Large-Scale, High-Throughput Phenotyping of the Postharvest Storage Performance of ‘Rustenburg’ Navel Oranges and the Development of Shelf-Life Prediction Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Postharvest Storage Conditions
2.3. Evaluations of Fruit Quality
2.3.1. Firmness
2.3.2. Weight Loss
2.3.3. Peel Color
2.3.4. Peel Damage, Decay and Internal Dryness
2.3.5. Total Soluble Solids (TSS) and Titratable Acidity (TA)
2.3.6. Vitamin C
2.3.7. Ethanol Levels
2.3.8. Flavor
2.3.9. Acceptance Scores
2.4. Statistical Analysis
2.5. Quality-Prediction Models
2.5.1. Data-Set Preparation
2.5.2. Prediction Models
2.5.3. Evaluation of the Models
2.5.4. Duplication as a Way to Deal with Unbalanced Data Sets
3. Results
3.1. Effects of Pre-Harvest and Postharvest Features on the Quality of ‘Rustenburg’ Navel Oranges
3.2. Quality-Prediction Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fruit: World Production by Type 2020|Statista, (n.d.). Available online: https://www.statista.com/statistics/264001/worldwide-production-of-fruit-by-variety/ (accessed on 19 May 2022).
- Ranganna, S.; Govindarajan, V.S.; Ramana, K.V.R.; Kefford, J.F. Citrus fruits–Varieties, chemistry, technology, and quality evaluation. Part II. Chemistry, technology, and quality evaluation. A. Chemistry. Crit. Rev. Food Sci. Nutr. 1983, 18, 313–386. [Google Scholar] [CrossRef] [PubMed]
- Marloth, R.H.; Basson, W.J. Relative Performance of Washington Navel Orange Selections and other Navel Varieties. J. Hortic. Sci. 1959, 34, 133–141. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Kan, C.; Chen, M.; Chen, J. Changes of peel color and fruit quality in navel orange fruits under different storage methods. Sci. Hortic. 2019, 256, 108522. [Google Scholar] [CrossRef]
- Kader, A.A. A Perspective on Postharvest Horticulture (1978–2003). HortScience 2003, 38, 1004–1008. [Google Scholar] [CrossRef] [Green Version]
- Arpaia, M. Preharvest factors influencing postharvest quality of tropical and subtropical fruit. HortScience 1994, 29, 982–985. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, S.; Sahay, S.; Imran, M.; Rashmi, K.; Mahesh, S.S. Pre-harvest Factors Influencing the Postharvest Quality of Fruits: A Review. Curr. J. Appl. Sci. Technol. 2017, 23, 1–12. [Google Scholar] [CrossRef]
- Tadeo, J.L.; Ortiz, J.M.; Estellés, A. Sugar changes in Clementine and orange fruit during ripening. J. Hortic. Sci. 1987, 62, 531–537. [Google Scholar] [CrossRef]
- Schirra, M.; D’Hallewin, G.; Cabras, P.; Angioni, A.; Garau, V.L. Seasonal Susceptibility of Tarocco Oranges to Chilling Injury As Affected by Hot Water and Thiabendazole Postharvest Dip Treatments. J. Agric. Food Chem. 1998, 46, 1177–1180. [Google Scholar] [CrossRef]
- Alferez, F.; Zacarías, L. Influence of fruit maturity in the susceptibility of Navelina oranges to develop postharvest non-chilling peel pitting. Food Sci. Technol. Int. 2014, 20, 183–191. [Google Scholar] [CrossRef]
- Khalid, S.; Malik, A.U.; Khan, A.S.; Khan, M.N.; Ullah, M.I.; Abbas, T.; Khalid, M.S. Tree age and fruit size in relation to postharvest respiration and quality changes in ‘Kinnow’ mandarin fruit under ambient storage. Sci. Hortic. 2017, 220, 183–192. [Google Scholar] [CrossRef]
- Kader, A.A.; Arpaia, M.L. Postharvest handling systems: Subtropical fruit. In Postharvest Technology of Horticultural Crops, 3rd ed.; University of California, Agriculture and Natural Resources: Oakland, CA, USA, 2002; pp. 375–384. [Google Scholar]
- Paull, R. Effect of temperature and relative humidity on fresh commodity quality. Postharvest Biol. Technol. 1999, 15, 263–277. [Google Scholar] [CrossRef]
- Hertog, L.A.T.M.; Uysal, I.; McCarthy, U.; Verlinden, B.M.; Nicolaï, B.M. Shelf life modelling for first-expired-first-out warehouse management. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20130306. [Google Scholar] [CrossRef] [PubMed]
- Jedermann, R.; Nicometo, M.; Uysal, I.; Lang, W. Reducing food losses by intelligent food logistics. Philos. Trans. R. Soc. London. Ser. A: Math. Phys. Eng. Sci. 2014, 372, 20130302. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chu, X.; Fu, Z.; Feng, J.; Mu, W. Shelf life prediction model of postharvest table grape using optimized radial basis function (RBF) neural network. Br. Food J. 2019, 121, 2919–2936. [Google Scholar] [CrossRef]
- Song, Y.; Hu, Q.; Wu, Y.; Pei, F.; Kimatu, B.M.; Su, A.; Yang, W. Storage time assessment and shelf-life prediction models for postharvest Agaricus bisporus. LWT 2019, 101, 360–365. [Google Scholar] [CrossRef]
- Jalali, A.; Linke, M.; Geyer, M.; Mahajan, P.V. Shelf life prediction model for strawberry based on respiration and transpiration processes. Food Packag. Shelf Life 2020, 25, 100525. [Google Scholar] [CrossRef]
- Salehi, F. Recent Advances in the Modeling and Predicting Quality Parameters of Fruits and Vegetables during Postharvest Storage: A Review. Int. J. Fruit Sci. 2020, 20, 506–520. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Ahern, A.; Carbone, C.; Temko, A.; Claesson, M.J.; Gasbarrini, A.; Tortora, G. Gut microbiome, big data and machine learning to promote precision medicine for cancer. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 635–648. [Google Scholar] [CrossRef]
- Neethirajan, S. The role of sensors, big data and machine learning in modern animal farming. Sens. Bio-Sens. Res. 2020, 29, 100367. [Google Scholar] [CrossRef]
- La Scalia, G.; Nasca, A.; Corona, O.; Settanni, L.; Micale, R. An Innovative Shelf Life Model Based on Smart Logistic Unit for an Efficient Management of the Perishable Food Supply Chain. J. Food Process Eng. 2017, 40, e12311. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Dukovska-Popovska, I.; Subramanian, N.; Chan, H.K.; Bai, R. Decision-making in cold chain logistics using data analytics: A literature review. Int. J. Logist. Manag. 2018, 29, 839–861. [Google Scholar] [CrossRef] [Green Version]
- Hiromi, K.; Kuwamoto, C.; Ohnishi, M. A rapid sensitive method for the determination of ascorbic acid in the excess of 2,6-dichlorophenolindophenol using a stopped-flow apparatus. Anal. Biochem. 1980, 101, 421–426. [Google Scholar] [CrossRef]
- Davis, P.L.; Chace, W.G. Determination of alcohol in citrus juice by gas chromatographic analysis of headspace. Hortscience 1969, 2, 168–169. [Google Scholar]
- Lundberg, S.M.; Lee, S.U. A unified approach to interpreting model predictions. Adv. Neural. Info Process. Syst. 2017, 30, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Louppe, G.; Wehenkel, L.; Sutera, A.; Geurts, P. Understanding variable importances in Forests of randomized trees. Adv. Neural Inf. Process. Syst. 2013, 26, 431–439. [Google Scholar]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Lewis-Beck, C.; Lewis-Beck, M. Applied Regression: An Introduction; Sage Publications: Thousand Oaks, CA, USA, 2015. [Google Scholar]
- Drucker, H.; Surges, C.J.C.; Kaufman, L.; Smola, A.; Vapnik, V. Support vector regression machines. Adv. Neural Info Process Syst. 1997, 1, 155–161. [Google Scholar]
- Awad, M.; Khanna, R. Support vector regression. In Efficient Learning Machines; Apress; Springer: New York, NY, USA, 2015; pp. 67–80. [Google Scholar] [CrossRef] [Green Version]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Chapman & Hall/CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Breiman, L. Bagging predictors. Mach. Learn. 1996, 24, 123–140. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Polikar, R. Ensemble based systems in decision making. IEEE Circuits Syst. Mag. 2006, 6, 21–44. [Google Scholar] [CrossRef]
- Lin, Y.; Guan, Y.; Asudeh, A.; Jagadish, H.V.; Michigan, Y.L.U.; Michigan, Y.G.U.; Illinois, A.A.U.; Michigan, H.V.J.U. Identifying insufficient data coverage in databases with multiple relations. Proc. VLDB Endow. 2020, 13, 2229–2242. [Google Scholar] [CrossRef]
- Efron, B. Estimating the error rate of a prediction rule: Improvement on cross-validation. J. Amer. Statist. Assoc. 1983, 78, 316–331. [Google Scholar] [CrossRef]
- Casagrande, E.; Génard, M.; Lurol, S.; Charles, F.; Plénet, D.; Lescourret, F. A process-based model of nectarine quality development during pre- and post-harvest. Postharvest Biol. Technol. 2021, 175, 111458. [Google Scholar] [CrossRef]
- Amodio, M.L.; Derossi, A.; Mastrandrea, L.; Colelli, G. A study of the estimated shelf life of fresh rocket using a non-linear model. J. Food Eng. 2015, 150, 19–28. [Google Scholar] [CrossRef]
- Wang, W.; Hu, W.; Ding, T.; Ye, X.; Liu, D. Shelf-life prediction of strawberry at different temperatures during storage using kinetic analysis and model development. J. Food Process. Preserv. 2018, 42, e13693. [Google Scholar] [CrossRef]
- Niu, Y.; Yun, J.; Bi, Y.; Wang, T.; Zhang, Y.; Liu, H.; Zhao, F. Predicting the shelf life of postharvest Flammulina velutipes at various temperatures based on mushroom quality and specific spoilage organisms. Postharvest Biol. Technol. 2020, 167, 111235. [Google Scholar] [CrossRef]
- Ktenioudaki, A.; O’Donnell, C.P.; do Nascimento Nunes, M.C. Modelling the biochemical and sensory changes of strawberries during storage under diverse relative humidity conditions. Postharvest Biol. Technol. 2019, 154, 148–158. [Google Scholar] [CrossRef]
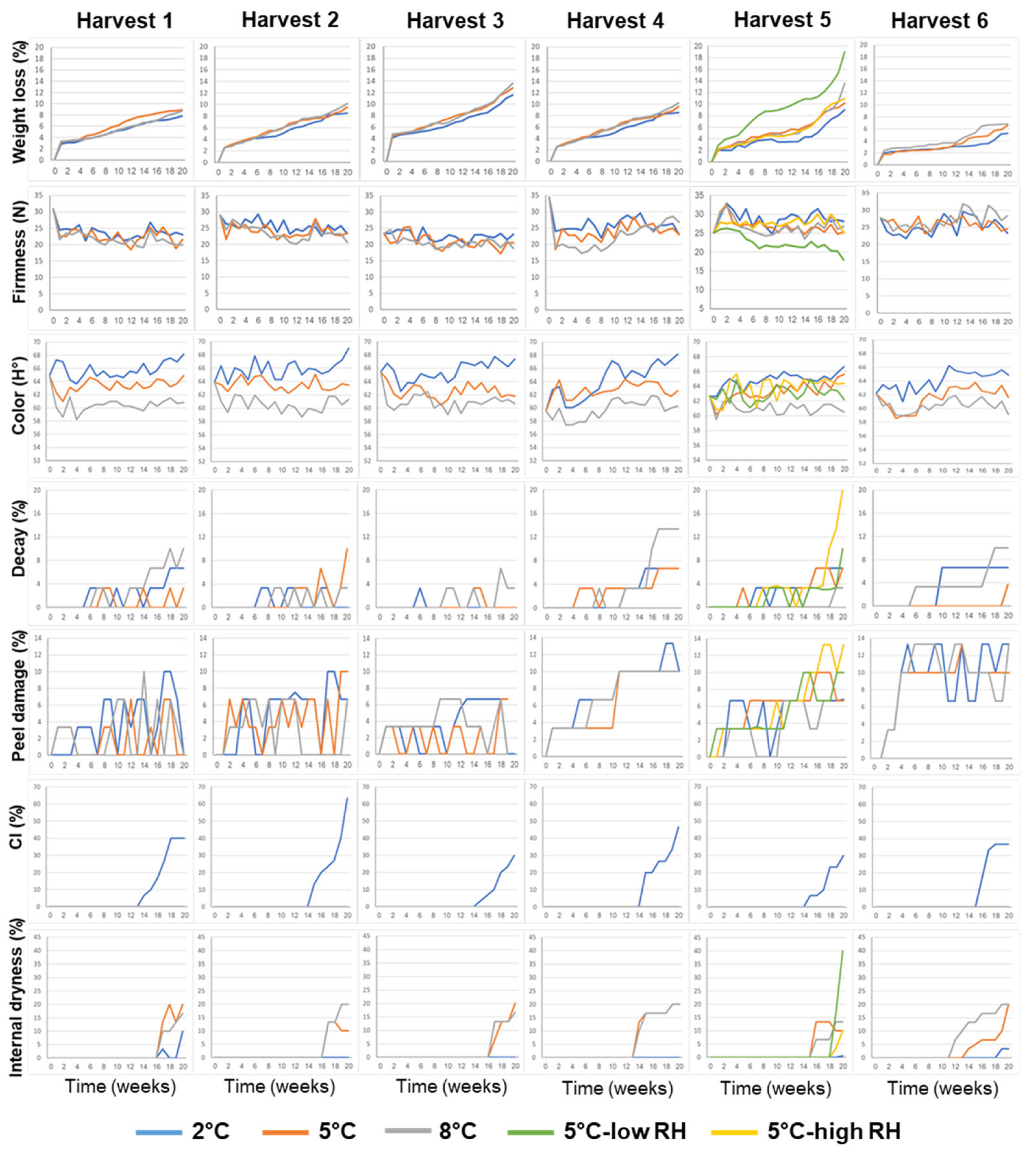
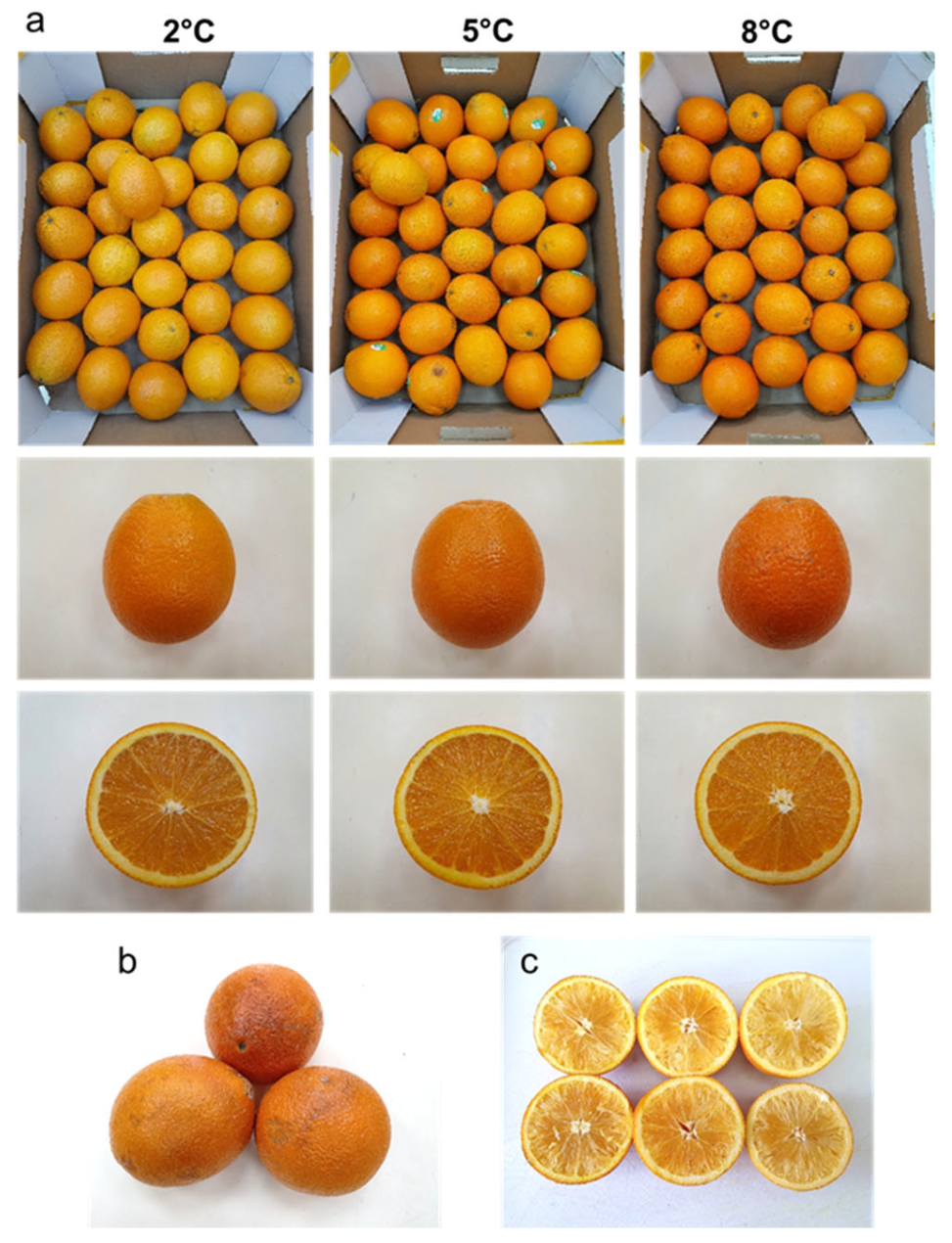
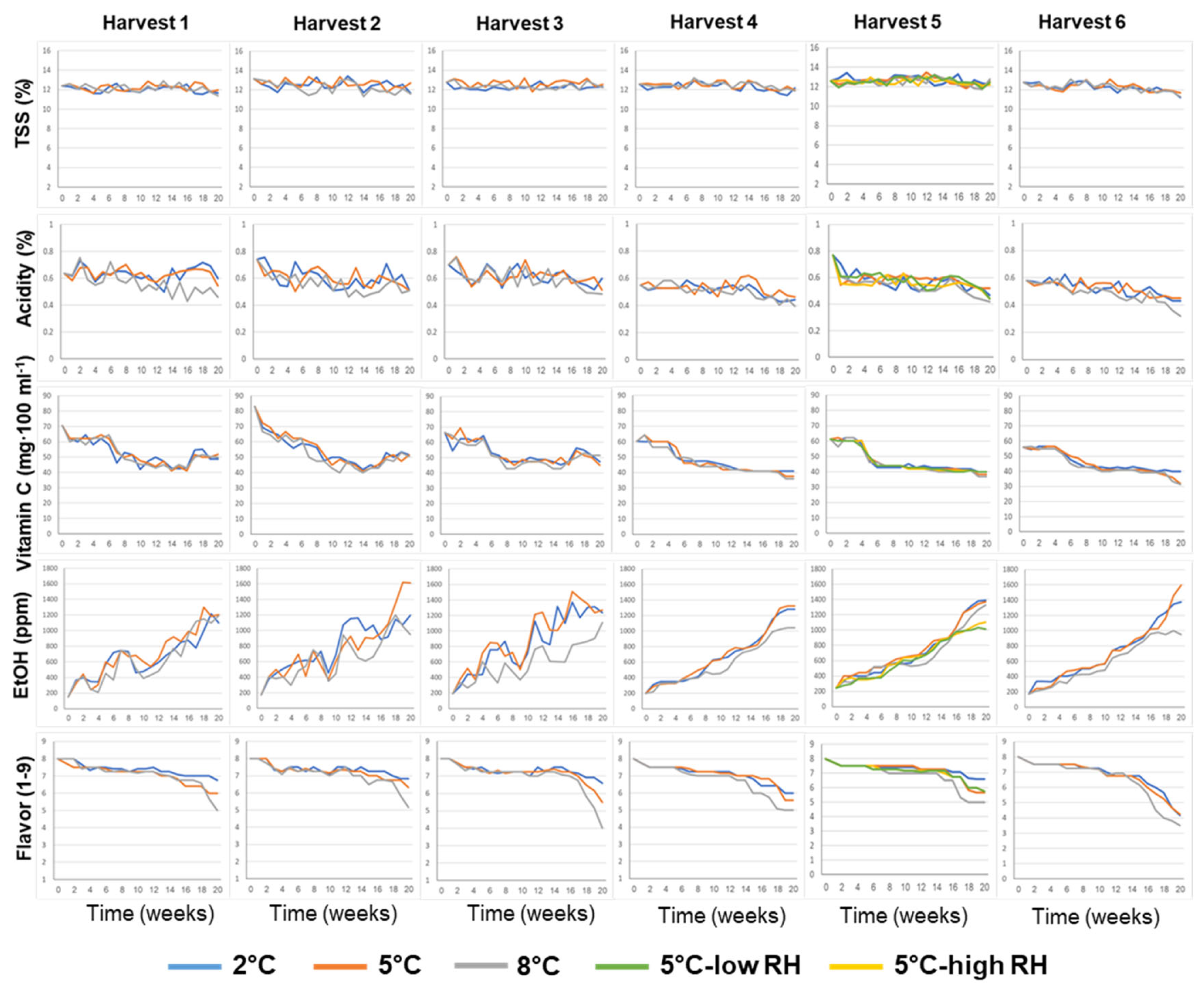
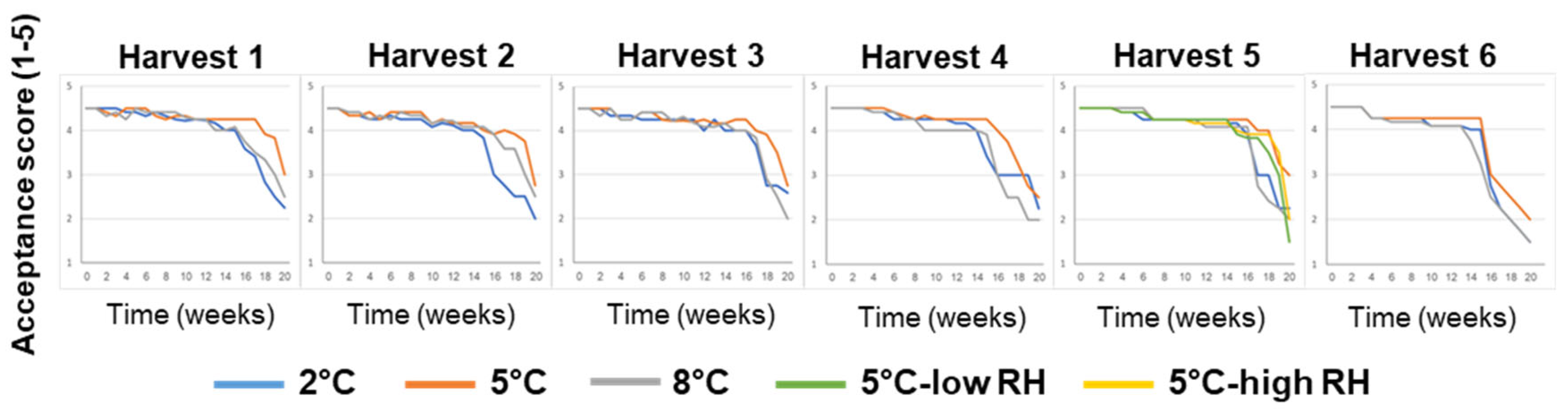
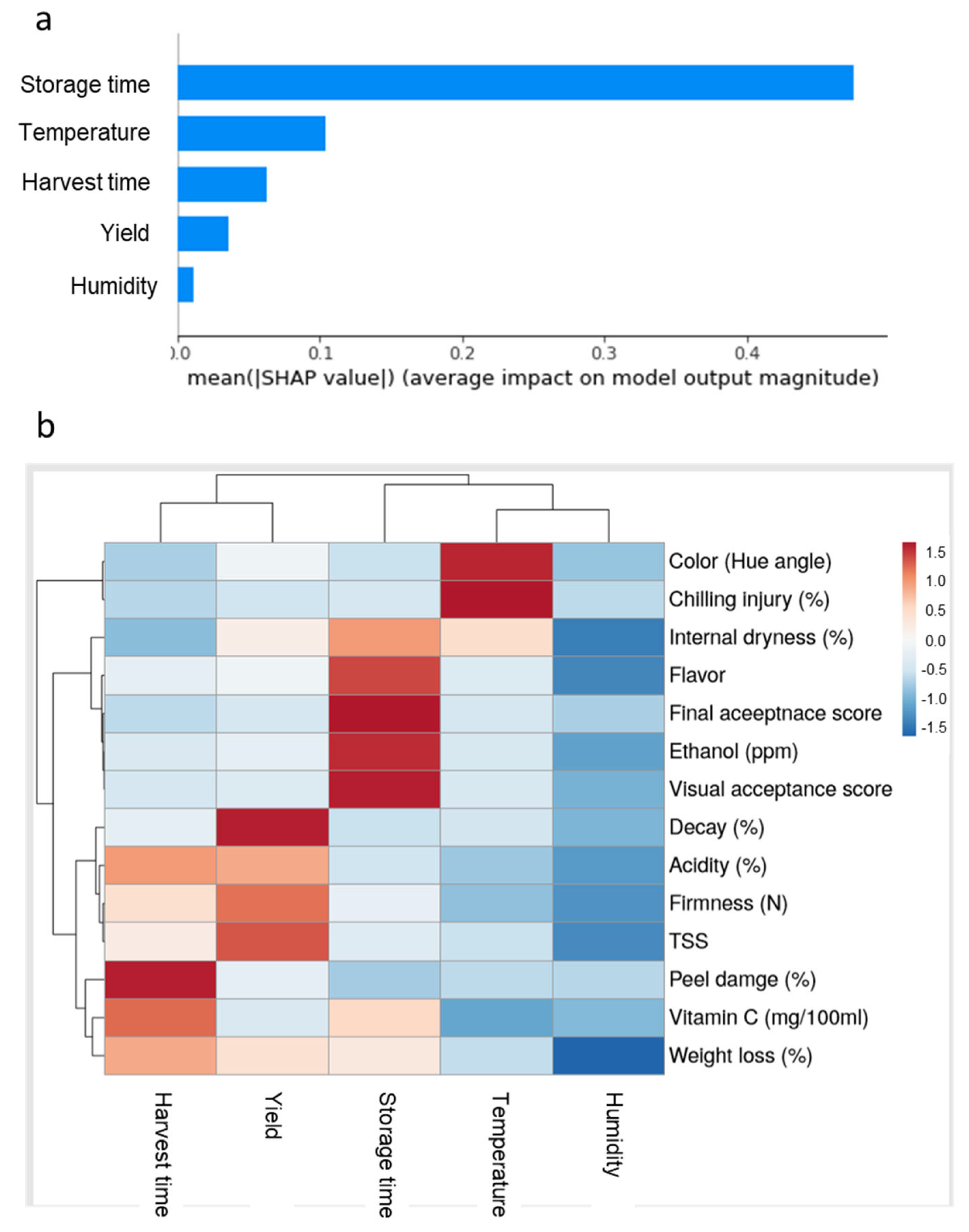
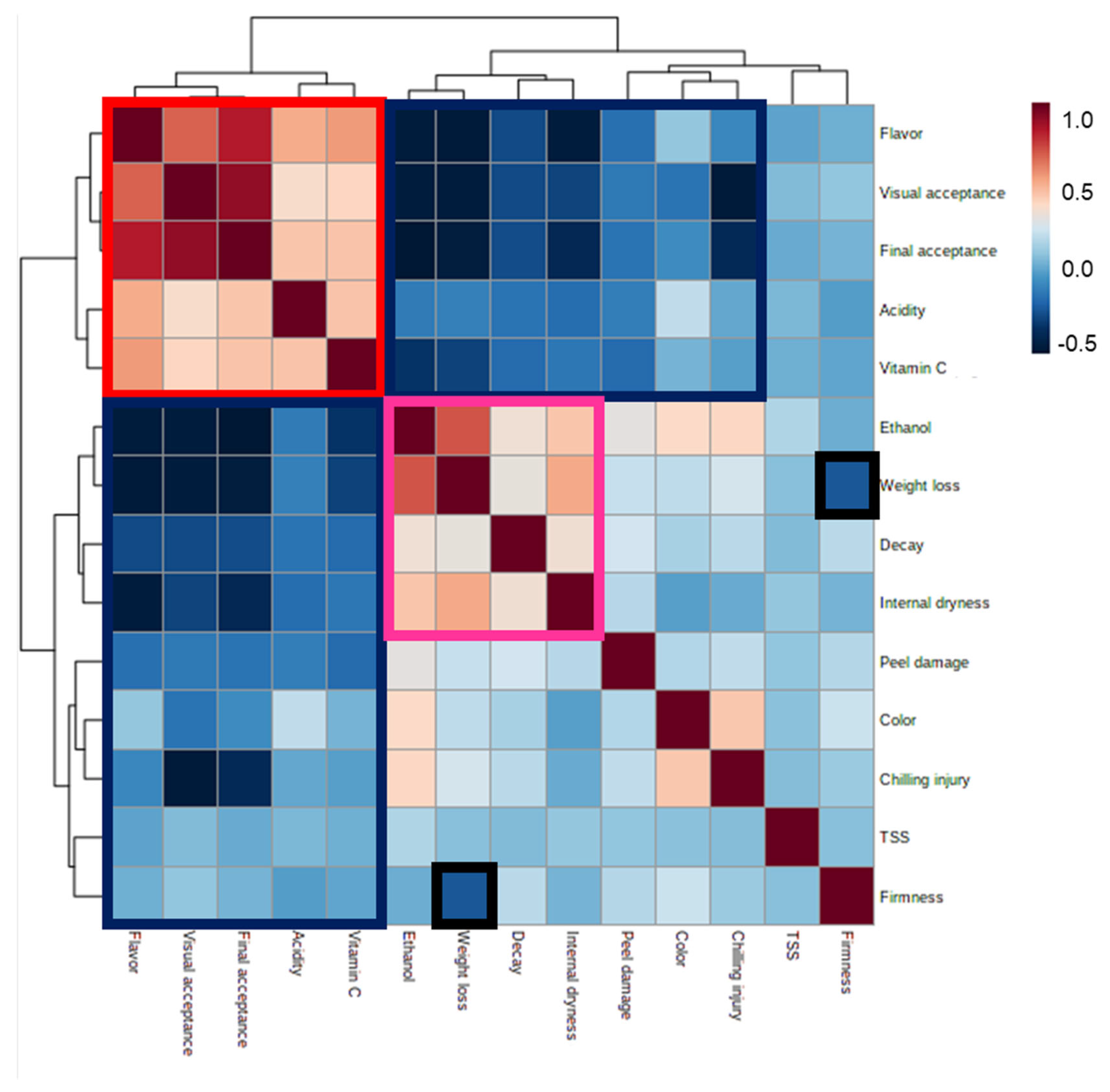
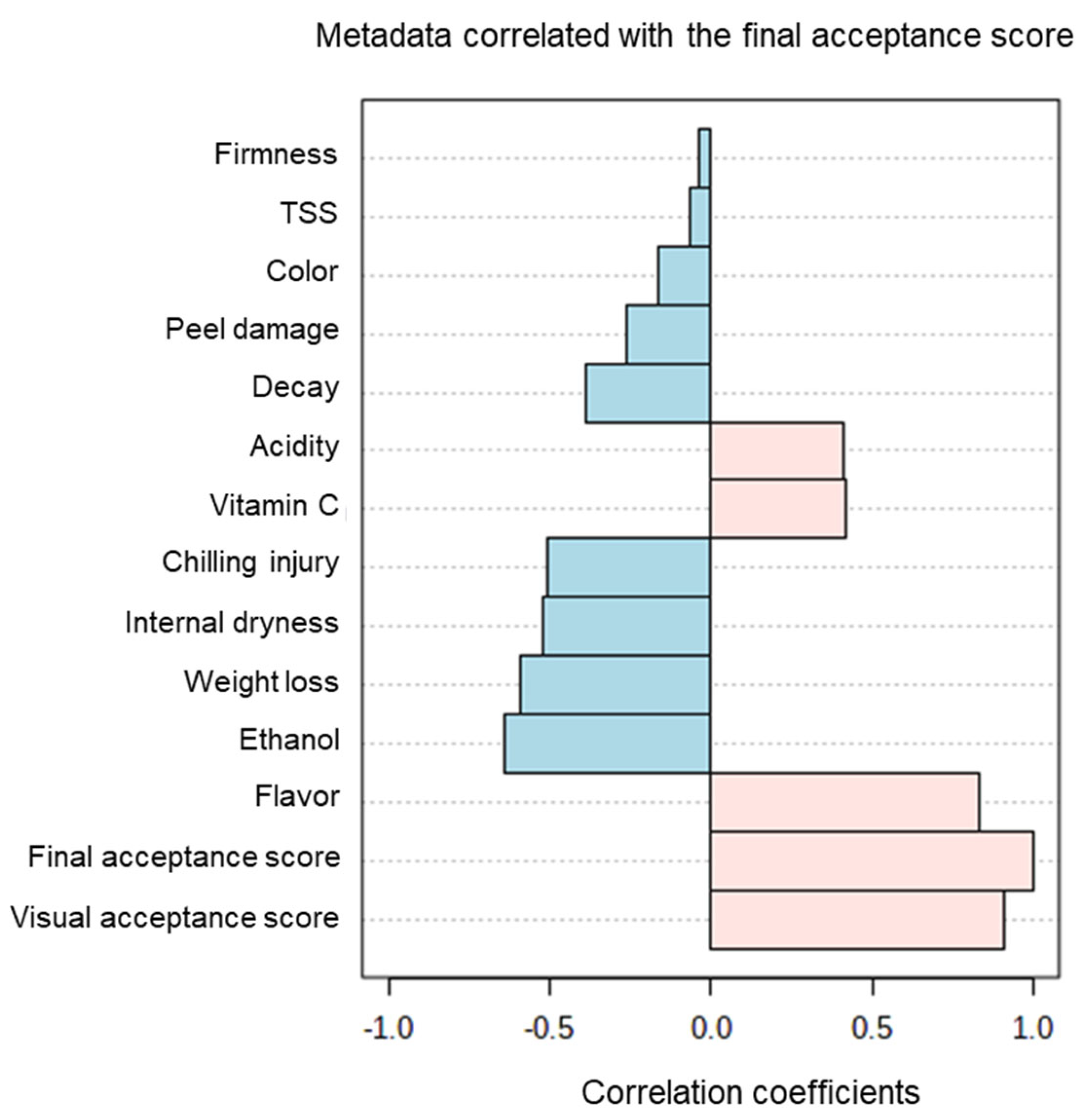
| Harvest Time (Weeks from Blooming) | Yield (Ton/Hectare) | |
|---|---|---|
| Harvest 1 (21 February 2021) | 48 | 48 |
| Harvest 2 (24 February 2021) | 48 | 44 |
| Harvest 3 (28 February 2021) | 49 | 18 |
| Harvest 4 (17 March 2021) | 51 | 35 |
| Harvest 5 (25 March 2021) | 52 | 18 |
| Harvest 6 (6 April 2021) | 54 | 33 |
| Harvest Time | Storage Time | Yield | Storage Temperature | Humidity | |
|---|---|---|---|---|---|
| Acidity | 6.55 × 1042 | 3.06 × 1033 | 2.01 × 1010 | 9.42 × 1015 | - |
| Vitamin C | 3.15 × 1044 | 4.20 × 10210 | 1.54 × 1013 | 7.17 × 1017 | 0.001 |
| Internal dryness | - | 6.98 × 10106 | 0.002 | 1.08 × 1013 | - |
| Decay | 4.86 × 106 | 2.97 × 1035 | 0.001 | 0.034 | 0.005 |
| Flavor | 7.92 × 1017 | 4.37 × 10268 | 3.55 × 105 | 3.08 × 1016 | - |
| Final acceptance score | 2.82 × 107 | 0 | 0.003 | 3.03 × 107 | - |
| Firmness | 7.56 × 1069 | 5.58 × 109 | - | 5.11 × 1016 | 2.07 × 1021 |
| Ethanol | 0.004 | 0 | 0.002 | 1.76 × 1017 | - |
| Visual acceptance score | - | 1.47 × 10279 | 0.035 | 1.09 × 1010 | - |
| Weight loss | 2.33 × 1027 | 4.82 × 10270 | 1.58 × 1016 | 3.77 × 1022 | 6.08 × 1012 |
| Peel damage | 3.24 × 1031 | 3.95 × 1016 | 8.33 × 106 | 3.28 × 106 | 0.019 |
| Hue angle | 3.47 × 108 | 4.15 × 1012 | 0.001 | 2.33 × 10238 | 0.006 |
| TSS | - | - | - | - | - |
| Chilling injury | - | 1.46 × 1044 | 0.030 | 5.12 × 1045 | 0.012 |
| Full Set | Low-Score Subset | |||
|---|---|---|---|---|
| Algorithm | RMSE | R2 | RMSE | R2 |
| MLR | 0.444 | 0.566 | 0.831 | −2.314 |
| SVR | 0.312 | 0.784 | 0.662 | −1.107 |
| RF | 0.200 | 0.910 | 0.404 | 0.217 |
| XGBoost | 0.195 | 0.914 | 0.380 | 0.305 |
| Full Set | Low-Score Subset | |||
|---|---|---|---|---|
| Number of Duplications | RMSE | R2 | RMSE | R2 |
| 0 | 0.195 | 0.914 | 0.380 | 0.305 |
| 1 | 0.201 | 0.907 | 0.358 | 0.385 |
| 2 | 0.206 | 0.903 | 0.344 | 0.433 |
| 3 | 0.210 | 0.898 | 0.337 | 0.456 |
| 4 | 0.214 | 0.894 | 0.333 | 0.469 |
| 5 | 0.217 | 0.891 | 0.329 | 0.479 |
| Full Set | Low-Score Subset | |||
|---|---|---|---|---|
| Subgroup | RMSE | R2 | RMSE | R2 |
| 1. Storage time | 0.371 | 0.687 | 0.473 | −0.074 |
| 2. Storage time + pre-harvest features (harvest time, yield) | 0.304 | 0.790 | 0.464 | −0.036 |
| 3. Storage time + postharvest features (temperature, humidity) | 0.340 | 0.739 | 0.535 | −0.376 |
| 4. Storage time + pre-harvest (harvest time, yield) + postharvest features (temperature, humidity) | 0.217 | 0.891 | 0.329 | 0.479 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owoyemi, A.; Porat, R.; Lichter, A.; Doron-Faigenboim, A.; Jovani, O.; Koenigstein, N.; Salzer, Y. Large-Scale, High-Throughput Phenotyping of the Postharvest Storage Performance of ‘Rustenburg’ Navel Oranges and the Development of Shelf-Life Prediction Models. Foods 2022, 11, 1840. https://doi.org/10.3390/foods11131840
Owoyemi A, Porat R, Lichter A, Doron-Faigenboim A, Jovani O, Koenigstein N, Salzer Y. Large-Scale, High-Throughput Phenotyping of the Postharvest Storage Performance of ‘Rustenburg’ Navel Oranges and the Development of Shelf-Life Prediction Models. Foods. 2022; 11(13):1840. https://doi.org/10.3390/foods11131840
Chicago/Turabian StyleOwoyemi, Abiola, Ron Porat, Amnon Lichter, Adi Doron-Faigenboim, Omri Jovani, Noam Koenigstein, and Yael Salzer. 2022. "Large-Scale, High-Throughput Phenotyping of the Postharvest Storage Performance of ‘Rustenburg’ Navel Oranges and the Development of Shelf-Life Prediction Models" Foods 11, no. 13: 1840. https://doi.org/10.3390/foods11131840
APA StyleOwoyemi, A., Porat, R., Lichter, A., Doron-Faigenboim, A., Jovani, O., Koenigstein, N., & Salzer, Y. (2022). Large-Scale, High-Throughput Phenotyping of the Postharvest Storage Performance of ‘Rustenburg’ Navel Oranges and the Development of Shelf-Life Prediction Models. Foods, 11(13), 1840. https://doi.org/10.3390/foods11131840








