Whole-Genome Sequencing of a Potential Ester-Synthesizing Bacterium Isolated from Fermented Golden Pomfret and Identification of Its Lipase Encoding Genes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Screening and Identification of Ester Synthase-Producing Bacteria
2.1.1. Isolation and Morphological Identification of Ester Synthase-Producing Microorganisms
2.1.2. Determination of Enzyme Activity and Total Ester Content
2.1.3. Morphological Observation and Identification of Ester Synthase-Producing Microorganisms
Morphological Observation of Ester Synthase-Producing Microorganisms
Identification of Ester Synthase-Producing Bacteria
2.2. Growth Characteristics and Ester Synthase Activity of SCSMX-3
2.3. Whole Genome Sequencing of SCSMX-3
2.4. Genome Composition Prediction and Gene Function Annotation
2.5. DNA and Protein Sequence Analysis
2.6. Statistical Analyses
3. Results and Discussion
3.1. Screening and Identification of Ester Synthase-Producing Strains
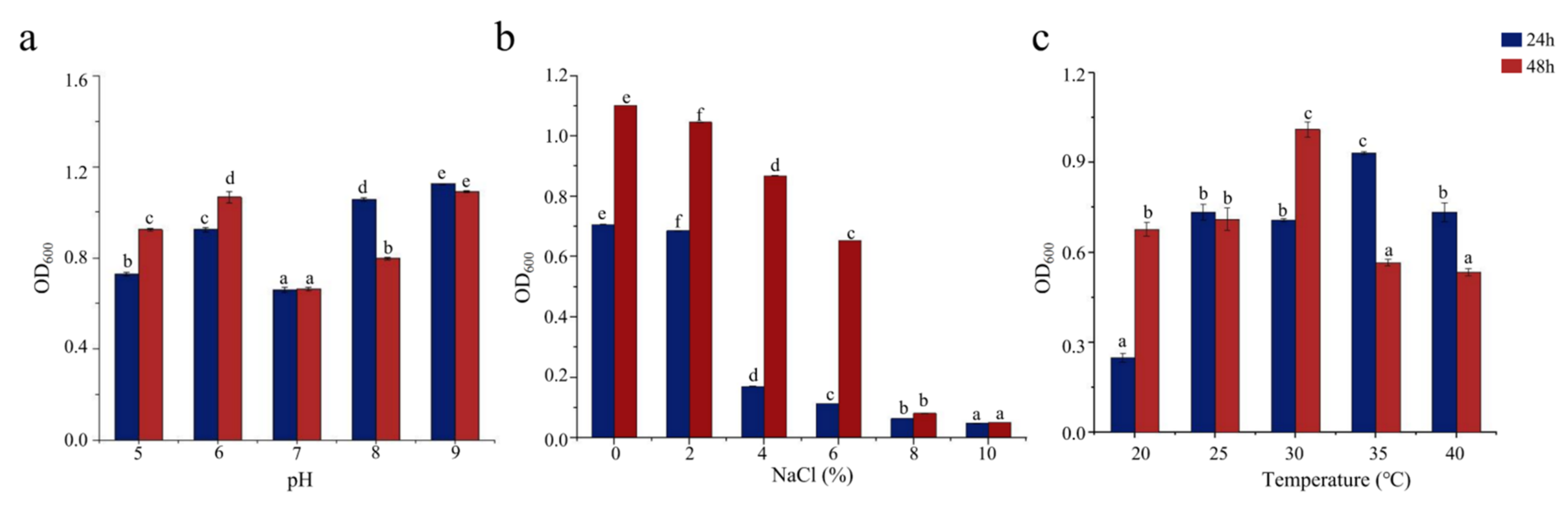
3.2. Growth Properties of SCSMX-3
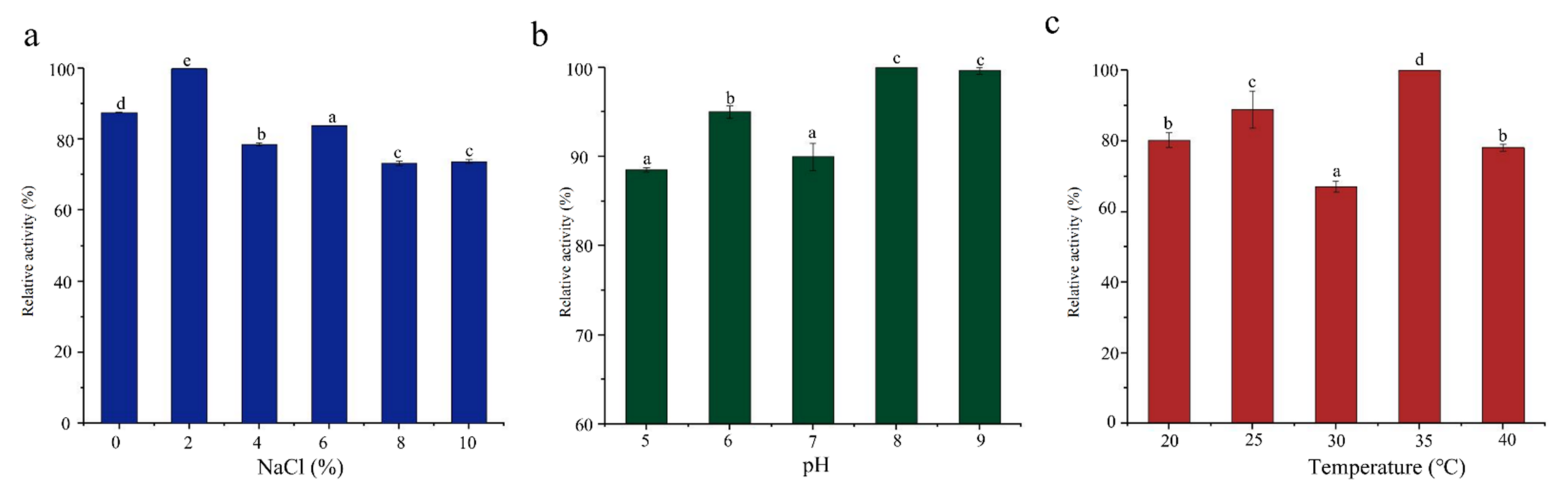
3.3. Characterization of the Ester Synthase Activity of SCSMX-3
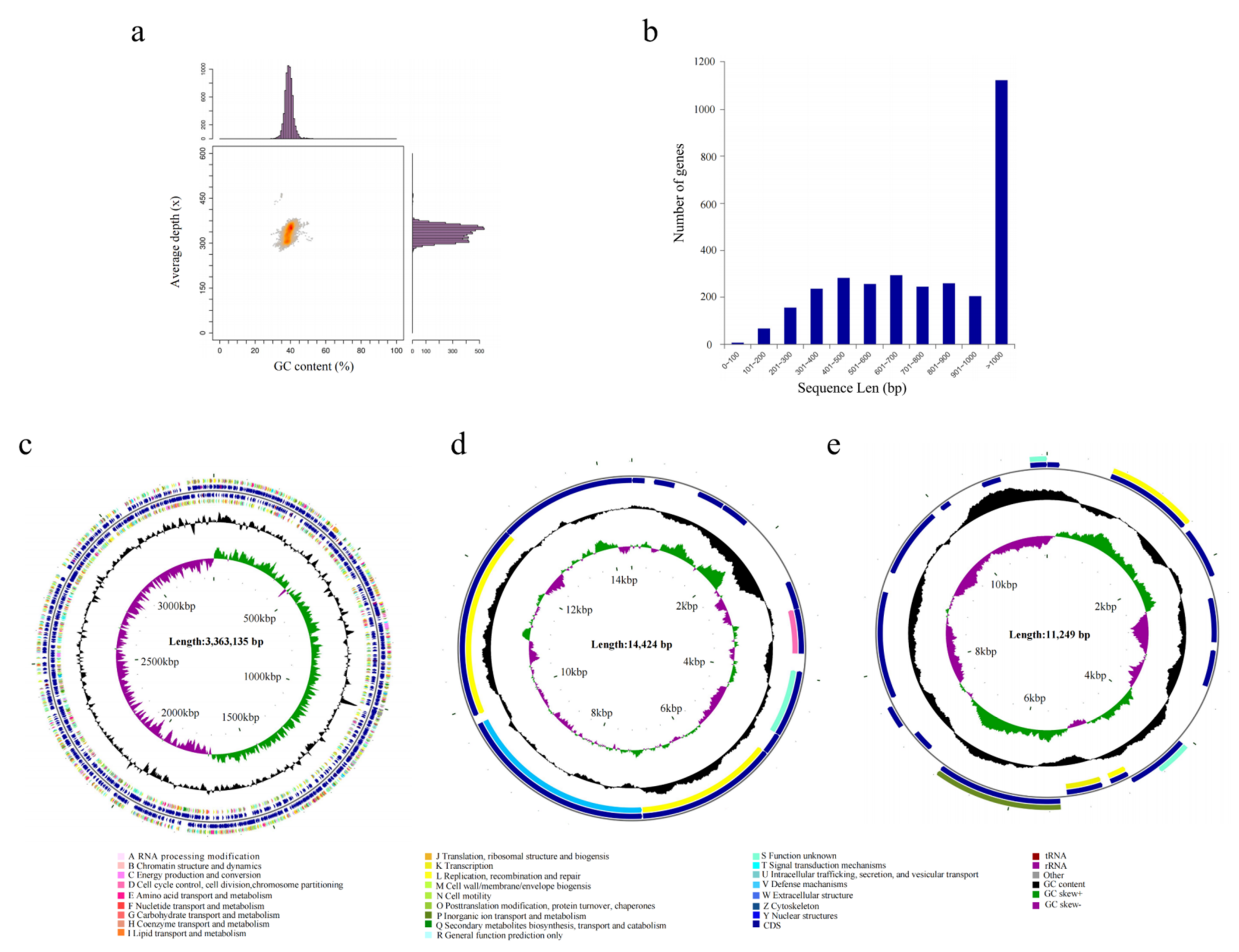
3.4. Complete-Genome Sequencing and Information Analysis
3.5. CRISPR Prediction
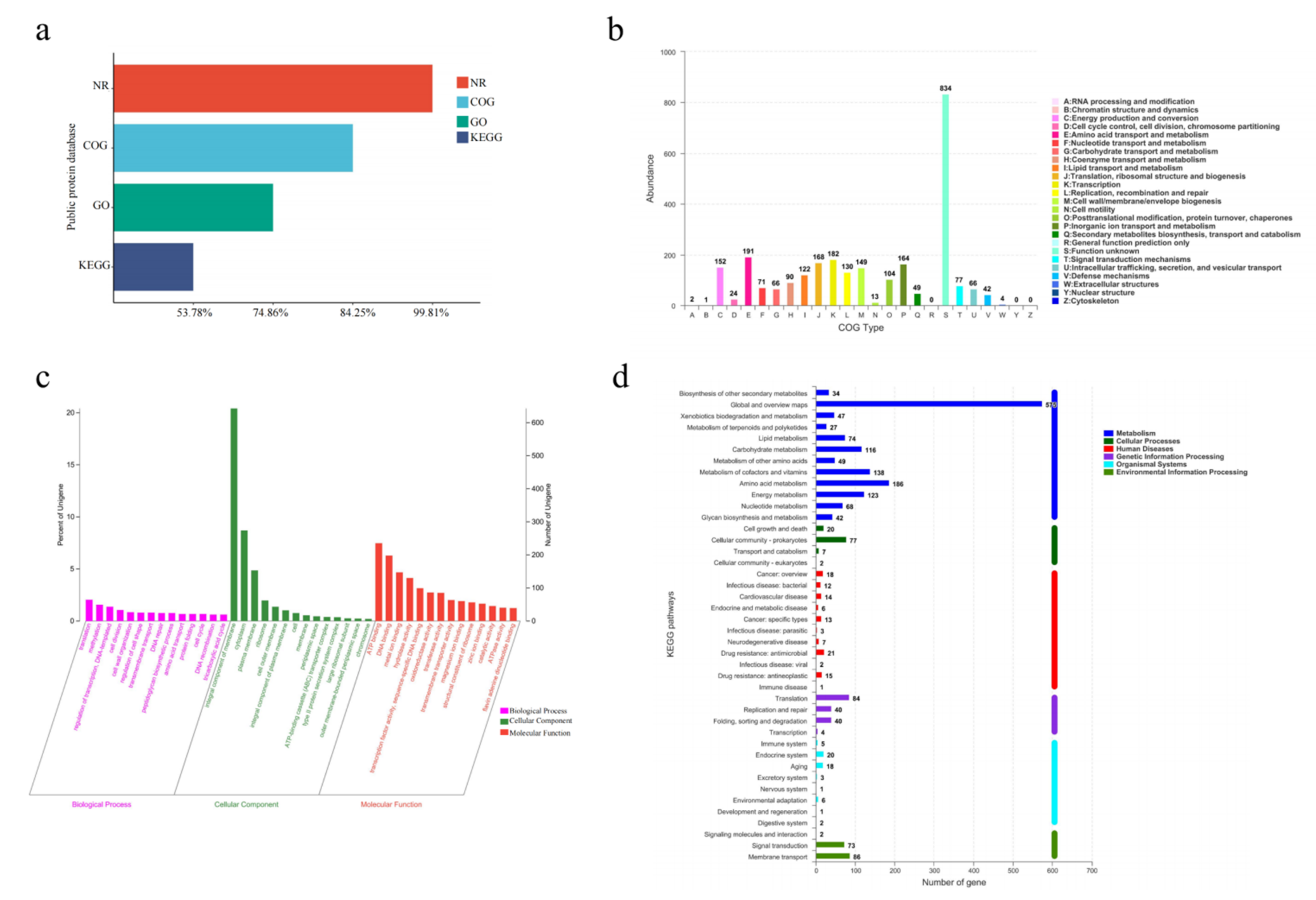
3.6. Genome Function Annotation
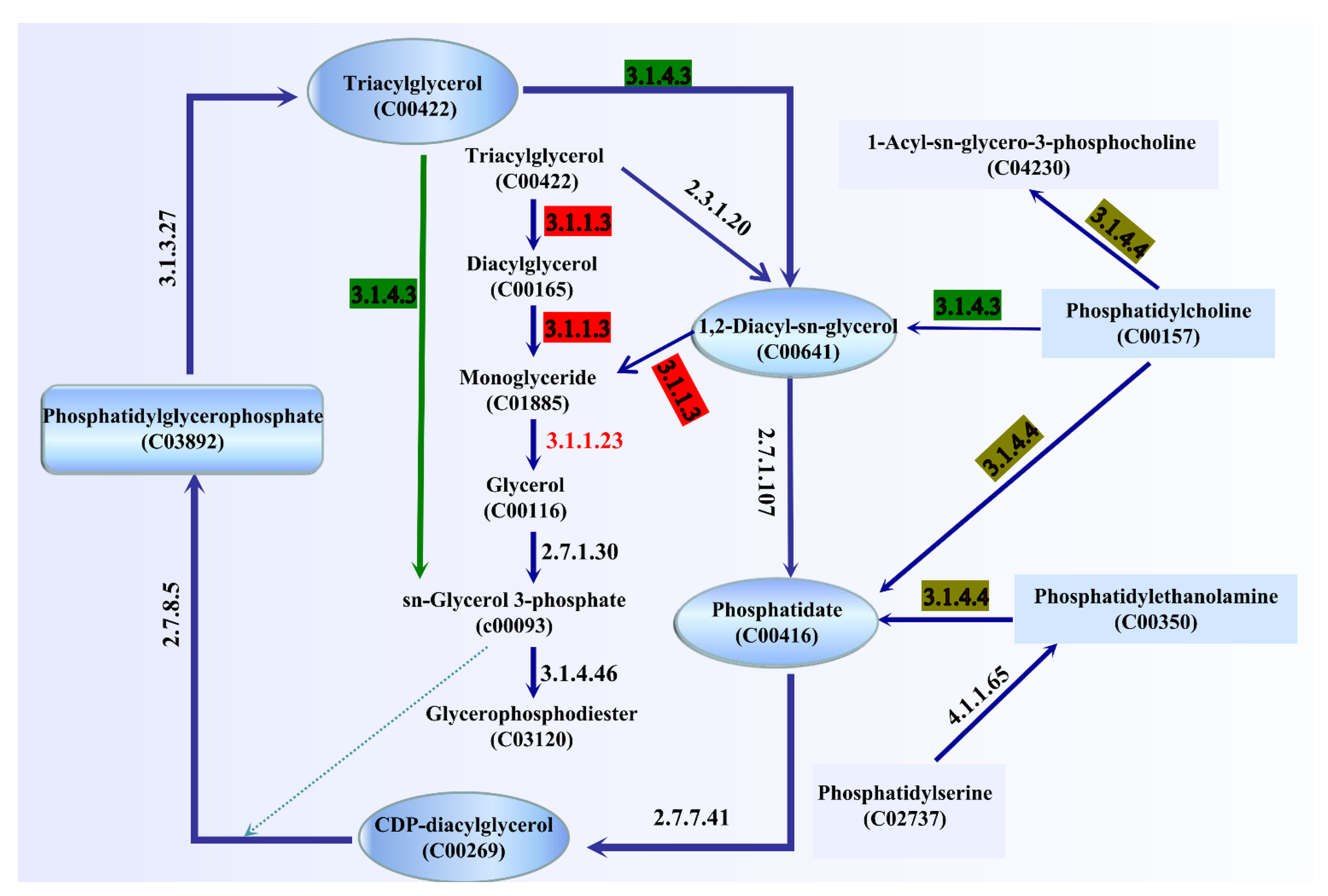
3.7. Analysis of Genes Related to Lipid Metabolism
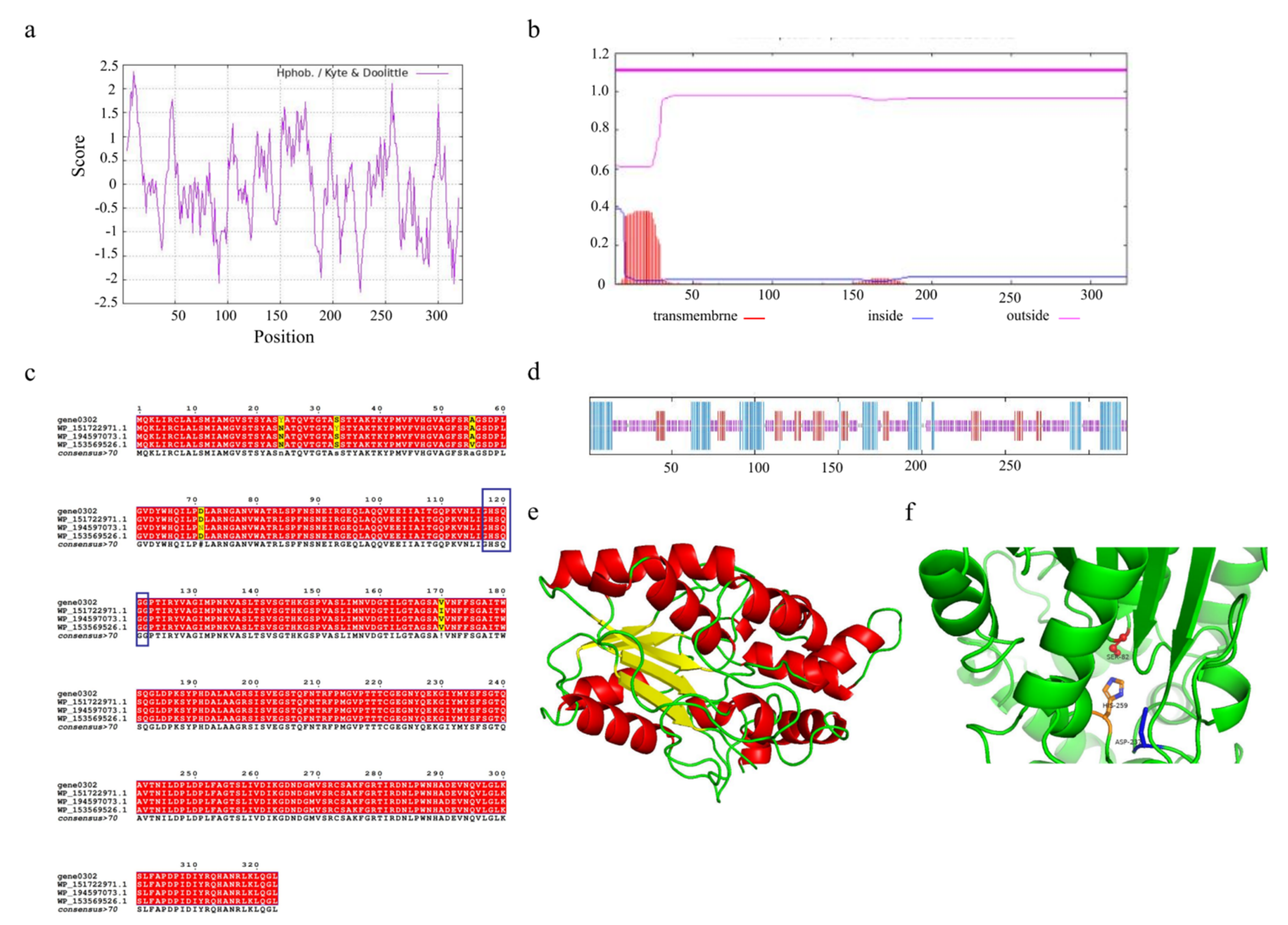
3.8. Genetic Informatic Analysis of the Triacylglycerol Lipase of A. venetianus SCSMX-3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zang, J.; Xu, Y.; Xia, W.; Yu, D.; Gao, P.; Jiang, Q.; Yang, F. Dynamics and diversity of microbial community succession during fermentation of Suan yu, a Chinese traditional fermented fish, determined by high throughput sequencing. Food Res. Int. 2018, 111, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Lin, H.; Ahmed, I.; Sui, J. Isolation and identification of the umami peptides from Trachinotus ovatus hydrolysate by consecutive chromatography and Nano-HPLC-MS/MS. LWT 2021, 141, 110887. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Wu, Y.; Li, C.; Li, L.; Yang, X.; Chen, S.; Zhao, Y.; Cen, J.; Yang, S.; et al. Investigation of fermentation-induced changes in the volatile compounds of Trachinotus ovatus (meixiangyu) based on molecular sensory and interpretable machine-learning techniques: Comparison of different fermentation stages. Food Res. Int. 2021, 150, 110739. [Google Scholar] [CrossRef] [PubMed]
- Ban, S.; Chen, L.; Fu, S.; Wu, Q.; Xu, Y. Modelling and predicting population of core fungi through processing parameters in spontaneous starter (Daqu) fermentation. Int. J. Food Microbiol. 2022, 363, 109493. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, Y.; Wang, Y.; Li, L.; Li, C.; Zhao, Y.; Yang, S. Contribution of autochthonous microbiota succession to flavor formation during Chinese fermented mandarin fish (Siniperca chuatsi). Food Chem. 2021, 348, 129107. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Zhao, Y.; Li, L.; Yang, X.; Wu, Y.; Chen, S.; Cen, J.; Yang, S.; Yang, D. Novel insight into the formation mechanism of volatile flavor in Chinese fish sauce (Yu-lu) based on molecular sensory and metagenomics analyses. Food Chem. 2020, 323, 126839. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Li, L.; Chen, S.; Wu, Y.; Qi, B. Microbial community changes induced by a newly isolated salt-tolerant Tetragenococcus muriaticus improve the volatile flavor formation in low-salt fish sauce. Food Res. Int. 2022, 156, 111153. [Google Scholar] [CrossRef]
- Gao, P.; Jiang, Q.; Xu, Y.; Xia, W. Biosynthesis of acetate esters by dominate strains, isolated from Chinese traditional fermented fish (Suan yu). Food Chem. 2018, 244, 44–49. [Google Scholar] [CrossRef]
- Xu, Y.; Li, L.; Regenstein, J.M.; Gao, P.; Zang, J.; Xia, W.; Jiang, Q. The contribution of autochthonous microflora on free fatty acids release and flavor development in low-salt fermented fish. Food Chem. 2018, 256, 259–267. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Y.; Wang, Y.; Li, L.; Yang, X.; Chen, S.; Zhao, Y.; Zhou, W. Microbial community changes induced by Pediococcus pentosaceus improve the physicochemical properties and safety in fermented tilapia sausage. Food Res. Int. 2021, 147, 110476. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Y.; Shao, Y.; Chen, W.; Chen, F.; Li, M. Cloning, expression and characterization of a novel cold-active and organic solvent-tolerant esterase from Monascus ruber M7. Extremophiles 2016, 20, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, J.; Liu, X.; Zhang, C.; Zhao, Z.; Li, X.; Sun, B. Flavor mystery of Chinese traditional fermented baijiu: The great contribution of ester compounds. Food Chem. 2022, 369, 130920. [Google Scholar] [CrossRef] [PubMed]
- Salgado, C.A.; dos Santos, C.I.A.; Vanetti, M.C.D. Microbial lipases: Propitious biocatalysts for the food industry. Food Biosci. 2022, 45, 101509. [Google Scholar] [CrossRef]
- Miguel-Ruano, V.; Rivera, I.; Rajkovic, J.; Knapik, K.; Torrado, A.; Otero, J.M.; Beneventi, E.; Becerra, M.; ánchez-Costa, M.; Hidalgo, A.; et al. Biochemical and Structural Characterization of a novel thermophilic esterase EstD11 provide catalytic insights for the HSL family. Comput. Struct. Biotechnol. J. 2021, 19, 1214–1232. [Google Scholar] [CrossRef] [PubMed]
- Bassegoda, A.; Pastor, F.I.J.; Diaz, P. Rhodococcus sp Strain CR-53 LipR, the First Member of a New Bacterial Lipase Family (Family X) Displaying an Unusual Y-Type Oxyanion Hole, Similar to the Candida antarctica Lipase Clan. Appl. Environ. Microbiol. 2012, 78, 1724–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speranza, B.; Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. A possible approach to assess acidification of meat starter cultures: A case study from some wild strains of Lactobacillus plantarum. J. Sci. Food Agric. 2017, 97, 2961–2968. [Google Scholar] [CrossRef]
- Saravanan, K.A.; Panigrahi, M.; Kumar, H.; Rajawat, D.; Nayak, S.S.; Bhushan, B.; Dutt, T. Role of genomics in combating COVID-19 pandemic. Gene 2022, 823, 146387. [Google Scholar] [CrossRef]
- Randhawa, S.S.; Pawar, R. Fish genomes and their evolution under the influence of ecology. Ecol. Complex. 2022, 49, 100980. [Google Scholar] [CrossRef]
- Scherlach, K.; Hertweck, C. Mining and unearthing hidden biosynthetic potential. Nat. Commun. 2021, 12, 3864. [Google Scholar] [CrossRef]
- Tepkasikul, P.; Santiyanont, P.; Booncharoen, A.; Abhisingha, M.; Mhuantong, W.; Chantarasakha, K.; Pitaksutheepong, C.; Visessanguan, W.; Tepaamorndech, S. The functional starter and its genomic insight for histamine degradation in fish sauce. Food Microbiol. 2022, 104, 103988. [Google Scholar] [CrossRef]
- Huang, X.; Gu, Y.; Zhou, H.; Xu, L.; Cao, H.; Gai, C. Acinetobacter venetianus, a potential pathogen of red leg disease in freshwater-cultured whiteleg shrimp Penaeus vannamei. Aquac. Rep. 2020, 18, 100543. [Google Scholar] [CrossRef]
- Ardö, Y. Flavour formation by amino acid catabolism. Biotechnol. Adv. 2006, 24, 238–242. [Google Scholar] [CrossRef]
- Tang, H.; Wang, H.; Wu, H.; Deng, J.; Liu, Y.; Wang, Y. Screening, Identification and Characterization of Aroma-Producing and Salt-Tolerant Yeast Strains from South Sichuan, China. Food Sci. 2020, 41, 12. [Google Scholar]
- Lu, Y.; Liang, H.; Chen, P.; Liu, Z.; Zhong, C. Screening and Characteristic Aroma Analysis of Aroma-producing Yeasts in High-temperature Daq. Food Res. Dev. 2021, 42, 11. [Google Scholar]
- Maruthupandy, M.; Rajivgandhi, G.; Muneeswaran, T.; Song, J.-M.; Manoharan, N. Biologically synthesized zinc oxide nanoparticles as nanoantibiotics against ESBLs producing gram negative bacteria. Microb. Pathog. 2018, 121, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Sarwar, A.; Naveed, M.; Shahzad, M.; Aqib Shabbir, M.; Dablool, A.S.; ud Din, J.; Ali Khan, A.; Naz, S.; Cui, H.; et al. Bio-Molecular analysis of selected food derived Lactiplantibacillus strains for CLA production reveals possibly a complex mechanism. Food Res. Int. 2022, 154, 111031. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Liu, B.; Xie, Y.; Li, Z.; Huang, W.; Yuan, J.; He, G.; Chen, Y.; Pan, Q.; Liu, Y.; et al. SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler. GigaScience 2012, 1, 2047–2217X-1-18. [Google Scholar] [CrossRef]
- Krawczyk, P.S.; Lipinski, L.; Dziembowski, A. PlasFlow: Predicting plasmid sequences in metagenomic data using genome signatures. Nucleic Acids Res. 2018, 46, 14. [Google Scholar] [CrossRef] [Green Version]
- Delcher, A.L.; Bratke, K.A.; Powers, E.C.; Salzberg, S.L. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 2007, 23, 673–679. [Google Scholar] [CrossRef]
- Lowe, T.M.; Chan, P.P. tRNAscan-SE On-line: Integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef]
- He, X.; Lu, T.; Zhou, X. Whole genome sequencing and comparative genomics analysis of Pectobacterium carotovorum identifies key pathogenic genes. Mol. Phylogenet. Evol. 2021, 162, 107114. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [Green Version]
- Albayati, S.H.; Masomian, M.; Ishak, S.N.; Mohamad Ali, M.S.; Thean, A.L.; Mohd Shariff, F.B.; Muhd Noor, N.D.; Raja Abd Rahman, R.N. Main Structural Targets for Engineering Lipase Substrate Specificity. Catalysts 2020, 10, 747. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, B.; Lan, Y.; Ma, T. Enhanced degradation of different crude oils by defined engineered consortia of Acinetobacter venetianus RAG-1 mutants based on their alkane metabolism. Bioresour. Technol. 2021, 327, 124787. [Google Scholar] [CrossRef]
- Tian, X.; Gao, P.; Xu, Y.; Xia, W.; Jiang, Q. Reduction of biogenic amines accumulation with improved flavor of low-salt fermented bream (Parabramis pekinensis) by two-stage fermentation with different temperature. Food Biosci. 2021, 44, 101438. [Google Scholar] [CrossRef]
- Banoth, L.; Devarapalli, K.; Paul, I.; Thete, K.N.; Pawar, S.V.; Chand Banerjee, U. Screening, isolation and selection of a potent lipase producing microorganism and its use in the kinetic resolution of drug intermediates. J. Indian Chem. Soc. 2021, 98, 100143. [Google Scholar] [CrossRef]
- Bharathi, D.; Rajalakshmi, G. Microbial lipases: An overview of screening, production and purification. Biocatal. Agric. Biotechnol. 2019, 22, 101368. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Esmail, G.A.; Ghilan, A.-K.M.; Arasu, M.V. Isolation and screening of Streptomyces sp. Al-Dhabi-49 from the environment of Saudi Arabia with concomitant production of lipase and protease in submerged fermentation. Saudi J. Biol. Sci. 2020, 27, 474–479. [Google Scholar] [CrossRef]
- Moonga, H.B.; Schoustra, S.E.; Linnemann, A.R.; van den Heuvel, J.; Shindano, J.; Smid, E.J. Influence of fermentation temperature on microbial community composition and physicochemical properties of mabisi, a traditionally fermented milk. LWT 2021, 136, 110350. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Ren, J.; Gu, P.; Li, T.; Wu, Y.; Zhang, B.; Zhu, B. Comparison on evolution of volatile compounds and aroma attributes in different pH-adjusted fermented bog bilberry syrup wines during bottle-aging period. Food Biosci. 2018, 22, 121–128. [Google Scholar] [CrossRef]
- Mojica, F.J.M.; Rodriguez-Valera, F. The discovery of CRISPR in archaea and bacteria. FEBS J. 2016, 283, 3162–3169. [Google Scholar] [CrossRef] [PubMed]
- Bland, C.; Ramsey, T.L.; Sabree, F.; Lowe, M.; Brown, K.; Kyrpides, N.C.; Hugenholtz, P. CRISPR Recognition Tool (CRT): A tool for automatic detection of clustered regularly interspaced palindromic repeats. BMC Bioinform. 2007, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katayama, T.; Maruyama, J.-i. CRISPR/Cpf1-mediated mutagenesis and gene deletion in industrial filamentous fungi Aspergillus oryzae and Aspergillus sojae. J. Biosci. Bioeng. 2022, 133, 353–361. [Google Scholar] [CrossRef]
- Salazar-Cerezo, S.; Kun, R.S.; de Vries, R.P.; Garrigues, S. CRISPR/Cas9 technology enables the development of the filamentous ascomycete fungus Penicillium subrubescens as a new industrial enzyme producer. Enzyme Microb. Technol. 2020, 133, 109463. [Google Scholar] [CrossRef]
- Wei, M.; Dhanasekaran, S.; Godana, E.A.; Yang, Q.; Sui, Y.; Zhang, X.; Ngolong Ngea, G.L.; Zhang, H. Whole-genome sequencing of Cryptococcus podzolicus Y3 and data-independent acquisition-based proteomic analysis during OTA degradation. Food Control 2022, 136, 108862. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Liu, D.-M.; Jia, X.-Z.; Liang, M.-H.; Lu, Y.; Liu, J. Whole genome sequencing of Lactobacillus plantarum DMDL 9010 and its effect on growth phenotype under nitrite stress. LWT 2021, 149, 111778. [Google Scholar] [CrossRef]
- Kumaran, D.; Bonanno, J.B.; Burley, S.K.; Swaminathan, S. Crystal structure of phosphatidylglycerophosphatase (PGPase), a putative membrane-bound lipid phosphatase, reveals a novel binuclear metal binding site and two “proton wires”. Proteins Struct. Funct. Bioinform. 2006, 64, 851–862. [Google Scholar] [CrossRef]
- Xiang, M.; Wang, L.; Yan, Q.; Jiang, Z.; Yang, S. High-level expression and characterization of a novel phospholipase C from Thielavia terrestris suitable for oil degumming. Int. J. Biol. Macromol. 2020, 156, 740–748. [Google Scholar] [CrossRef]
- Borrelli, G.M.; Trono, D. Recombinant Lipases and Phospholipases and Their Use as Biocatalysts for Industrial Applications. Int. J. Mol. Sci. 2015, 16, 20774–20840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rupwate, S.D.; Rupwate, P.S.; Rajasekharan, R. Regulation of lipid biosynthesis by phosphatidylinositol-specific phospholipase C through the transcriptional repression of upstream activating sequence inositol containing genes. FEBS Lett. 2012, 586, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, L.; Shi, G. Secretory expression of a phospholipase A2 from Lactobacillus casei DSM20011 in Kluyveromyces lactis. J. Biosci. Bioeng. 2015, 120, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Cerminati, S.; Paoletti, L.; Aguirre, A.; Peiru, S.; Menzella, H.G.; Castelli, M.E. Industrial uses of phospholipases: Current state and future applications. Appl. Microbiol. Biotechnol. 2019, 103, 2571–2582. [Google Scholar] [CrossRef]
- Kashyap, A.; Gupta, R. Disrupting putative N-glycosylation site N17 in lipase Lip11 of Yarrowia lipolytica yielded a catalytically efficient and thermostable variant accompanying conformational changes. Enzyme Microb. Technol. 2021, 151, 109922. [Google Scholar] [CrossRef]
- Pearce, R.; Zhang, Y. Deep learning techniques have significantly impacted protein structure prediction and protein design. Curr. Opin. Struct. Biol. 2021, 68, 194–207. [Google Scholar]
- Yoo, P.D.; Zhou, B.B.; Zomaya, A.Y. Machine learning techniques for protein secondary structure prediction: An overview and evaluation. Curr. Bioinform. 2008, 3, 74–86. [Google Scholar] [CrossRef]

| Type | Total Number | Average Length (bp) | Total Length (bp) | GC Content (%) |
|---|---|---|---|---|
| Gene | 3150 | 943.93 | 2,973,366 | 40.09% |
| tRNA | 73 | 76.835 | 5609 | - |
| 5s_rRNA | 6 | 109 | 654 | - |
| 16s_rRNA | 6 | 1531.833 | 9191 | - |
| 23s_rRNA | 6 | 2888 | 17,328 | - |
| sRNA | 17 | 132.647 | 2255 | - |
| Location | CRISPR ID | Start | End | Duplicate Sequences Average Length (bp) | Duplicate Sequences Average Length (bp) | Spaced Sequences Average Length (bp) |
|---|---|---|---|---|---|---|
| Chromosome | CRISPR1 | 378,376 | 378,604 | 4 | 31 | 35 |
| Chromosome | CRISPR2 | 438,986 | 440,099 | 20 | 31 | 26 |
| Chromosome | CRISPR3 | 441,911 | 442,682 | 14 | 31 | 26 |
| Chromosome | CRISPR4 | 2,033,076 | 2,033,818 | 11 | 23 | 49 |
| Chromosome | CRISPR5 | 2,034,295 | 2,034,592 | 4 | 46 | 38 |
| Chromosome | CRISPR6 | 2,038,236 | 2,039,478 | 15 | 28 | 58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wu, Y.; Wang, Y. Whole-Genome Sequencing of a Potential Ester-Synthesizing Bacterium Isolated from Fermented Golden Pomfret and Identification of Its Lipase Encoding Genes. Foods 2022, 11, 1954. https://doi.org/10.3390/foods11131954
Wang H, Wu Y, Wang Y. Whole-Genome Sequencing of a Potential Ester-Synthesizing Bacterium Isolated from Fermented Golden Pomfret and Identification of Its Lipase Encoding Genes. Foods. 2022; 11(13):1954. https://doi.org/10.3390/foods11131954
Chicago/Turabian StyleWang, Huifang, Yanyan Wu, and Yueqi Wang. 2022. "Whole-Genome Sequencing of a Potential Ester-Synthesizing Bacterium Isolated from Fermented Golden Pomfret and Identification of Its Lipase Encoding Genes" Foods 11, no. 13: 1954. https://doi.org/10.3390/foods11131954
APA StyleWang, H., Wu, Y., & Wang, Y. (2022). Whole-Genome Sequencing of a Potential Ester-Synthesizing Bacterium Isolated from Fermented Golden Pomfret and Identification of Its Lipase Encoding Genes. Foods, 11(13), 1954. https://doi.org/10.3390/foods11131954






