Recovery and Concentration of Polyphenols from Roasted Hazelnut Skin Extract Using Macroporous Resins
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polyphenol Extraction
2.3. Total Polyphenolic Content
2.4. Antioxidant Capacity
2.5. ABTS+ Assay
2.6. Reversed Phase-HPLC-Diode Array Detector Analysis
2.7. Static Adsorption/Desorption Evaluation
2.8. Dynamic Adsorption/Desorption
2.9. Statistical Analysis
3. Results
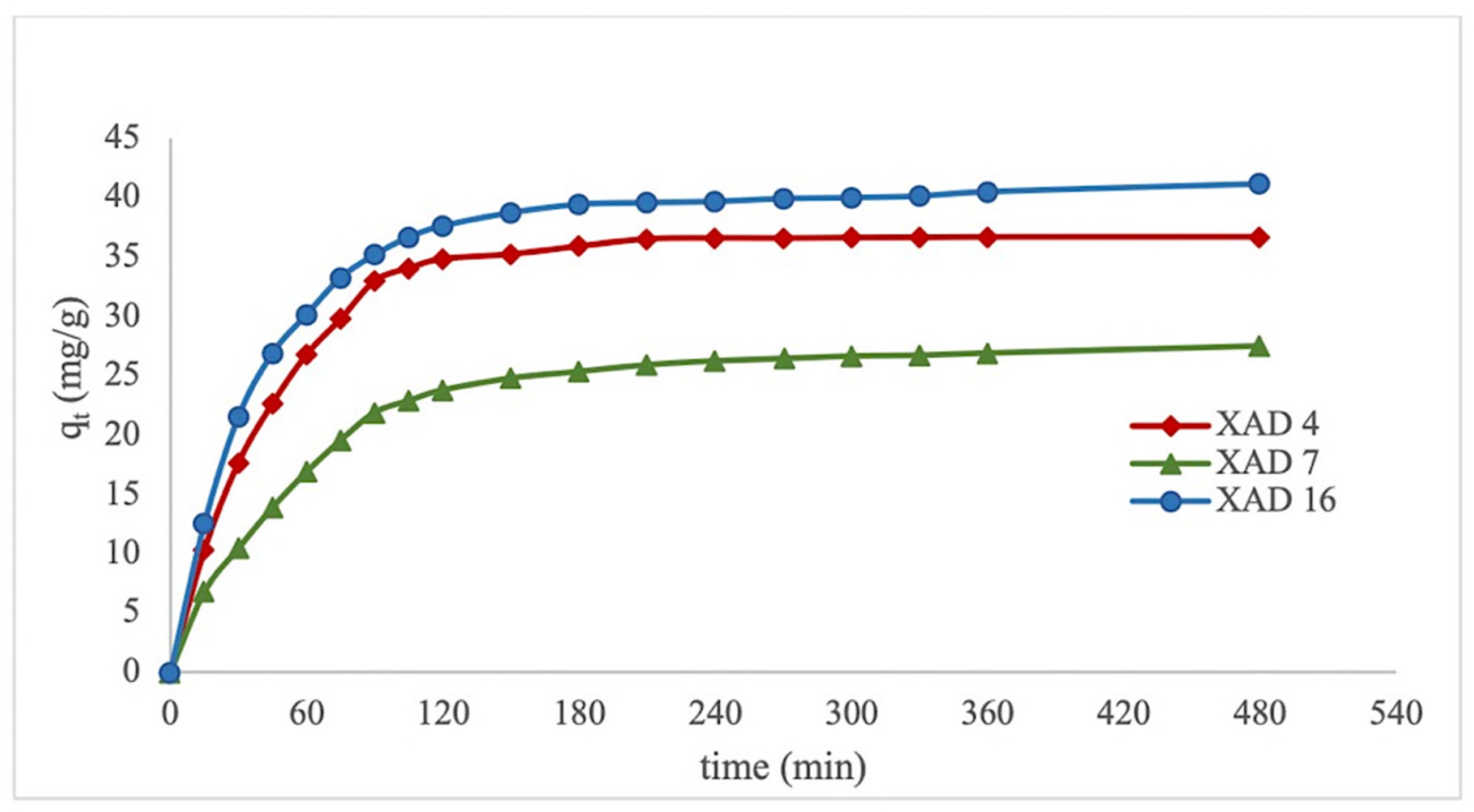
3.1. Static Adsorption/Desorption
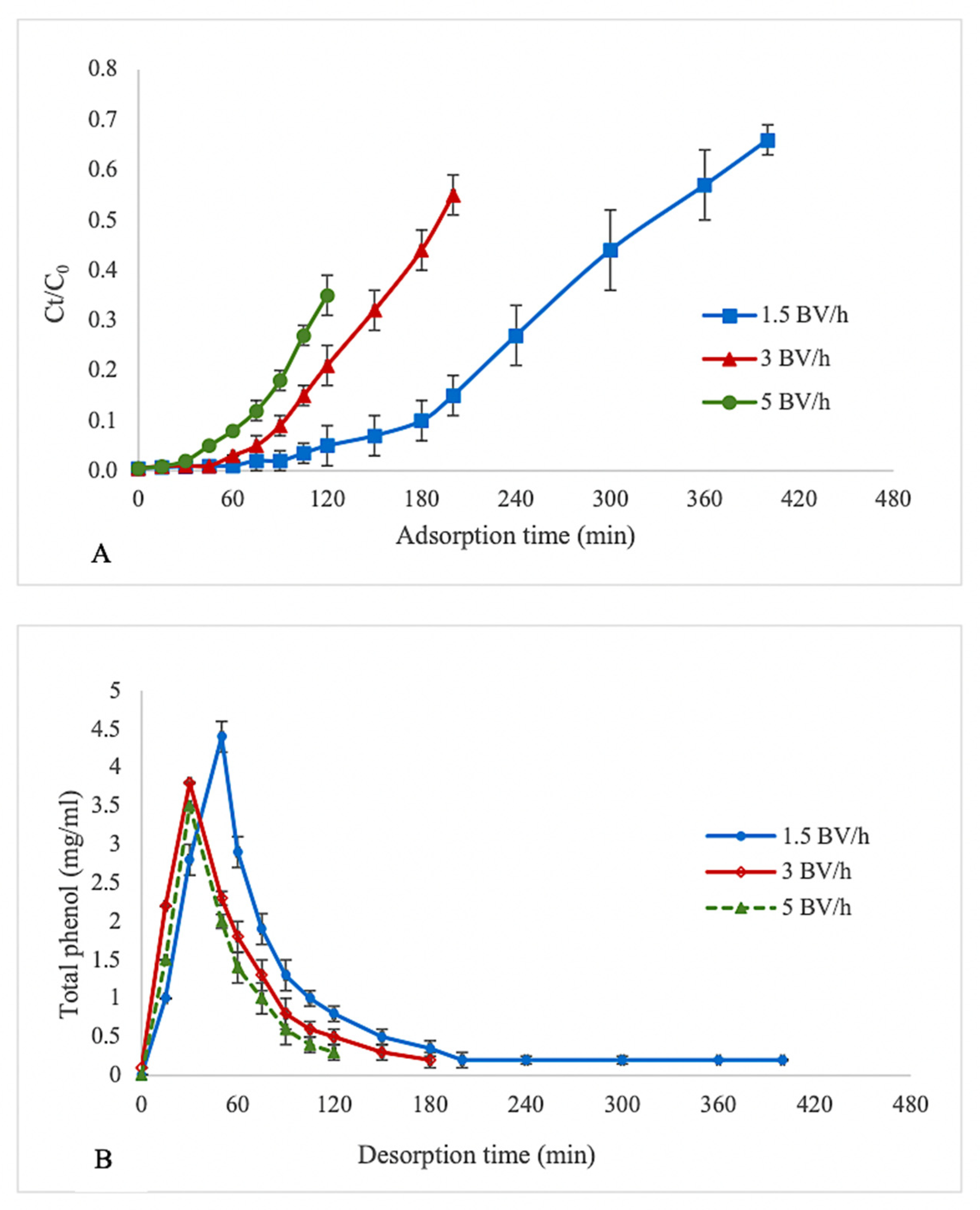
3.2. Dynamic Adsorption/Desorption
3.3. Phenolic Content and Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spagnuolo, L.; della Posta, S.; Fanali, C.; Dugo, L.; de Gara, L. Antioxidant and Antiglycation Effects of Polyphenol Compounds Extracted from Hazelnut Skin on Advanced Glycation End-products (Ages) Formation. Antioxidants 2021, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, M.; Belviso, S.; Dal Bello, B.; Ghirardello, D.; Giordano, M.; Rolle, L.; Gerbi, V.; Zeppa, G. Influence of the Addition of Different Hazelnut Skins on the Physicochemical, Antioxidant, Polyphenol and Sensory Properties Ofyogurt. LWT Food Sci. Technol. 2015, 63, 1145–1154. [Google Scholar] [CrossRef]
- Pérez-Larrán, P.; Díaz-Reinoso, B.; Moure, A.; Alonso, J.L.; Domínguez, H. Adsorption Technologies to Recover and Concentrate Food Polyphenols. Curr. Opin. Food Sci. 2018, 23, 165–172. [Google Scholar] [CrossRef]
- Zeppa, G.; Belviso, S.; Bertolino, M.; Cavallero, M.C.; Dal Bello, B.; Ghirardello, D.; Giordano, M.; Giorgis, M.; Grosso, A.; Rolle, L.; et al. The Effect of Hazelnut Roasted Skin from Different Cultivars on the Quality Attributes, Polyphenol Content and Texture of Fresh Egg Pasta. J. Sci. Food Agric. 2015, 95, 1678–1688. [Google Scholar] [CrossRef] [PubMed]
- Taş, N.G.; Gökmen, V. Bioactive Compounds in Different Hazelnut Varieties and Their Skins. J. Food Compos. Anal. 2015, 43, 203–208. [Google Scholar] [CrossRef]
- Locatelli, M.; Travaglia, F.; Coïsson, J.D.; Martelli, A.; Stévigny, C.; Arlorio, M. Total Antioxidant Activity of Hazelnut Skin (Nocciola Piemonte PGI): Impact of Different Roasting Conditions. Food Chem. 2010, 119, 1647–1655. [Google Scholar] [CrossRef]
- Pelvan, E.; Olgun, E.Ö.; Karadağ, A.; Alasalvar, C. Phenolic Profiles and Antioxidant Activity of Turkish Tombul Hazelnut Samples (Natural, Roasted, and Roasted Hazelnut Skin). Food Chem. 2018, 244, 102–108. [Google Scholar] [CrossRef]
- del Rio, D.; Calani, L.; Dall’Asta, M.; Brighenti, F. Polyphenolic Composition of Hazelnut Skin. J. Agric. Food Chem. 2011, 59, 9935–9941. [Google Scholar] [CrossRef]
- Hou, M.; Zhang, L. Adsorption/Desorption Characteristics and Chromatographic Purification of Polyphenols from Vernonia Patula (Dryand.) Merr. Using Macroporous Adsorption Resin. Ind. Crops Prod. 2021, 170, 113729. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, S.; Peng, M.; She, Z.; Yang, Q.; Huang, T. Adsorption and Desorption Characteristics of Polyphenols from Eucommia Ulmoides Oliv. Leaves with Macroporous Resin and Its Inhibitory Effect on α-Amylase and α-Glucosidase. Ann. Transl. Med. 2020, 8, 1004. [Google Scholar] [CrossRef]
- Leyton, A.; Vergara-Salinas, J.R.; Pérez-Correa, J.R.; Lienqueo, M.E. Purification of Phlorotannins from Macrocystis Pyrifera Using Macroporous Resins. Food Chem. 2017, 237, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, K.S.; Yilmaz, C.; Durmaz, G.; Gokmen, V. Hazelnut Skin Powder: A New Brown Colored Functional Ingredient. Food Res. Int. 2014, 65, 291–297. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Guglielmetti, A.; Zeppa, G. Pulsed Electric Field Assisted Extraction of Bioactive Compounds from Cocoa Bean Shell and Coffee Silverskin. Food Bioprocess Technol. 2018, 11, 818–835. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Yu, Q.; Fan, L.; Li, J. A Novel Process for Asparagus Polyphenols Utilization by Ultrasound Assisted Adsorption and Desorption Using Resins. Ultrason. Sonochem. 2020, 63, 104920. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Yang, J.; Liu, R.; Lu, Q.; Ding, Y.; Zhang, J.; Deng, J. A Comparative Study on the Adsorption and Desorption Characteristics of Flavonoids from Honey by Six Resins. Food Chem. 2018, 268, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Mu, T.; Sun, H. Preparative Purification of Polyphenols from Sweet Potato (Ipomoea batatas L.) Leaves by AB-8 Macroporous Resins. Food Chem. 2015, 172, 166–174. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Kinetic Models: Physical Meanings, Applications, and Solving Methods. J. Hazard. Mater. 2020, 390, 121156. [Google Scholar] [CrossRef]
- Park, J.J.; Lee, W.Y. Adsorption and Desorption Characteristics of a Phenolic Compound from Ecklonia Cava on Macroporous Resin. Food Chem. 2021, 338, 128150. [Google Scholar] [CrossRef]
- Le, T.T.; Framboisier, X.; Aymes, A.; Ropars, A.; Frippiat, J.P.; Kapel, R. Identification and Capture of Phenolic Compounds from a Rapeseed Meal Protein Isolate Production Process By-Product by Macroporous Resin and Valorization Their Antioxidant Properties. Molecules 2021, 26, 5853. [Google Scholar] [CrossRef]
- Buran, T.J.; Sandhu, A.K.; Li, Z.; Rock, C.R.; Yang, W.W.; Gu, L. Adsorption/Desorption Characteristics and Separation of Anthocyanins and Polyphenols from Blueberries Using Macroporous Adsorbent Resins. J. Food Eng. 2014, 128, 167–173. [Google Scholar] [CrossRef]
- Wang, X.; Su, J.; Chu, X.; Zhang, X.; Kan, Q.; Liu, R.; Fu, X. Adsorption and Desorption Characteristics of Total Flavonoids from Acanthopanax Senticosus on Macroporous Adsorption Resins. Molecules 2021, 26, 4162. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.L.; Moure, A.; Domínguez, H.; Parajó, J.C. Batch and Fixed Bed Column Studies on Phenolic Adsorption from Wine Vinasses by Polymeric Resins. J. Food Eng. 2017, 209, 52–60. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Gu, L. Adsorption/Desorption Characteristics and Separation of Anthocyanins from Muscadine (Vitis Rotundifolia) Juice Pomace by Use of Macroporous Adsorbent Resins. J. Agric. Food Chem. 2013, 61, 1441–1448. [Google Scholar] [CrossRef]
- Iheanacho, O.C.; Nwabanne, J.T.; Obi, C.C.; Onu, C.E. Packed Bed Column Adsorption of Phenol onto Corn Cob Activated Carbon: Linear and Nonlinear Kinetics Modeling. S. Afr. J. Chem. Eng. 2021, 36, 80–93. [Google Scholar] [CrossRef]
- Ma, C.; Tao, G.; Tang, J.; Lou, Z.; Wang, H.; Gu, X.; Hu, L.; Yin, M. Preparative Separation and Purification of Rosavin in Rhodiola Rosea by Macroporous Adsorption Resins. Sep. Purif. Technol. 2009, 69, 22–28. [Google Scholar] [CrossRef]
- Li, B.; Wang, C.; Chen, X.; Lyu, J.; Guo, S. Highly Specific Separation for Antitumor Spiropreussione A from Endophytic Fungal [Preussia Sp.] Fermentation Broth by One-Step Macroporous Resins AB-8 Treatment. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 938, 1–7. [Google Scholar] [CrossRef]
- Vavouraki, A. Removal of Polyphenols from Olive Mill Wastewater by FPX 66 Resin: Part II. Adsorption Kinetics and Equilibrium Studies. Int. J. Waste Resour. 2020, 10, 374. [Google Scholar] [CrossRef][Green Version]
- Johnson, R.; Mitchell, A.E. Use of Amberlite Macroporous Resins to Reduce Bitterness in Whole Olives for Improved Processing Sustainability. J. Agric. Food Chem. 2019, 67, 1546–1553. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, P. Extraction and Purification Conditions of Anthocyanin from the Fruit of Aronia Melanocarpa. Beijing Linye Daxue Xuebao J. Beijing For. Univ. 2016, 38, 118–124. [Google Scholar] [CrossRef]
| XAD 4 | XAD 7 | XAD 16 | |
|---|---|---|---|
| Polarity | non polar | moderately polar | non polar |
| Chemical structure | Hydrophobic polyaromatic | Acrylic ester | Hydrophobic polyaromatic |
| Dry density (g/mL) | 1.08–1.02 | 1.24–1.05 | 1.08–1.02 |
| Surf. Area (m2/g) | 725 | 450 | 900 |
| Pore diameter (nm) | 5 | 9 | 10 |
| Pore mesh size | 20–60 | 20–60 | 20–60 |
| Pore volum (mL/g) | 0.98 | 1.14 | 0.82 |
| Particle size (mm) | 0.3–1.2 | 0.3–1.2 | 0.3–1.2 |
| Resin Amount (g) | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Significance | |
| XAD 4 | 13.87 ± 0.74 Be | 24.82 ± 1.20 Bd | 34.82 ± 0.58 Bc | 51.89 ± 0.79 Bb | 58.73 ± 0.75 Ba | *** |
| XAD 7 | 10.95 ± 1.16 Ce | 20.65 ± 0.55 Cd | 24.25 ± 1.03 Cc | 38.23 ± 1.09 Cb | 42.66 ± 0.89 Ca | *** |
| XAD 16 | 15.58 ± 0.47 Ae | 29.03 ± 1.87 Ad | 38.93 ± 1.95 Ac | 58.81 ± 1.17 Ab | 65.06 ± 0.14 Aa | *** |
| Significance | *** | *** | *** | *** | *** | |
| Resin Amount (g) | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| XAD 4 | 17.70 ± 0.95 Be | 20.82 ± 0.36 Ad | 23.38 ± 0.39 Bc | 26.61 ± 0.40 Bb | 36.14 ± 0.46 Ba | *** |
| XAD 7 | 13.97 ± 1.48 Cd | 15.25 ± 0.40 Bd | 16.28 ± 0.69 Cc | 19.60 ± 0.56 Cb | 26.25 ± 0.54 Ca | *** |
| XAD 16 | 19.88 ± 0.61 Ae | 22.39 ± 1.44 Ad | 26.14 ± 1.30 Ac | 30.16 ± 0.60 Ab | 40.05 ± 0.55 Aa | *** |
| Significance | *** | *** | *** | *** | *** | |
|
Ethanol Concentration (%) | Resin Amount (g) | Significance | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| XAD 4 | 99.99 | 35.27 ± 0.48 Be | 44.70 ± 1.41 Bd | 58.46 ± 0.98 Bc | 65.85 ± 2.90 Bb | 72.53 ± 0.78 Ba | *** |
| 70 | 40.70 ± 2.04 Ad | 51.07 ± 1.94 Ac | 64.26 ± 1.66 Ab | 73.94 ± 1.45 Aa | 76.64 ± 0.93 Aa | *** | |
| 50 | 32.40 ± 1.18 Ce | 40.28 ± 1.21 Cd | 50.37 ± 1.70 Cc | 58.17 ± 2.28 Cb | 65.10 ± 1.93 Ca | *** | |
| Significance | *** | *** | *** | *** | *** | ||
| XAD 7 | 99.99 | 14.73 ± 0.46 Ce | 19.57 ± 0.37 Cd | 30.26 ± 0.77 Cc | 38.34 ± 3.13 Cb | 43.58 ± 1.43 Ca | *** |
| 70 | 17.76 ± 0.91 Be | 29.92 ± 0.77 Bd | 37.49 ± 0.79 Bc | 44.46 ± 0.92 Bb | 48.75 ± 2.13 Ba | *** | |
| 50 | 19.89 ± 0.59 Ae | 33.45 ± 1.08 Ad | 40.57 ± 0.75 Ac | 48.64 ± 0.83 Ab | 54.37 ± 1.65 Aa | *** | |
| Significance | *** | *** | *** | *** | *** | ||
| XAD 16 | 99.99 | 39.46 ± 1.50 Be | 47.49 ± 1.19 Bd | 58.97 ± 1.23 Bc | 71.38 ± 2.28 Bb | 75.80 ± 2.35 Ba | *** |
| 70 | 45.06 ± 1.47 Ae | 53.05 ± 2.31 Ad | 65.65 ± 1.22 Ab | 76.79 ± 2.41 Ab | 81.17 ± 1.19 Aa | *** | |
| 50 | 35.66 ± 0.70 Ce | 43.89 ± 0.66 Cd | 53.28 ± 0.92 Cb | 61.99 ± 2.60 Cb | 67.01 ± 2.46 Ca | *** | |
| Significance | *** | *** | *** | *** | *** | ||
| Pseudo-First Order | Pseudo-Second Order | ||||||
|---|---|---|---|---|---|---|---|
| qe exp. | k1 | qe | r2 | k2 | qe | r2 | |
| XAD 4 | 36.68 | 0.0210 | 33.08 | 0.9853 | 0.00065 | 38.17 | 0.9947 |
| XAD 7 | 27.49 | 0.0105 | 18.43 | 0.9560 | 0.00065 | 29.07 | 0.9972 |
| XAD 16 | 41.15 | 0.0105 | 20.12 | 0.9014 | 0.00061 | 41.32 | 0.9950 |
| Raw Extract | Purified Extract | Increment% * | |
|---|---|---|---|
| Gallic acid (µg/mL) | 9.16 ± 0.74 | 30.85 ± 2.01 | 237 |
| Protocatechuic acid (µg/mL) | 2.80 ± 0.22 | 8.18± 0.12 | 192 |
| Catechin (µg/mL) | 4.39 ± 0.34 | 22.06 ± 0.44 | 402 |
| Epicatechin (µg/mL) | 2.72 ± 0.31 | 14.83 ± 1.05 | 445 |
| Quercitin (µg/mL) | 9.34 ± 0.76 | 47.23 ± 2.25 | 406 |
| DPPH (mM TE/mL) | 13.44 ± 0.85 | 83.51 ± 1.25 | 521 |
| ABTS (mM TE/mL) | 8.71 ± 1.23 | 51.83 ± 1.45 | 495 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seif Zadeh, N.; Zeppa, G. Recovery and Concentration of Polyphenols from Roasted Hazelnut Skin Extract Using Macroporous Resins. Foods 2022, 11, 1969. https://doi.org/10.3390/foods11131969
Seif Zadeh N, Zeppa G. Recovery and Concentration of Polyphenols from Roasted Hazelnut Skin Extract Using Macroporous Resins. Foods. 2022; 11(13):1969. https://doi.org/10.3390/foods11131969
Chicago/Turabian StyleSeif Zadeh, Negin, and Giuseppe Zeppa. 2022. "Recovery and Concentration of Polyphenols from Roasted Hazelnut Skin Extract Using Macroporous Resins" Foods 11, no. 13: 1969. https://doi.org/10.3390/foods11131969
APA StyleSeif Zadeh, N., & Zeppa, G. (2022). Recovery and Concentration of Polyphenols from Roasted Hazelnut Skin Extract Using Macroporous Resins. Foods, 11(13), 1969. https://doi.org/10.3390/foods11131969







