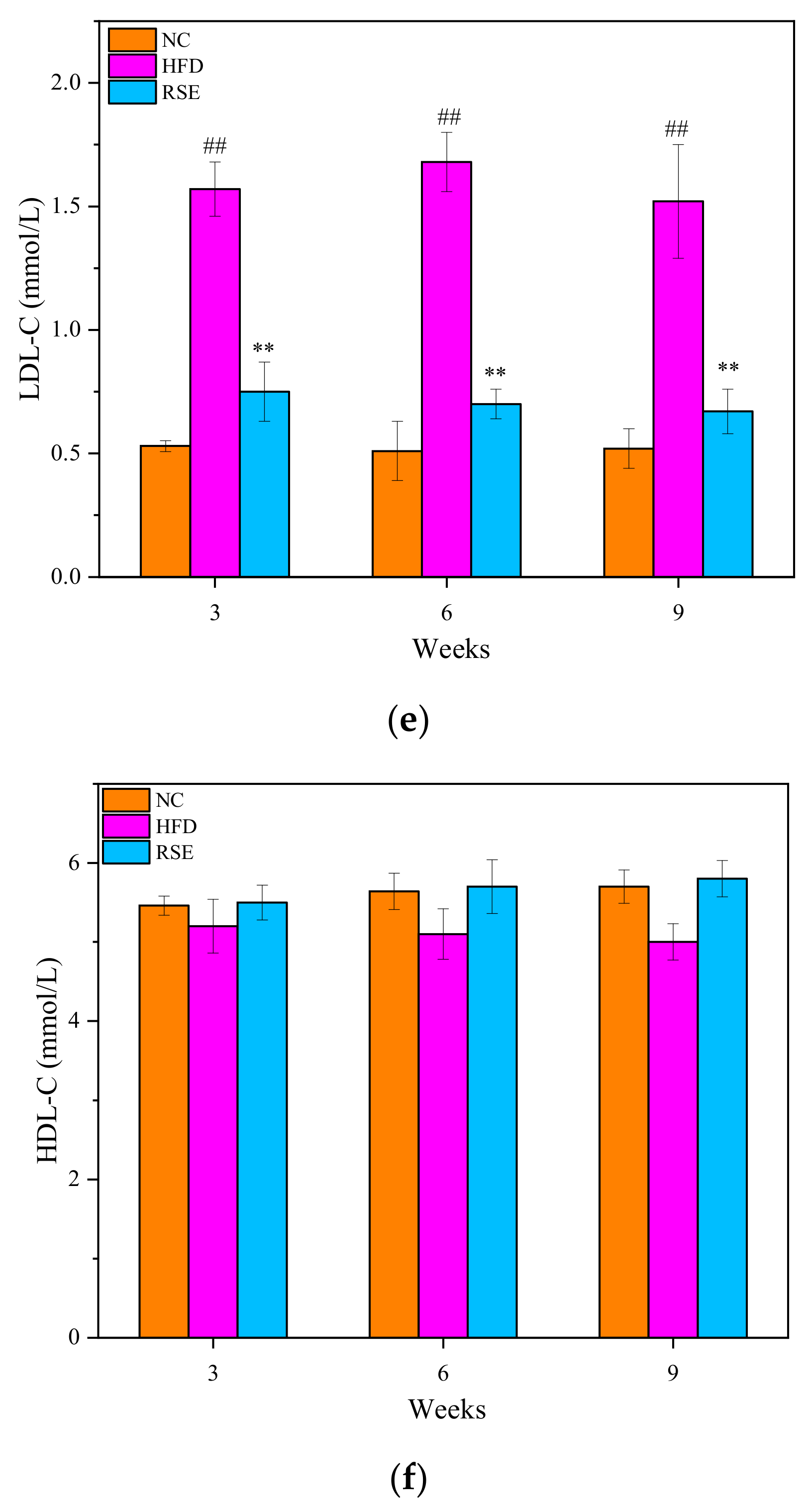
3.2. RSE Attenuated the Features of Obesity and Dyslipidaemia in HFD-Fed Mice
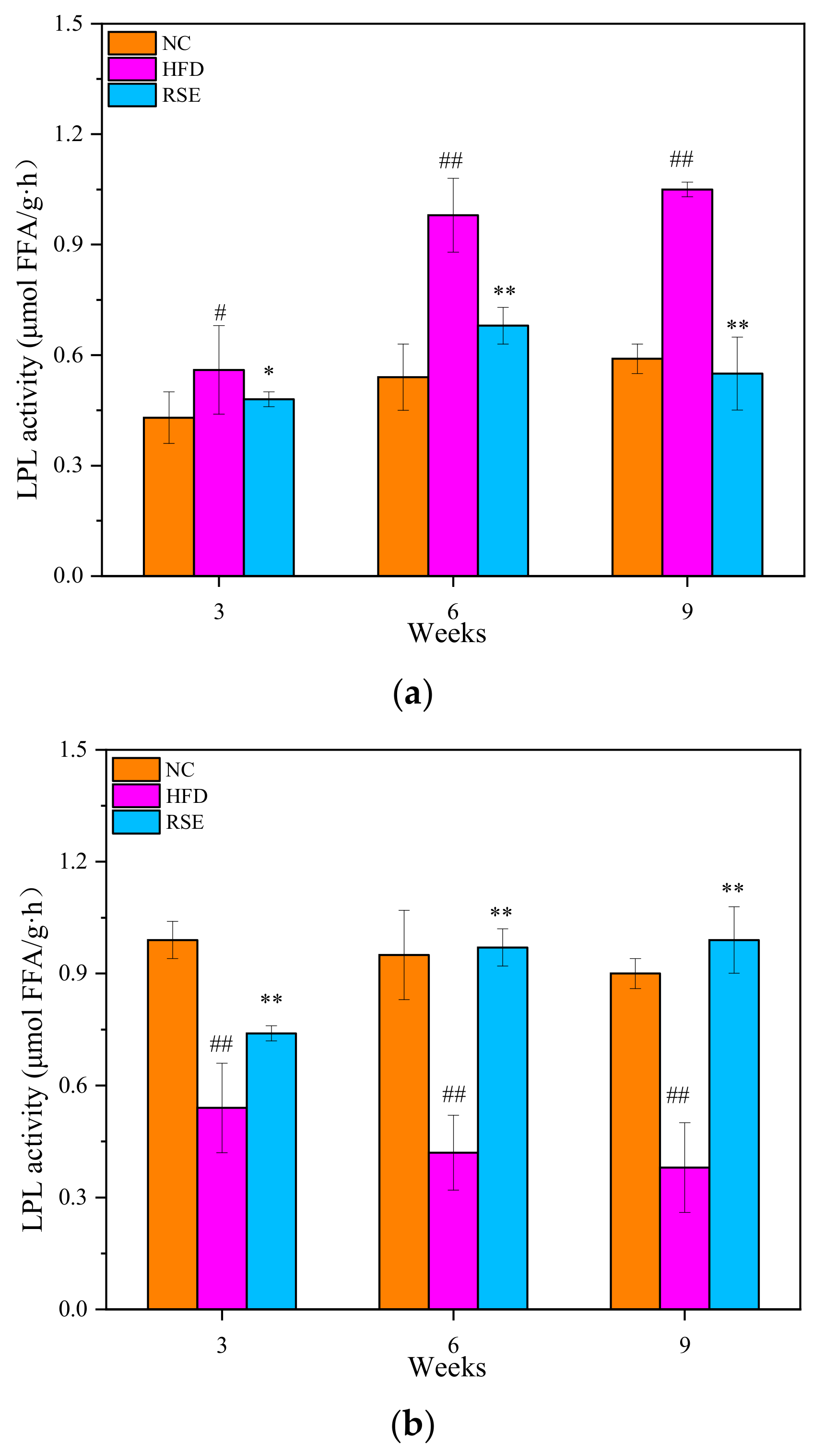
3.3. RSE Regulated the LPL Activity in HFD-Fed Mice
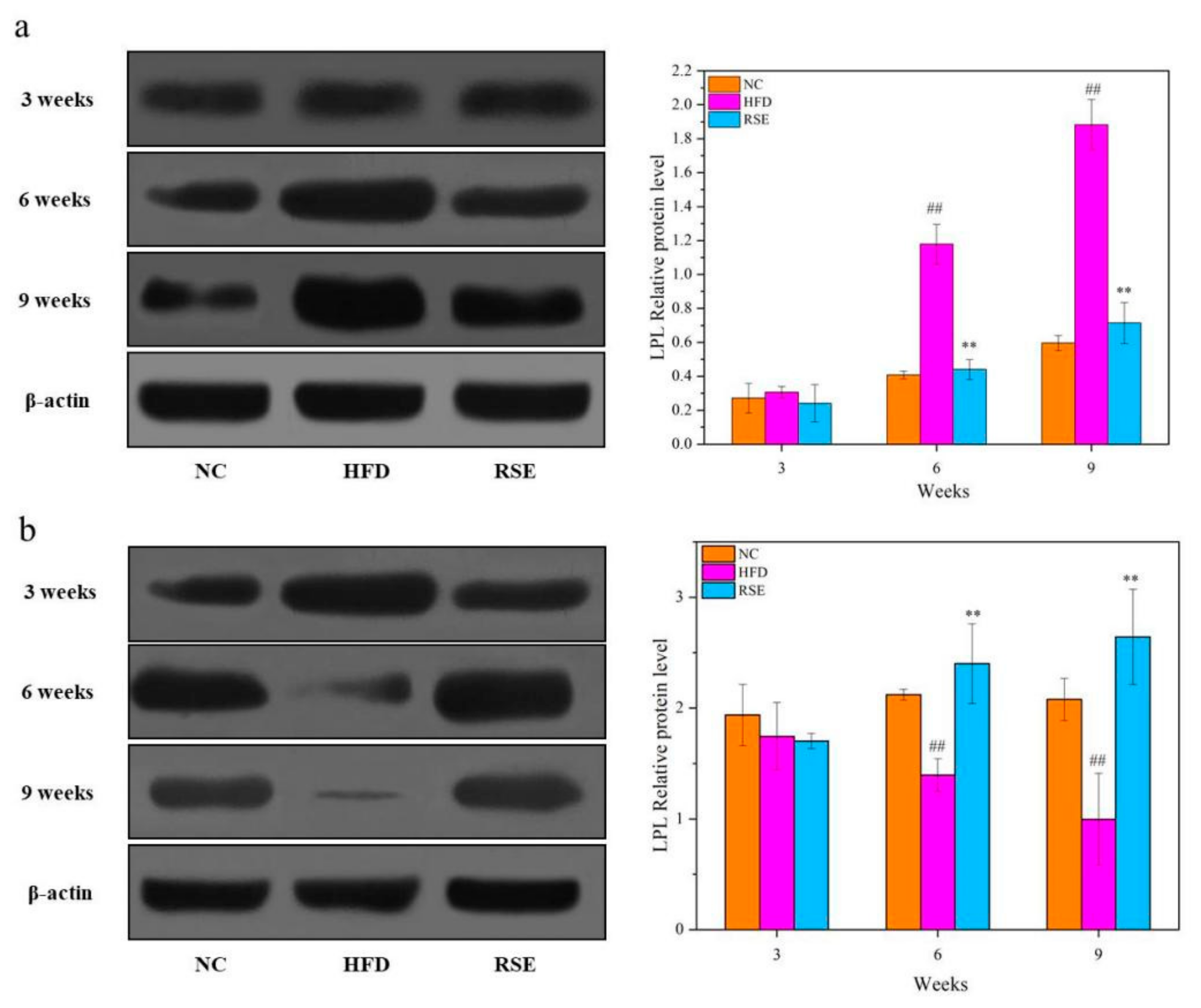
3.4. RSE Regulated the LPL Protein Expression in HFD-Fed Mice
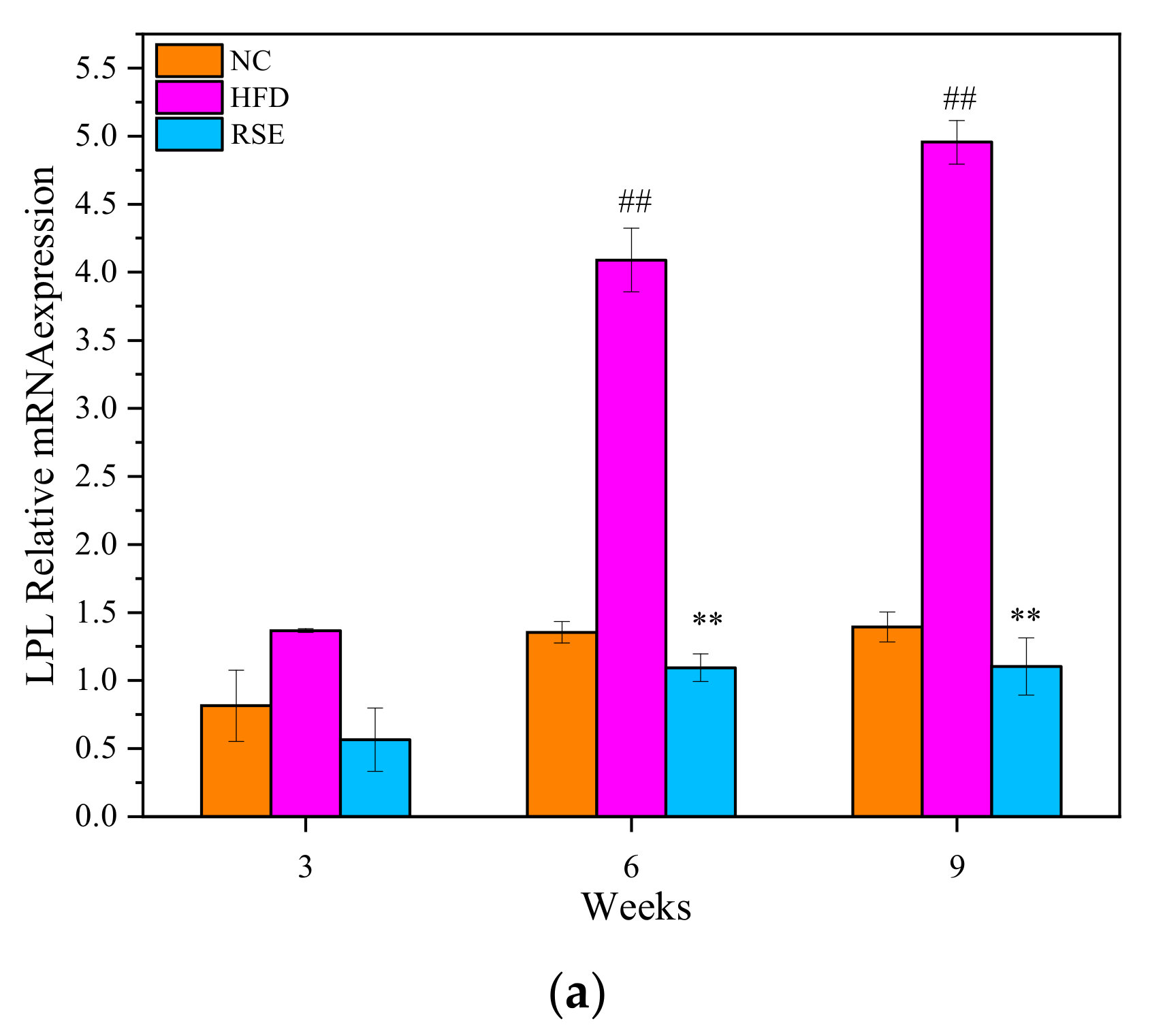
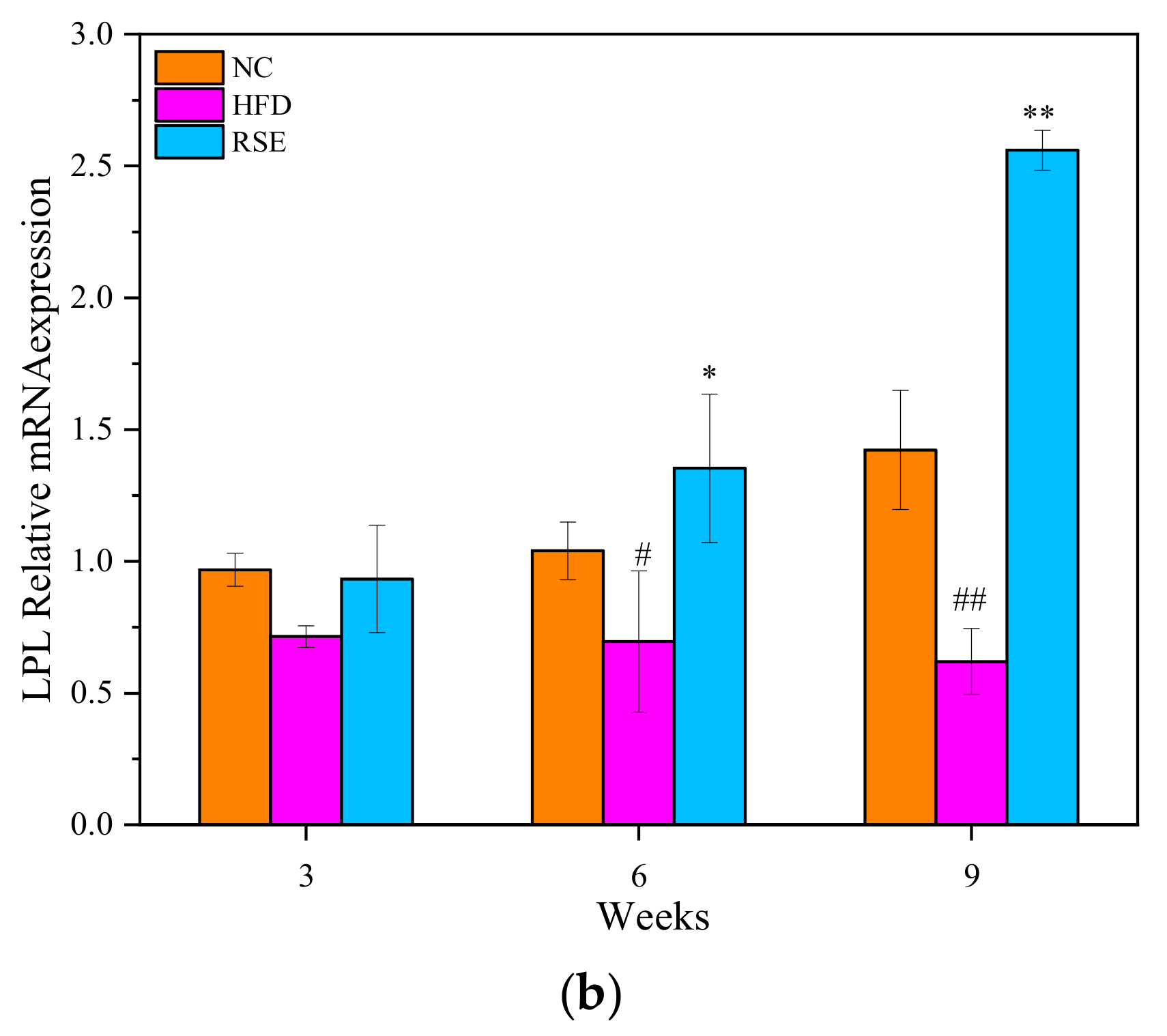
3.5. RSE Regulated the LPL mRNA Expression in HFD-Fed Mice
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gates, P. Arteriosclerosis with superimposed atherosclerosis is the cause not the consequence of essential hypertension. Med. Hypotheses 2020, 144, 110236. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, R.; Persand, D.; Maddie, N.; Carrillo-Sepulveda, M.A. Vascular Glucagon-like Peptide 1 (GLP-1) Signaling Is Reduced In Obesity-related Hypertension In Female Rats. Hypertension 2021, 78, A59. [Google Scholar] [CrossRef]
- Pillon, N.J.; Loos, R.J.; Marshall, S.M.; Zierath, J.R. Metabolic consequences of obesity and type 2 diabetes: Balancing genes and environment for personalized care. Cell 2021, 184, 1530–1544. [Google Scholar] [CrossRef] [PubMed]
- Montserrat-de la Paz, S.; Pérez-Pérez, A.; Vilariño-García, T.; Jiménez-Cortegana, C.; Muriana, F.J.G.; Millán-Linares, M.C.; Sánchez-Margalet, V. Nutritional modulation of leptin expression and leptin action in obesity and obesity-associated complications. J. Nutr. Biochem. 2021, 89, 108561. [Google Scholar] [CrossRef]
- Basu, D.; Goldberg, I.J. Regulation of lipoprotein lipase-mediated lipolysis of triglycerides. Curr. Opin. Lipidol. 2020, 31, 154. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Zhou, Y.; Zhang, Z.; Xie, Z.; Zhang, J.; Wan, X. Green tea polyphenols alleviate obesity in broiler chickens through the regulation of lipid-metabolism-related genes and transcription factor expression. J. Agric. Food Chem. 2013, 61, 8565–8572. [Google Scholar] [CrossRef]
- Liu, L.; Yasen, M.; Tang, D.; Ye, J.; Aisa, H.A.; Xin, X. Polyphenol-enriched extract of Rosa rugosa Thunb regulates lipid metabolism in diabetic rats by activation of AMPK pathway. Biomed. Pharmacother. 2018, 100, 29–35. [Google Scholar] [CrossRef]
- Serisier, S.; Leray, V.; Poudroux, W.; Magot, T.; Nguyen, P. Green tea improves, at nutritional doses, insulin sensitivity and plasma lipids concentrations in obese dog by increase PPARγ expression. FASEB J. 2007, 21, A5. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, Y.; Zeng, M.; Wang, Z.; Qin, F.; Wang, Y.; Chen, J.; He, Z. Lotus (Nelumbo nucifera Gaertn.) leaf: A narrative review of its Phytoconstituents, health benefits and food industry applications. Trends Food Sci. Technol. 2021, 112, 631–650. [Google Scholar] [CrossRef]
- Guo, Y.; Ding, X.; Ni, Y. The combination of NIR spectroscopy and HPLC chromatography for differentiating lotus seed cultivars and quantitative prediction of four main constituents in lotus with the aid of chemometrics. Anal. Methods 2017, 9, 6420–6429. [Google Scholar] [CrossRef]
- Yen, G.-C.; Duh, P.-D.; Su, H.-J.; Yeh, C.-T.; Wu, C.-H. Scavenging effects of lotus seed extracts on reactive nitrogen species. Food Chem. 2006, 94, 596–602. [Google Scholar] [CrossRef]
- Li, T.; Li, Q.; Wu, W.; Li, Y.; Hou, D.; Xu, H.; Zheng, B.; Zeng, S.; Shan, Y.; Lu, X.; et al. Lotus seed skin proanthocyanidin extract exhibits potent antioxidant property via activation of the Nrf2–ARE pathway. Acta Biochim. Biophys. Sin. 2018, 51, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Z.; Zheng, Y.-B.; Chen, T.-Q.; Yi, J.; Qin, L.-P.; Rahman, K.; Lin, W.-X. Evaluation of the quality of lotus seed of Nelumbo nucifera Gaertn from outer space mutation. Food Chem. 2007, 105, 540–547. [Google Scholar] [CrossRef]
- Kredy, H.M.; Huang, D.; Xie, B.; He, H.; Yang, E.; Tian, B.; Xiao, D. Flavonols of lotus (Nelumbo nucifera, Gaertn.) seed epicarp and their antioxidant potential. Eur. Food Res. Technol. 2010, 231, 387–394. [Google Scholar] [CrossRef]
- Cao, J.; Yu, X.; Deng, Z.; Pan, Y.; Zhang, B.; Tsao, R.; Li, H. Chemical compositions, antiobesity, and antioxidant effects of proanthocyanidins from Lotus seed epicarp and Lotus seed pot. J. Agric. Food Chem. 2018, 66, 13492–13502. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Golding, J.B.; Stathopoulos, C.E.; Nguyen, M.H.; Roach, P.D. Optimizing conditions for the extraction of catechins from green tea using hot water. J. Sep. Sci. 2011, 34, 3099–3106. [Google Scholar] [CrossRef]
- Castellano-Castillo, D.; Moreno-Indias, I.; Fernández-García, J.C.; Alcaide-Torres, J.; Moreno-Santos, I.; Ocana, L.; Gluckman, E.; Tinahones, F.; Queipo-Ortuño, M.I.; Cardona, F. Adipose tissue LPL methylation is associated with triglyceride concentrations in the metabolic syndrome. Clin. Chem. 2018, 64, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Xu, W.; Fu, X.; Huang, Y.; Wen, Y.; Xu, Q.; He, X.; Wang, K.; Huang, S.; Lv, Z. Blood miR-1275 is associated with risk of ischemic stroke and inhibits macrophage foam cell formation by targeting ApoC2 gene. Gene 2020, 731, 144364. [Google Scholar] [CrossRef]
- Benchoula, K.; Parhar, I.S.; Hwa, W.E. The molecular mechanism of vgf in appetite, lipids, and insulin regulation. Pharmacol. Res. 2021, 172, 105855. [Google Scholar] [CrossRef]
- Bessesen, D.H.; Rupp, C.L.; Eckel, R.H. Trafficking of dietary fat in lean rats. Obes. Res. 1995, 3, 191–203. [Google Scholar] [CrossRef]
- Vienberg, S.G.; Kleinridders, A.; Suzuki, R.; Kahn, C.R. Differential effects of angiopoietin-like 4 in brain and muscle on regulation of lipoprotein lipase activity. Mol. Metab. 2015, 4, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tan, F.; Zhang, T.; Xie, B.; Ran, L.; Zhao, X. The anti-obesity effect of lotus leaves on high-fat-diet-induced obesity by modulating lipid metabolism in C57BL/6J mice. Appl. Biol. Chem. 2020, 63, 61. [Google Scholar] [CrossRef]
- Imessaoudene, A.; Merzouk, H.; Berroukeche, F.; Mokhtari, N.; Bensenane, B.; Cherrak, S.; Merzouk, S.A.; Elhabiri, M. Beneficial effects of quercetin–iron complexes on serum and tissue lipids and redox status in obese rats. J. Nutr. Biochem. 2016, 29, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Presby, D.M.; Rudolph, M.C.; Sherk, V.D.; Jackman, M.R.; Foright, R.M.; Jones, K.L.; Houck, J.A.; Johnson, G.C.; Higgins, J.A.; Neufer, P.D. Lipoprotein lipase overexpression in skeletal muscle attenuates weight regain by potentiating energy expenditure. Diabetes 2021, 70, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Del Bas, J.M.; Fernández-Larrea, J.; Blay, M.; Ardèvol, A.; Salvadó, M.J.; Arola, L.; Bladé, C. Grape seed procyanidins improve atherosclerotic risk index and induce liver CYP7A1 and SHP expression in healthy rats. FASEB J. 2005, 19, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, O.-K.; Yoon, H.-G.; Park, J.; You, Y.; Kim, K.; Lee, Y.-H.; Choi, K.-C.; Lee, J.; Jun, W. Anti-obesity effect of extract from fermented Curcuma longa L. through regulation of adipogenesis and lipolysis pathway in high-fat diet-induced obese rats. Food. Nutr. Res. 2016, 60, 30428. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Luo, Y.C.; Ji, B.P.; Li, B.; Guo, Y.; Li, Y.; Su, W.; Xiao, Z.L. Effect of polysaccharide from Auricularia auricula on blood lipid metabolism and lipoprotein lipase activity of ICR mice fed a cholesterol-enriched diet. J. Food Sci. 2008, 73, H103–H108. [Google Scholar] [CrossRef]
- Kusunoki, M.; Tsutsumi, K.; Iwata, K.; Yin, W.; Nakamura, T.; Ogawa, H.; Nomura, T.; Mizutani, K.; Futenma, A.; Utsumi, K.; et al. NO-1886 (ibrolipim), a lipoprotein lipase activator, increases the expression of uncoupling protein 3 in skeletal muscle and suppresses fat accumulation in high-fat diet–induced obesity in rats. Metabolism 2005, 54, 1587–1592. [Google Scholar] [CrossRef]
- Wei, X.; Wang, D.; Yang, Y.; Xia, M.; Li, D.; Li, G.; Zhu, Y.; Xiao, Y.; Ling, W. Cyanidin-3-O-β-glucoside improves obesity and triglyceride metabolism in KK-Ay mice by regulating lipoprotein lipase activity. J. Sci. Food Agric. 2011, 91, 1006–1013. [Google Scholar] [CrossRef]
- Ahmad, B.; Friar, E.P.; Vohra, M.S.; Garrett, M.D.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Mechanisms of action for the anti-obesogenic activities of phytochemicals. Phytochemistry 2020, 180, 112513. [Google Scholar] [CrossRef]
- Fan, C.; Yan, J.; Qian, Y.; Wo, X.; Gao, L. Regulation of lipoprotein lipase expression by effect of hawthorn flavonoids on peroxisome proliferator response element pathway. J. Pharmacol. 2006, 100, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, Y.-S.; Kang, O.-H.; Kim, S.-B.; Mun, S.-H.; Kang, D.-H.; Yang, D.-W.; Choi, J.-G.; Lee, Y.-M.; Kang, D.-K.; Lee, H.-S. Quercetin prevents adipogenesis by regulation of transcriptional factors and lipases in OP9 cells. Int. J. Mol. Med. 2015, 35, 1779–1785. [Google Scholar] [PubMed]
- Quesada, H.; Del Bas, J.; Pajuelo, D.; Díaz, S.; Fernandez-Larrea, J.; Pinent, M.; Arola, L.; Salvadó, M.; Bladé, C. Grape seed proanthocyanidins correct dyslipidemia associated with a high-fat diet in rats and repress genes controlling lipogenesis and VLDL assembling in liver. Int. J. Obes. 2009, 33, 1007–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paudel, K.R.; Panth, N. Phytochemical profile and biological activity of Nelumbo nucifera. Evid.-Based Complement. Altern. Med. 2015, 2015, 789124. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.-Z.; Wu, W.; Jiao, L.-L.; Yang, P.-F.; Guo, M.-Q. Analysis of flavonoids in lotus (Nelumbo nucifera) leaves and their antioxidant activity using macroporous resin chromatography coupled with LC-MS/MS and antioxidant biochemical assays. Molecules 2015, 20, 10553–10565. [Google Scholar] [CrossRef] [Green Version]
| No. | Proposed Compound | RT (min) | Observed m/z | Adduct | Theoretical m/z | Formula | Ontology | Major Fragment Ions m/z |
|---|---|---|---|---|---|---|---|---|
| 1 | 2-Acetylacteoside | 1.315 | 689.2020 | [M+Na]+ | 689.2000 | C31H38O16 | Coumaric acids and derivatives | 527, 525, 509, 467, 365, 347 |
| 2 | Luteolin-6-C-glucoside | 1.433 | 447.0968 | [M-H]− | 447.0933 | C21H20O11 | Flavonoid C-glycosides | 401, 357, 327, 297, 285 |
| 3 | 1-(3,4-Dimethoxycinnamoyl) piperidine | 1.434 | 298.1414 | [M+Na]+ | 298.1400 | C16H21NO3 | Cinnamic acids and derivatives | 283, 282, 254, 240, 190, 107 |
| 4 | Apigenin 6-C-glucoside 8-C-arabinoside | 1.472 | 563.1463 | [M-H]− | 563.1406 | C26H28O14 | Flavone C and C-glycosides | 473, 443, 383, 353 |
| 5 | 6-Methylcoumarin | 1.593 | 161.0593 | [M+H]+ | 161.0600 | C10H8O2 | Coumarins and derivatives | 143, 115 |
| 6 | (+)-Gallocatechin | 1.671 | 305.0669 | [M-H]− | 305.0667 | C15H14O7 | Flavanol | 125 |
| 7 | (E)-3-[2-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl) oxan-2-yl] oxyphenyl] prop-2-enoic acid | 1.671 | 371.1010 | [M+FA-H]− | 371.0980 | C15H18O8 | Coumaric acids and derivatives | 163, 119 |
| 8 | 6-methoxy-4-methyl-2H-chromen-2-one | 1.673 | 191.0861 | [M+H]+ | 191.0800 | C11H10O3 | Coumarins and derivatives | 189 |
| 9 | Chalcone | 1.713 | 209.0936 | [M+H]+ | 209.0961 | C15H12O | Retrochalcones | 189 |
| 10 | 2,5-dihydroxybenzoic acid | 1.751 | 153.0198 | [M-H]− | 153.0193 | C7H6O4 | Hydroxybenzoic acid derivatives | 152, 124, 109, 107 |
| 11 | Casticin | 1.832 | 397.0908 | [M+Na]+ | 397.0900 | C19H18O8 | 7-O-methylated flavonoids | 283 |
| 12 | Procyanidin B2 | 1.909 | 577.1404 | [M-H]− | 577.1351 | C30H26O12 | Biflavonoids and polyflavonoids | 289, 245, 205, 125 |
| 13 | Praeruptorin A | 1.912 | 409.0904 | [M+Na]+ | 409.0890 | C21H22O7 | Angular pyranocoumarins | 325 |
| 14 | 6″-O-Acetylglycitin | 1.912 | 511.1231 | [M+Na]+ | 511.1200 | C24H24O11 | Isoflavonoid O-glycosides | 475, 379 |
| 15 | 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-2,3-dihydro-4H-chromen-4-one | 1.951 | 325.0706 | [M+Na]+ | 325.0700 | C16H14O6 | 4′-O-methylated flavonoids | 325 |
| 16 | Procyanidin C1 | 1.989 | 865.2050 | [M-H]− | 865.198 | C45H38O18 | Biflavonoids and polyflavonoids | 739, 695, 577, 407, 287 |
| 17 | Catechin | 2.276 | 289.0721 | [M-H]− | 289.0718 | C15H14O6 | Flavanol | 245, 203, 151, 125, 109 |
| 18 | Protocatechuic aldehyde | 2.752 | 137.0244 | [M-H]− | 137.0244 | C7H6O3 | Hydroxybenzaldehydes | 136, 119, 109, 108 |
| 19 | Methyl rosmarinate | 4.943 | 397.0910 | [M+Na]+ | 397.0900 | C19H18O8 | Coumaric acids and derivatives | 379, 349, 307, 295 |
| 20 | Swertisin | 4.943 | 469.1074 | [M+Na]+ | 469.1100 | C22H22O10 | Flavonoid C-glycosides | 433, 375 |
| 21 | (-)-Epigallocatechin | 5.024 | 329.0643 | [M+Na]+ | 329.0640 | C15H14O7 | Flavonal | 311, 300, 286, 283, 255, 149 |
| 22 | Isoshaftoside | 5.064 | 587.1323 | [M+Na]+ | 587.1300 | C26H28O14 | Flavonoid 8-C-glycosides | 569, 551, 509, 497 |
| 23 | Myricetin-3-Galactoside | 5.237 | 479.0872 | [M-H]− | 479.0831 | C21H20O13 | Flavonoid-3-O-glycosides | 433, 316, 271 |
| 24 | Homoorientin | 5.316 | 447.0942 | [M-H]− | 447.0933 | C21H20O11 | Flavonoid C-glycosides | 369, 357,327, 299, 298, 297, 285 |
| 25 | 7-hydroxy-3-phenyl-4H-chromen-4-one | 5.353 | 239.0678 | [M+H]+ | 239.0700 | C15H10O3 | Isoflavones | 224, 181 |
| 26 | 2-Hydroxychalcone | 5.393 | 225.0904 | [M+H]+ | 225.0910 | C15H12O2 | Retrochalcones | 197, 182 |
| 27 | Quercetin | 5.513 | 303.0482 | [M+H]+ | 303.0500 | C15H10O7 | Flavonols | 285, 274, 257, 247, 229, 201, 153, 137 |
| 28 | Rutin | 5.513 | 633.1376 | [M+Na]+ | 633.1405 | C27H30O16 | Flavonols O-glycoside | 331 |
| 29 | Quercetin-3-O-rutinoside | 5.593 | 609.1476 | [M-H]- | 609.1461 | C27H30O16 | Flavonoid O-glycosides | 343, 301, 300, 271, 255, 242, 178 |
| 30 | 8-(azepan-1-ium-1-ylmethyl)-3-(2,4-dimethoxyphenyl)-2-oxo-2H-chromen-7-olate | 5.593 | 432.1784 | [M+Na]+ | 432.1800 | C24H27NO5 | Hydroxyisoflavonoids | 297, 174 |
| 31 | 4′,7-Di-O-methylnaringenin | 5.633 | 323.0882 | [M+Na]+ | 323.0900 | C17H16O5 | 7-O-methylated flavonoids | 295 |
| 32 | Spiraeoside | 5.633 | 465.1124 | [M+H]+ | 465.1030 | C21H20O12 | Flavonoid O-glycosides | 303 |
| 33 | 2-(3,4-dihydroxyphenyl)-5-hydroxy-10-isopropyl-9,10-dihydropyrano [2,3-f] chromene-4,8-dione | 5.644 | 405.0939 | [M+Na]+ | 405.0900 | C21H18O7 | Pyranoflavonoids | 387, 377 |
| 34 | liquiritin | 5.644 | 441.1163 | [M+Na]+ | 441.1200 | C21H22O9 | Flavonoid O-glycosides | 423, 405, 379, 351, 325 |
| 35 | Vitexin | 5.671 | 431.1003 | [M-H]− | 431.0984 | C21H20O10 | Flavonoid 8-C-glycosides | 341, 323, 311, 283 |
| 36 | 7-hydroxy-3-phenyl-4H-chromen-4-one | 5.683 | 239.0677 | [M+H]+ | 239.0700 | C15H10O3 | Isoflavones | 211, 183, 165, 153 |
| 37 | 5,7-dihydroxy-2-(4-hydroxyphenyl)-6-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]-8-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]chromen-4-one | 5.710 | 577.1602 | [M-H]− | 577.1570 | C27H30O14 | Flavonoid 8-C-glycosides | 487, 473, 457, 383, 353, 179 |
| 38 | 3-Hydroxy-4-methoxycinnamic acid (isoferulic acid) | 5.750 | 193.0518 | [M-H]− | 193.0506 | C10H10O4 | Hydroxycinnamic acids | 178, 134 |
| 39 | Isoquercitin | 5.750 | 463.0902 | [M-H]− | 463.0880 | C21H20O12 | Flavonoid-3-O-glycosides | 301, 300, 271, 255 |
| 40 | Quercetin-3-Glucuronide | 5.750 | 477.0706 | [M-H]− | 477.0675 | C21H18O13 | Flavonoid-3-O-glucuronides | 301, 255, 179, 151 |
| 41 | Hyperoside | 5.750 | 927.1992 | [2M-H]− | 927.1837 | C21H20O12 | Flavonoid-3-O-glycosides | 463, 301 |
| 42 | Sinapic acid | 5.789 | 223.0645 | [M-H]− | 223.0612 | C11H12O5 | Hydroxycinnamic acids | 193, 149 |
| 43 | Kaempferol | 5.803 | 287.0529 | [M+H]+ | 287.0550 | C15H10O6 | Flavonols | 287, 269, 241, 161, 153, 135 |
| 44 | 4′,5,7-Trihydroxy-6,8-diprenylisoflavone | 5.803 | 429.1610 | [M+Na]+ | 429.1700 | C25H26O5 | 6-prenylated isoflavanones | 309 |
| 45 | (2R,3R,4S,5R,6R)-2-(acetoxymethyl)-6-((3-(benzo[d][1,3]dioxol-5-yl)-6-ethyl-4-oxo-4H-chromen-7-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate | 5.962 | 663.1678 | [M+Na]+ | 663.1600 | C32H32O14 | Isoflavonoid O-glycosides | 417 |
| 46 | taxifolin | 5.868 | 303.0550 | [M-H]− | 303.0510 | C15H12O7 | Sinapic acid derivative | 285, 177, 125 |
| 47 | 3-[(2S,3R,4S,5R,6R)-3,5-dihydroxy-6-(hydroxymethyl)-4-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-2-yl]oxy-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one | 5.868 | 593.1551 | [M-H]− | 593.1512 | C27H30O15 | Flavanone glycosides | 547, 285, 255 |
| 48 | Isorhamnetin | 5.962 | 317.0674 | [M+H]+ | 317.0656 | C16H12O7 | Flavonol | 302, 285, 274, 245, 229, 153 |
| 49 | Isorhamnetin-3-O-rutinoside | 6.027 | 623.1674 | [M-H]− | 623.1617 | C28H32O16 | Flavonol O-glycosides | 315, 314, 300 |
| 50 | 9-(2,3-dihydroxypropoxy)-9-oxononanoic acid | 6.187 | 261.1359 | [M-H]− | 261.1348 | C12H22O6 | Flavonol O-glycosides | 187, 125 |
| 51 | Genistein | 6.121 | 271.0629 | [M+H]+ | 271.0601 | C15H10O5 | Isoflavones | 153 |
| 52 | Diosmetin | 6.200 | 301.0710 | [M+H]+ | 301.0700 | C16H12O6 | 4′-O-methylated flavonoids | 286, 258, 229 |
| 53 | 4′-O-Glucosylvitexin | 6.680 | 617.1353 | [M+Na]+ | 617.1400 | C27H30O15 | Flavonoid O-glycosides | 555 |
| 54 | Sinapoylcholine | 7.320 | 310.1599 | [M]+ | 310.1643 | C16H24NO5 | Coumaric acids and derivatives | 278, 253 |
| 55 | 3,4-Dihydrocoumarin | 11.650 | 149.0583 | [M+H]+ | 149.0597 | C9H8O2 | 3,4-dihydrocoumarins | 121, 103 |
| Group | 3 Weeks | 6 Weeks | 9 Weeks |
|---|---|---|---|
| NC | 4.76 ± 1.134 | 11.61 ± 2.84 | 12.26 ± 3.67 |
| HFD | 24.61 ± 9.49 ## | 24.35 ± 7.67 ## | 29.30 ± 8.95 ## |
| RSE | 14.35 ± 4.42 ##** | 17.38 ± 5.35 ##** | 19.67 ± 3.07 ##** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Gao, H.; Liu, F.; Gong, L. Red-Skin Extracts of Lotus Seeds Alleviate High-Fat-Diet Induced Obesity via Regulating Lipoprotein Lipase Activity. Foods 2022, 11, 2085. https://doi.org/10.3390/foods11142085
Xu H, Gao H, Liu F, Gong L. Red-Skin Extracts of Lotus Seeds Alleviate High-Fat-Diet Induced Obesity via Regulating Lipoprotein Lipase Activity. Foods. 2022; 11(14):2085. https://doi.org/10.3390/foods11142085
Chicago/Turabian StyleXu, Hong, Hang Gao, Feiyue Liu, and Lingxiao Gong. 2022. "Red-Skin Extracts of Lotus Seeds Alleviate High-Fat-Diet Induced Obesity via Regulating Lipoprotein Lipase Activity" Foods 11, no. 14: 2085. https://doi.org/10.3390/foods11142085
APA StyleXu, H., Gao, H., Liu, F., & Gong, L. (2022). Red-Skin Extracts of Lotus Seeds Alleviate High-Fat-Diet Induced Obesity via Regulating Lipoprotein Lipase Activity. Foods, 11(14), 2085. https://doi.org/10.3390/foods11142085