Benefits and Risks Associated with Meat Consumption during Key Life Processes and in Relation to the Risk of Chronic Diseases
Abstract
:1. Introduction
2. Meat Consumption and the Risk of Iron Deficiency and Chronic Non-Communicable Diseases
2.1. Iron-Deficiency Anaemia
2.2. Meat Consumption and Non-Communicable Chronic Diseases
2.2.1. Cardiovascular Diseases
2.2.2. Type 2 Diabetes
2.2.3. Cancer
| Meat Type | Colorectal Cancers RR per 100 g/day Red Meat/Red and Processed Meat and per 50 g/day Processed Meat 1 | Colorectal Cancers RR Highest vs. Lowest Intake 3 | ||||
|---|---|---|---|---|---|---|
| All CRC | Colon | Rectal | All CRC | Colon | Rectal | |
| Red and processed meat | 1.10 (1.02–1.18) | 1.19 (1.10–1.30) | 1.17 (0.99–1.39) | 1.17 (1.08–1.26) | 1.21 (1.09–1.34) | 1.26 (1.09–1.45) |
| Red meat | 1.12 2 (1.00–1.25) | 1.22 (1.06–1.39) | 1.13 (0.96–1.34) | 1.10 (1.03–1.17) | 1.17 (1.09–1.25) | 1.22 (1.01–1.46) |
| Processed meat | 1.16 2 (1.08–1.26) | 1.23 (1.11–1.35) | 1.08 (1.00–1.18) | 1.18 (1.13–1.24) | 1.21 (1.13–1.29) | 1.22 (1.09–1.36) |
2.2.4. Dementia
3. Meat Consumption and Key Life Processes
3.1. Skeletal Muscle and Bone Health and Maintenance
3.1.1. Skeletal Muscle
3.1.2. Bone Mass
3.1.3. Meat Consumption during Pregnancy
4. General Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ritchie, H.; Roser, M. Meat and Dairy Production. Published online at OurWorldInData.org. 2017. Available online: https://ourworldindata.org/meat-production. (accessed on 1 February 2022).
- Bonnet, C.; Bouamra-Mechemache, Z.; Réquillart, V.; Treich, N. Regulating meat consumption to improve health, the environment and animal welfare. Food Policy 2020, 97, 101847. [Google Scholar] [CrossRef]
- Basu, S. The transitional dynamics of caloric ecosystems: Changes in the food supply around the world. Crit. Public Health 2015, 25, 248–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milford, A.B.; Le Mouël, C.; Bodirsky, B.L.; Rolinski, S. Drivers of meat consumption. Appetite 2019, 141, 104313. [Google Scholar] [CrossRef] [PubMed]
- Salter, A. The effects of meat consumption on global health. Sci. Tech. Rev. 2018, 37, 1. [Google Scholar] [CrossRef] [PubMed]
- Kemper, J.A. Motivations, barriers, and strategies for meat reduction at different family lifecycle stages. Appetite 2020, 150, 104644. [Google Scholar] [CrossRef]
- IARC. Monograph on the Evaluation of Carcinogenic Risks to Humans: Red and Processed Meat; IARC: Lyon, France, 2018; Volume 114, p. 503. [Google Scholar]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Waldvogel-Abramowski, S.; Waeber, G.; Gassner, C.; Buser, A.; Frey, B.M.; Favrat, B. Physiology of iron metabolism. Transfus. Med. Hemother. 2014, 41, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Jáuregui-Lobera, I. Iron deficiency and cognitive functions. Neuropsychiatr. Dis. Treat. 2014, 10, 2087–2095. [Google Scholar] [CrossRef] [Green Version]
- Malczewska-Lenczowska, J.; Sitkowski, D.; Surała, O.; Orysiak, J.; Szczepańska, B.; Witek, K. The Association between Iron and Vitamin D Status in Female Elite Athletes. Nutrients 2018, 10, 167. [Google Scholar] [CrossRef] [Green Version]
- Zughaier, S.M.; Alvarez, J.A.; Sloan, J.H.; Konrad, R.J.; Tangpricha, V. The role of vitamin D in regulating the iron-hepcidin-ferroportin axis in monocytes. J. Clin. Transl. Endocrinol. 2014, 1, e19–e25. [Google Scholar] [CrossRef]
- Alon, D.B.; Chaimovitz, C.; Dvilansky, A.; Lugassy, G.; Douvdevani, A.; Shany, S.; Nathan, I. Novel role of 1,25(OH)(2)D(3) in induction of erythroid progenitor cell proliferation. Exp. Hematol. 2002, 30, 403–409. [Google Scholar] [CrossRef]
- Worldwide Prevalence of Anemia 1993–2005. WHO Global Database on Anemia. 2008. Available online: http://apps.who.int/iris/bitstream/handle/10665/43894/9789241596657_eng.pdf;jsessionid=669E918BBD67BDB3910260FC7320986C?sequence=1 (accessed on 2 February 2022).
- IIPS; ICF. International Institute for Population Sciences and ICF, National Family Health Survey (NFHS-4), 2015–2016; IIPS: Mumbai, India, 2017. [Google Scholar]
- Roberts, C.; Steer, T.; Maplethorpe, N.; Cox, L.; Meadows, S.; Nicholson, S.; Page, P.; Swan, P. National Diet and Nutrition Survey. Results from Years 7–8 (Combined) of the Rolling Programme (2014/15 to 2015/16); PHE Publication Gateway Number: 2017851; Public Health England: London, UK, 2018. [Google Scholar]
- Jamieson, J.A.; Weiler, H.A.; Kuhnlein, H.V.; Egeland, G.M. Prevalence of unexplained anaemia in Inuit men and Inuit post-menopausal women in northern Labrador: International polar year Inuit health survey. Can. J. Public Health 2016, 107, e81–e87. [Google Scholar] [CrossRef] [PubMed]
- Bæch, S.B.; Hansen, M.; Bukhave, K.; Jensen, M.; Sørensen, S.S.; Kristensen, L. Non heme-iron absorption from a phytate-rich meal is increased by the addition of small amounts of pork meat. Am. J. Clin. Nutr. 2003, 77, 173–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristensen, M.B.; Hels, O.; Morberg, C.; Marving, J.; Bügel, S.; Tetens, I. Pork meat increases iron absorption from a 5-day fully controlled diet when compared to a vegetarian diet with similar vitamin C and phytic acid content. Br. J. Nutr. 2005, 94, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Hooda, J.; Shah, A.; Zhang, L. Heme, an essential nutrient from dietary proteins, critically impacts diverse physiological and pathological processes. Nutrients 2014, 6, 1080. [Google Scholar] [CrossRef] [Green Version]
- Anderson, G.J.; Frazer, D.M. Current understanding of iron homeostasis. Am. J. Clin. Nutr. 2017, 106, 1559S–1566S. [Google Scholar] [CrossRef] [Green Version]
- McCance and Widdowson’s Composition of Foods, Integrated Dataset 2021; PHE publications gateway number: GW-2010; Public Health England: London, UK, 2021.
- Ghosh, S.; Sinha, S.; Thomas, T.; Sachdev, H.S.; Kurpad, A.V. Revisiting dietary iron requirement and deficiency in Indian women: Implications for food iron fortification and supplementation. J. Nutr. 2019, 149, 366–371. [Google Scholar] [CrossRef] [Green Version]
- Heath, A.L.M.; Fairweather-Tate, S.J. Clinical implications of changes in the modern diet: Iron intake, absorption and status. Best Pract. Res. Clin. Haematol. 2002, 15, 225–241. [Google Scholar] [CrossRef]
- DEFRA. Family Food Survey 2018/19, UK Household Purchases. Family Food Datasets. 2020. Available online: www.gov.uk (accessed on 20 December 2021).
- Eurostat Cardiovascular Diseases Statistics—Statistics Explained. 2021. Available online: http://www.europa.eu (accessed on 21 December 2021).
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef]
- Statistica. Prevalence of Diabetes in Adult Population in Europe 2019, by Country. 2019. Available online: http://www.eu.support@statista.com (accessed on 22 March 2022).
- Wolters, F.J.; Chibnik, L.B.; Waziry, R.; Anderson, R.; Berr, C.; Beiser, A.; Hofman, A. Twenty-seven-year time trends in dementia incidence in Europe and the United States: The Alzheimer Cohorts Consortium. Neurology 2020, 95, e519–e531. [Google Scholar] [CrossRef]
- O’Connor, L.E.; Herrick, K.A.; Parsons, R.; Reedy, J. Heterogeneity in meat food groups can meaningfully alter population-level intake estimates of red meat and poultry. Front. Nutr. 2021, 8, 778369. [Google Scholar] [CrossRef]
- World Health Organization. Q&A on the Carcinogenicity of the Consumption of Red Meat and Processed Meat. 2017. Available online: http://www.who.int/features/qa/cancer-red-meat/en/ (accessed on 14 December 2021).
- Micha, R.; Wallace, S.K.; Mozaffarian, D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: A systematic review and meta-analysis. Circulation 2010, 121, 2271–2283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellavia, A.; Stilling, F.; Wolk, A. High red meat intake and all-cause cardiovascular and cancer mortality: Is the risk modified by fruit and vegetable intake? Am. J. Clin. Nutr. 2016, 104, 1137–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abete, I.; Romaguera, D.; Vieira, A.R.; Lopez de Munain, A.; Norat, T. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: A meta-analysis of cohort studies. Br. J. Nutr. 2014, 112, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Iqbal, K.; Andriolo, V.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food Groups and Risk of Hypertension: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. 2017, 8, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, R.; Dehghan, M.; Mente, A.; Rangarajan, S.; Wielgosz, A.; Avezum, A.; Seron, P.; AlHabib, K.F.; Lopez-Jaramillo, P.; Swaminathan, S.; et al. Associations of unprocessed and processed meat intake with mortality and cardiovascular disease in 21 countries [Prospective Urban Rural Epidemiology (PURE) Study]: A prospective cohort study. Am. J. Clin. Nutr. 2021, 114, 1049–1058. [Google Scholar] [CrossRef]
- Jakobsen, M.U.; Bysted, A.; Mejborn, H.; Stockmarr, A.; Trolle, E. Intake of unprocessed and processed meat and the association with cardiovascular disease: An overview of systematic reviews. Nutrients 2021, 13, 3303. [Google Scholar] [CrossRef]
- InterAct Consortium. Association between dietary meat consumption and incident type 2 diabetes: The EPIC-InterAct study. Diabetologia 2013, 56, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Manson, J.E.; Willett, W.C.; Hu, F.B. Changes in red meat consumption and subsequent risk of type 2 diabetes mellitus: Three cohorts of US men and women. JAMA Intern. Med. 2013, 173, 1328–1335. [Google Scholar] [CrossRef]
- Neuenschwander, M.; Ballon, A.; Weber, K.S.; Norat, T.; Aune, D.; Schwingshackl, L.; Schlesinger, S. Role of diet in type 2 diabetes incidence: Umbrella review of meta-analyses of prospective observational studies. BMJ 2019, 366, l2368. [Google Scholar] [CrossRef] [Green Version]
- Hobbs Grimmer, D.A.; Givens, D.I.; Lovegrove, J.A. Associations between red meat, processed red meat and total red and processed red meat consumption, nutritional adequacy and markers of health and cardio metabolic diseases in British adults: A cross sectional analysis using data from UK National Diet and Nutrition Survey. Eur. J. Nutr. 2021, 60, 2979–2997. [Google Scholar] [CrossRef]
- Ley, S.H.; Sun, Q.; Willett, W.C.; Eliassen, A.H.; Wu, K.; Pan, A.; Grodstein, F.; Hu, F.B. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. Am. J. Clin. Nutr. 2014, 99, 352–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiker, N.R.W.; Bertram, H.C.; Mejborn, H.; Dragsted, L.O.; Kristensen, L.; Ruiz Carrascal, J.; Bügel, S.; Astrup, A. Meat and human health—Current knowledge and research gaps. Foods 2021, 10, 1556. [Google Scholar] [CrossRef]
- WCRF/AICR. World Cancer Research Fund/American Institute for Cancer Research: Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective; American Institute for Cancer Research: Washington, DC, USA, 2007. [Google Scholar]
- WCRF/AICR. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Report. The Associations between Food, Nutrition and Physical Activity, and the Risk of Colorectal Cancer; Imperial College: London, UK, 2010. [Google Scholar]
- WCRF/ACIR. Update Project Expert Report 2018. Diet, Nutrition, Physical Activity and Colorectal Cancer. Diet and Cancer Report; WCRF International: London, UK, 2018. [Google Scholar]
- WCRF/ACIR. Continuous Update Project Expert Report 2018. Meat, Fish, and Dairy Products and the Risk of Cancer. Available online: www.dietandcancerreport.org (accessed on 20 March 2022).
- NHS Choices. Meat in Your Diet. 2021. Available online: https://www.nhs.uk/live-well/eat-well/meat-nutrition/ (accessed on 17 January 2020).
- Farvid, M.S.; Sidahmed, E.; Spence, N.D.; Angua, K.M.; Rosner, B.A.; Barnett, J.B. Consumption of red meat and processed meat and cancer incidence: A systematic review and meta analysis of prospective studies. Eur. J. Epidemiol. 2021, 36, 937–951. [Google Scholar] [CrossRef] [PubMed]
- Händel, M.N.; Rohde, J.F.; Jacobsen, R.; Heitmann, B.L. Processed Meat Consumption and the Risk of Cancer: A Critical Evaluation of the Constraints of Current Evidence from Epidemiological Studies. Nutrients 2021, 13, 3601. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization Dementia Fact Sheet. 2021. Available online: https://www.who.int/en/news-room/fact-sheets/detail/dementia (accessed on 2 March 2022).
- Alzheimer’s Disease International. Updated estimates to the World Alzheimer Report. 2017. Available online: https://www.alzint.org/u/numbers-people-with-dementia-2017.pdf (accessed on 2 March 2022).
- Albanese, E.; Dangour, A.D.; Uauy, R.; Acosta, D.; Guerra, M.; Gallardo Guerra, S.S.; Huang, Y.; Jacob, K.S.; de Rodriguez, J.L.; Noriega, L.H.; et al. Dietary fish and meat intake and dementia in Latin America, China, and India: A 10/66 Dementia Research Group population-based study. Am. J. Clin. Nutr. 2009, 90, 392–400. [Google Scholar] [CrossRef] [Green Version]
- Grant, W.B. Trends in diet and Alzheimer’s disease during the nutrition transition in Japan and developing countries. J. Alzheimer’s Dis. 2014, 38, 611–620. [Google Scholar] [CrossRef]
- Zhang, H.; Greenwood, D.C.; Risch, H.A.; Bunce, D.; Hardie, L.J.; Cade, J.E. Meat consumption and risk of incident dementia: Cohort study of 493,888 UK Biobank participants. Am. J. Clin. Nutr. 2021, 114, 175–184. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef]
- Jaiswal, N.; Gavin, M.G.; Quinn, W.J., III; Luongo, T.S.; Gelfer, R.G.; Baur, J.A.; Titchenell, P.M. The role of skeletal muscle Akt in the regulation of muscle mass and glucose homeostasis. Mol. Metab. 2019, 28, 1–13. [Google Scholar] [CrossRef]
- Lonnie, M.; Hooker, E.; Brunstrom, J.M.; Corfe, B.M.; Green, M.A.; Watson, A.W.; Johnstone, A.M. Protein for life: Review of optimal protein intake, sustainable dietary sources and the effect on appetite in ageing adults. Nutrients 2018, 10, 360. [Google Scholar] [CrossRef] [Green Version]
- Daly, R.M.; O’Connell, S.L.; Mundell, N.L.; Grimes, C.A.; Dunstan, D.W.; Nowson, C.A. Protein-enriched diet, with the use of lean red meat, combined with progressive resistance training enhances lean tissue mass and muscle strength and reduces circulating IL-6 concentrations in elderly women: A cluster randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 899–910. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.; Monteferrario, F.; Peroni, G.; Repaci, E.; Allieri, F.; Perna, S. Novel insights on nutrient management of sarcopenia in elderly. BioMed Res. Int. 2015, 2015, 524948. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, S.H.; Witard, O.C. Characterising the muscle anabolic potential of dairy, meat and plant-based protein sources in older adults. Proc. Nutr. Soc. 2018, 77, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.L.; Mata, F.; Morales, J.S.; Castillo-García, A.; Lucia, A. Does beef protein supplementation improve body composition and exercise performance? A systematic review and meta-analysis of randomized controlled trials. Nutrients 2019, 11, 1429. [Google Scholar] [CrossRef] [Green Version]
- Watts, N.B.; Lewiecki, E.M.; Miller, P.D.; Baim, S. National Osteoporosis Foundation 2008 Clinician’s Guide to Prevention and Treatment of Osteoporosis and the World Health Organization Fracture Risk Assessment Tool (FRAX): What they mean to the bone densitometrist and bone technologist. J. Clin. Densitom. 2008, 11, 473–477. [Google Scholar] [CrossRef]
- Darling, A.L.; Manders, R.J.; Sahni, S.; Zhu, K.; Hewitt, C.E.; Prince, R.L.; Lanham-New, S.A. Dietary protein and bone health across the life-course: An updated systematic review and meta-analysis over 40 years. Osteoporos. Int. 2019, 30, 741–761. [Google Scholar] [CrossRef] [Green Version]
- Sellmeyer, D.E.; Stone, K.L.; Sebastian, A.; Cummings, S.R. A high ratio of dietary animal to vegetable protein increases the rate of bone loss and the risk of fracture in postmenopausal women. Am. J. Clin. Nutr. 2001, 73, 118–122. [Google Scholar]
- Wyness, L. The role of red meat in the diet: Nutrition and health benefits. Proc. Nutr. Soc. 2016, 75, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Jarman, M.; Mathe, N.; Ramazani, F.; Pakseresht, M.; Robson, P.J.; Johnson, S.T.; Bell, R.C. Dietary patterns prior to pregnancy and associations with pregnancy complications. Nutrients 2018, 10, 914. [Google Scholar] [CrossRef] [Green Version]
- Mizgier, M.; Jarzabek-Bielecka, G.; Mruczyk, K. Maternal diet and gestational diabetes mellitus development. J. Matern. Fetal Neonatal Med. 2021, 34, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Haugen, M.; Meltzer, H.M.; Brantsaeter, A.L. Mediterranean-type diet and risk of preterm birth among women in the Norwegian Mother and Child Cohort Study (MoBa): A prospective cohort study. Acta Obs. Gynecol. Scand. 2008, 87, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Saunders, L.; Guldner, L.; Costet, N. Effect of a Mediterranean diet during pregnancy on fetal growth and preterm delivery: Results from a French Caribbean Mother-Child Cohort Study (TIMOUN). Paediatr. Perinat. Epidemiol. 2014, 28, 235–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Schulze, M.B.; Solomon, C.G.; Hu, F.B. A prospective study of dietary patterns, meat intake and the risk of gestational diabetes mellitus. Diabetologia 2006, 49, 2604–2613. [Google Scholar] [CrossRef] [Green Version]
- Marí-Sanchis, A.; Díaz-Jurado, G.; Basterra-Gortari, F.J.; de la Fuente-Arrillaga, C.; Martínez-González, M.A.; Bes-Rastrollo, M. Association between pre-pregnancy consumption of meat, iron intake, and the risk of gestational diabetes: The SUN project. Eur. J. Nutr. 2018, 57, 939–949. [Google Scholar] [CrossRef]
- Liang, Y.; Gong, Y.; Zhang, X.; Yang, D.; Zhao, D.; Quan, L.; Cheng, G. Dietary protein intake, meat consumption, and dairy consumption in the year preceding pregnancy and during pregnancy and their associations with the risk of gestational diabetes mellitus: A prospective cohort study in southwest China. Front. Endocrinol. 2018, 9, 596. [Google Scholar] [CrossRef]
- Pogoda, J.M.; Preston-Martin, S. Maternal cured meat consumption during pregnancy and risk of paediatric brain tumour in offspring: Potentially harmful levels of intake. Public Health Nutr. 2001, 4, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Lundgren, S.N.; Madan, J.C.; Emond, J.A.; Morrison, H.G.; Christensen, B.C.; Karagas, M.R.; Hoen, A.G. Maternal diet during pregnancy is related with the infant stool microbiome in a delivery mode-dependent manner. Microbiome 2018, 6, 109. [Google Scholar] [CrossRef] [Green Version]
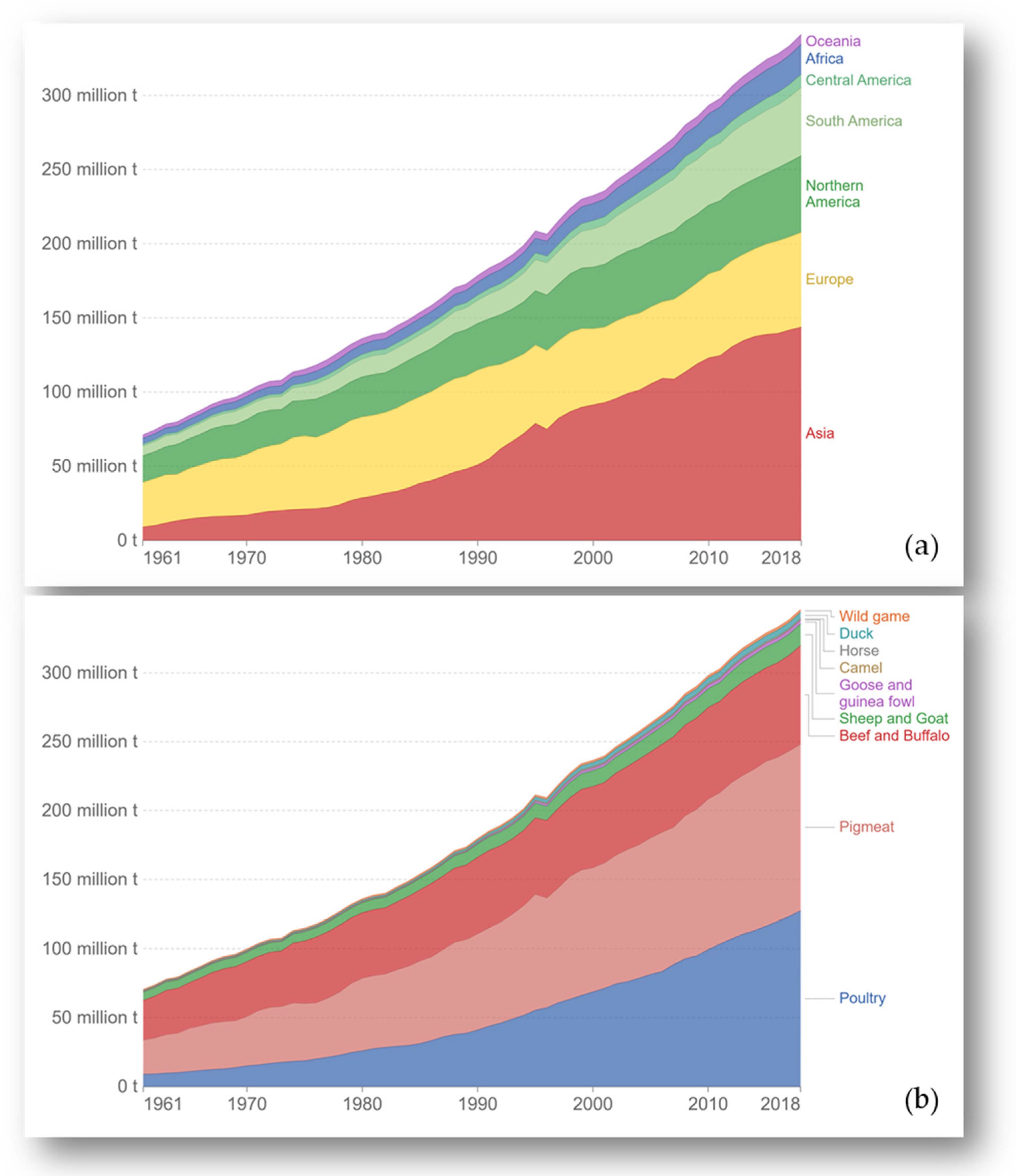
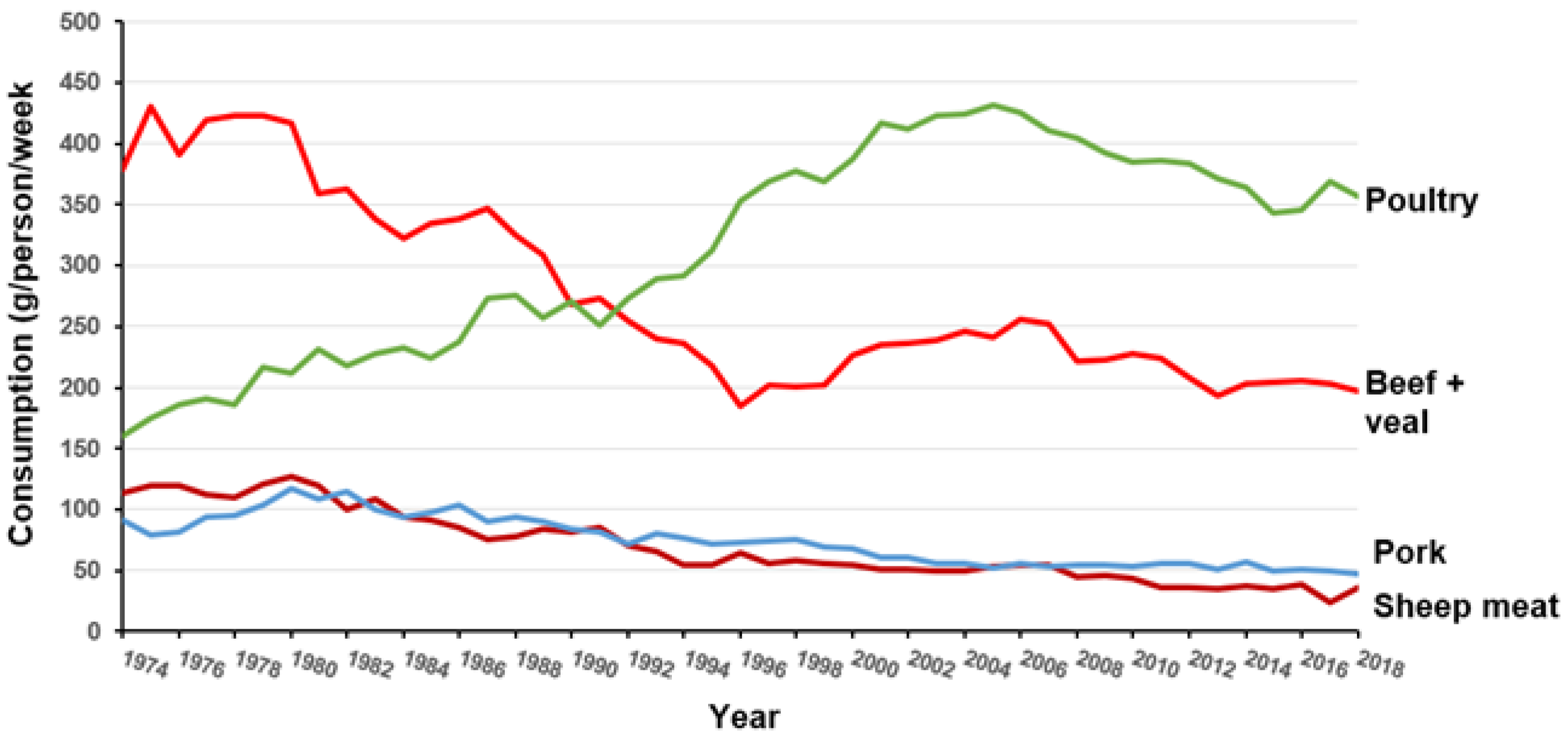
| Meat Type | Iron Concentration (mg/100 g) |
|---|---|
| Red meat | |
| Pork steaks, grilled, lean | 1.1 |
| Lamb leg steaks, grilled, lean | 2.2 |
| Beef rump steak, grilled, lean | 3.6 |
| Venison, roasted | 5.1 |
| White meat | |
| Turkey, light meat, roasted | 0.50 |
| Turkey, dark meat, roasted | 1.2 |
| Chicken, light meat, roasted | 0.40 |
| Chicken, dark meat, roasted | 0.80 |
| Fish | |
| Cod, flesh only, grilled | 0.10 |
| Haddock, flesh only, grilled | 0.17 |
| Salmon, flesh only, grilled | 0.45 |
| Systematic Review Used | Number of Cohort Studies | Outcome | Comparison Used | Risk Ratio (95% CI 1) |
|---|---|---|---|---|
| Unprocessed red meat | ||||
| Zeraatkar et al. (2019) | 3 | CVD | Dose–response, per 50 g/day | 1.01 (0.99, 1.02) |
| Kim et al. (2017) | 6 | Stroke | High vs. low intake | 1.11 (1.03, 1.20) |
| Zeraatkar et al. (2019) | 6 | Stroke | Dose–response, per 50 g/day | 1.01 (1.00, 1.01) |
| Unprocessed poultry meat | ||||
| Kim et al. (2017) | 3 | Stroke | High vs. low intake | 0.87 (0.78, 0.96) |
| Processed meat | ||||
| Zeraatkar et al. (2019) | 3 | CVD | Dose–response, per 50g/day | 1.01 (0.97, 1.05) |
| Bechthold et al., (2019) | 3 | IHD | Dose–response, per 50g/day | 1.27 (1.09, 1.49) |
| Kim et al. (2017) | 6 | Stroke | High vs. low intake | 1.17 (1.08, 1.25) |
| Bechthold et al. (2019) | 6 | Stroke | Dose–response, per 50g/day | 1.17 (1.02, 1.34) |
| Zeraatkar et al. (2019) | 6 | Stroke | Dose–response, per 50g/day | 1.02 (1.01. 1.04) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giromini, C.; Givens, D.I. Benefits and Risks Associated with Meat Consumption during Key Life Processes and in Relation to the Risk of Chronic Diseases. Foods 2022, 11, 2063. https://doi.org/10.3390/foods11142063
Giromini C, Givens DI. Benefits and Risks Associated with Meat Consumption during Key Life Processes and in Relation to the Risk of Chronic Diseases. Foods. 2022; 11(14):2063. https://doi.org/10.3390/foods11142063
Chicago/Turabian StyleGiromini, Carlotta, and D. Ian Givens. 2022. "Benefits and Risks Associated with Meat Consumption during Key Life Processes and in Relation to the Risk of Chronic Diseases" Foods 11, no. 14: 2063. https://doi.org/10.3390/foods11142063
APA StyleGiromini, C., & Givens, D. I. (2022). Benefits and Risks Associated with Meat Consumption during Key Life Processes and in Relation to the Risk of Chronic Diseases. Foods, 11(14), 2063. https://doi.org/10.3390/foods11142063






