Optimization of a Simultaneous Enzymatic Hydrolysis to Obtain a High-Glucose Slurry from Bread Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Bread
2.2. Enzymatic Hydrolysis
2.2.1. Enzymatic Sequential Hydrolysis: Liquefaction (LIQ)
2.2.2. Enzymatic Sequential Hydrolysis: Saccharification (SAC)
2.3. Enzymatic Simultaneous Hydrolysis (SH)
2.4. Analytical Methods
2.4.1. Determination of Reducing Sugars (RS)
2.4.2. Viscosity Analysis
2.4.3. Glucose Analysis (GL)
2.5. Modelling and Experimental Design
3. Results and Discussion
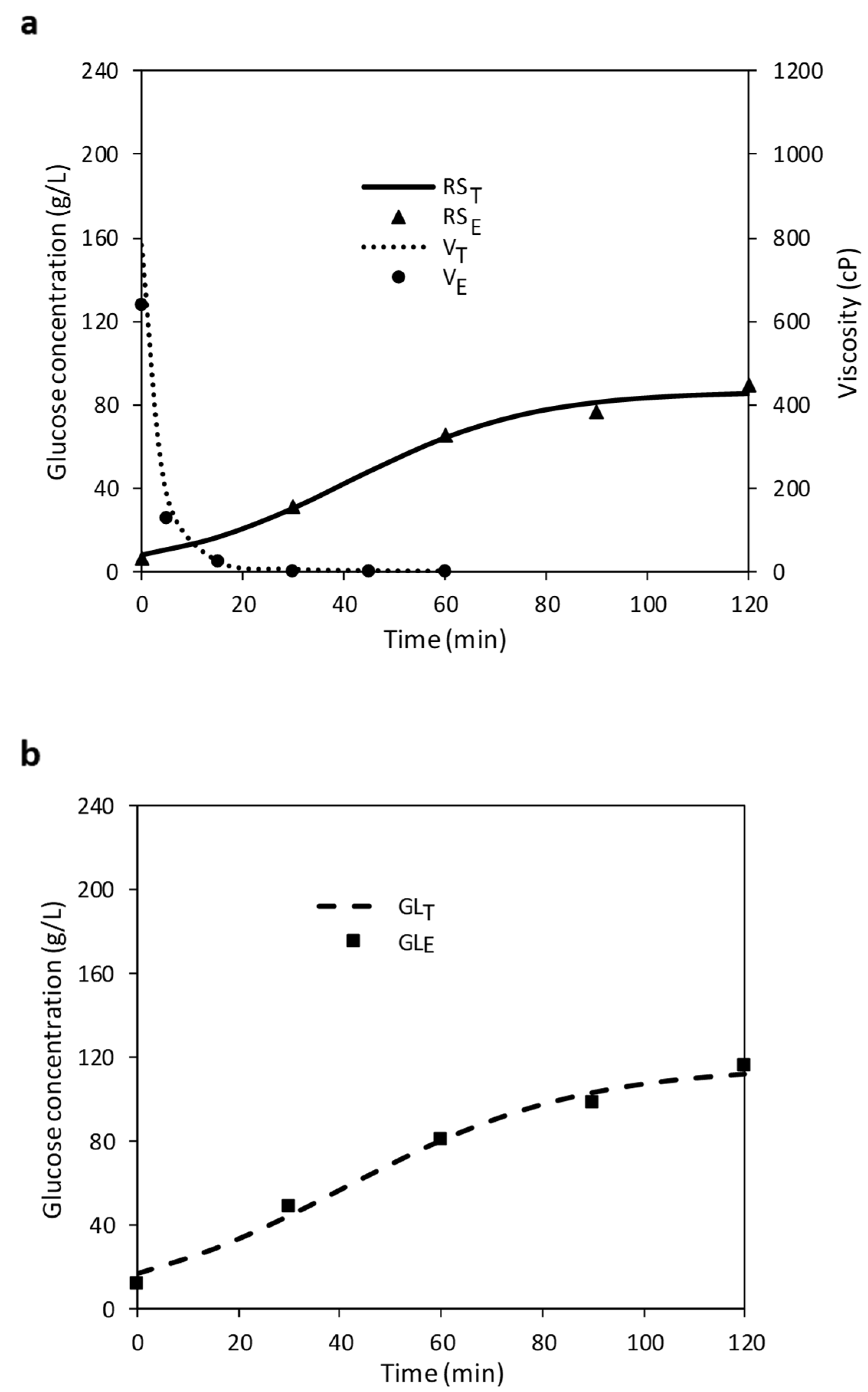
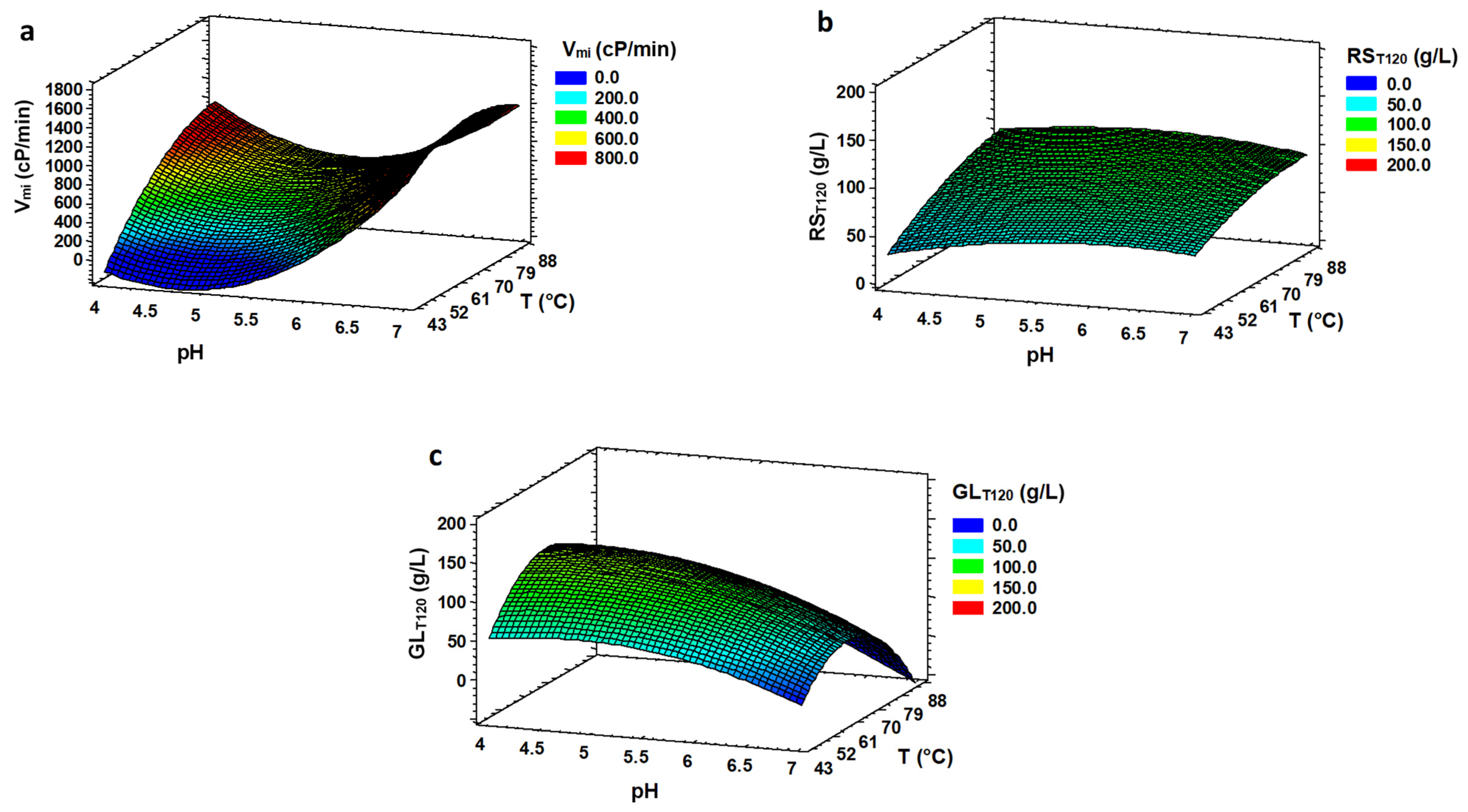
3.1. Optimization of Liquefaction (LIQ)
3.2. Optimization of Saccharification (SAC)
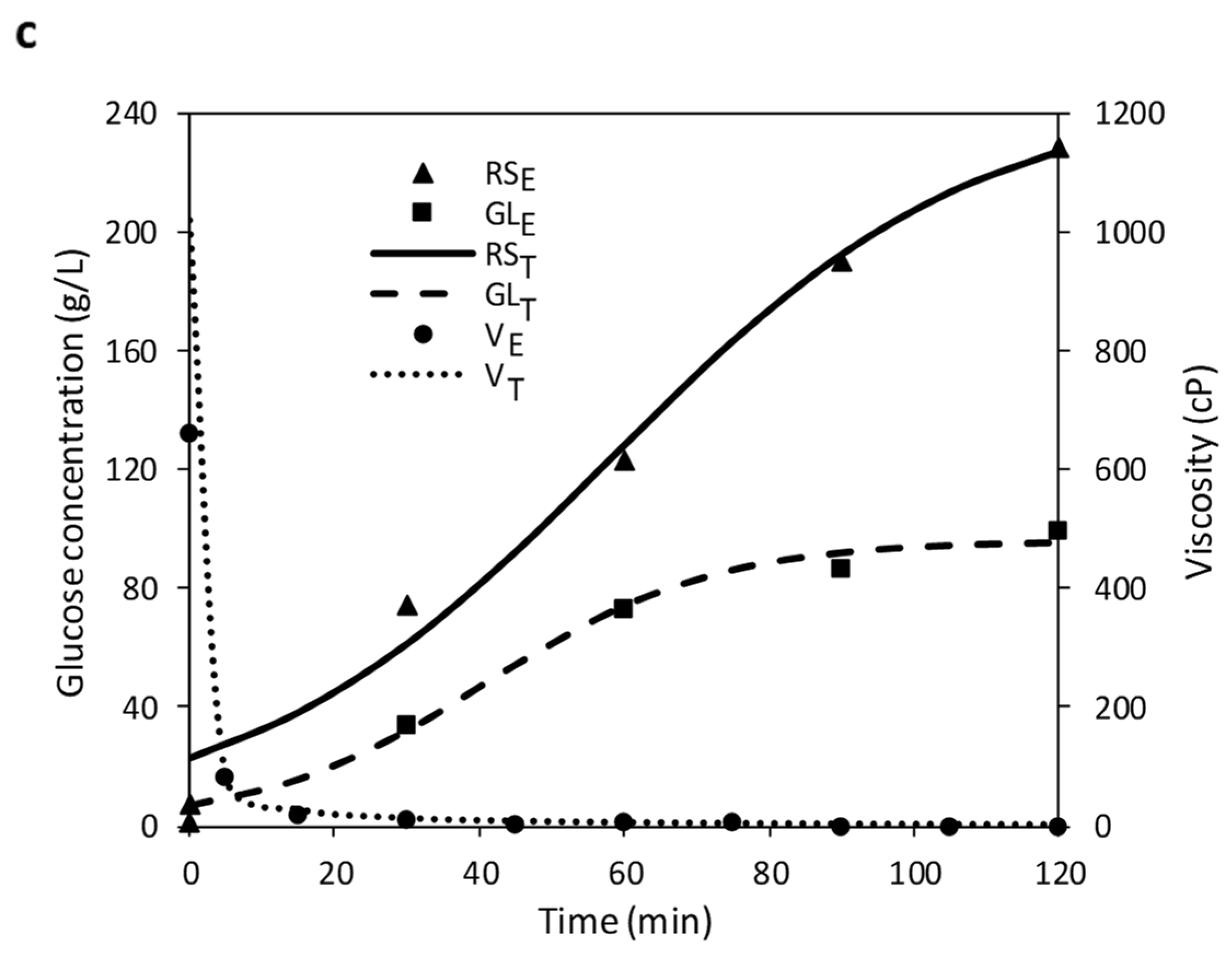
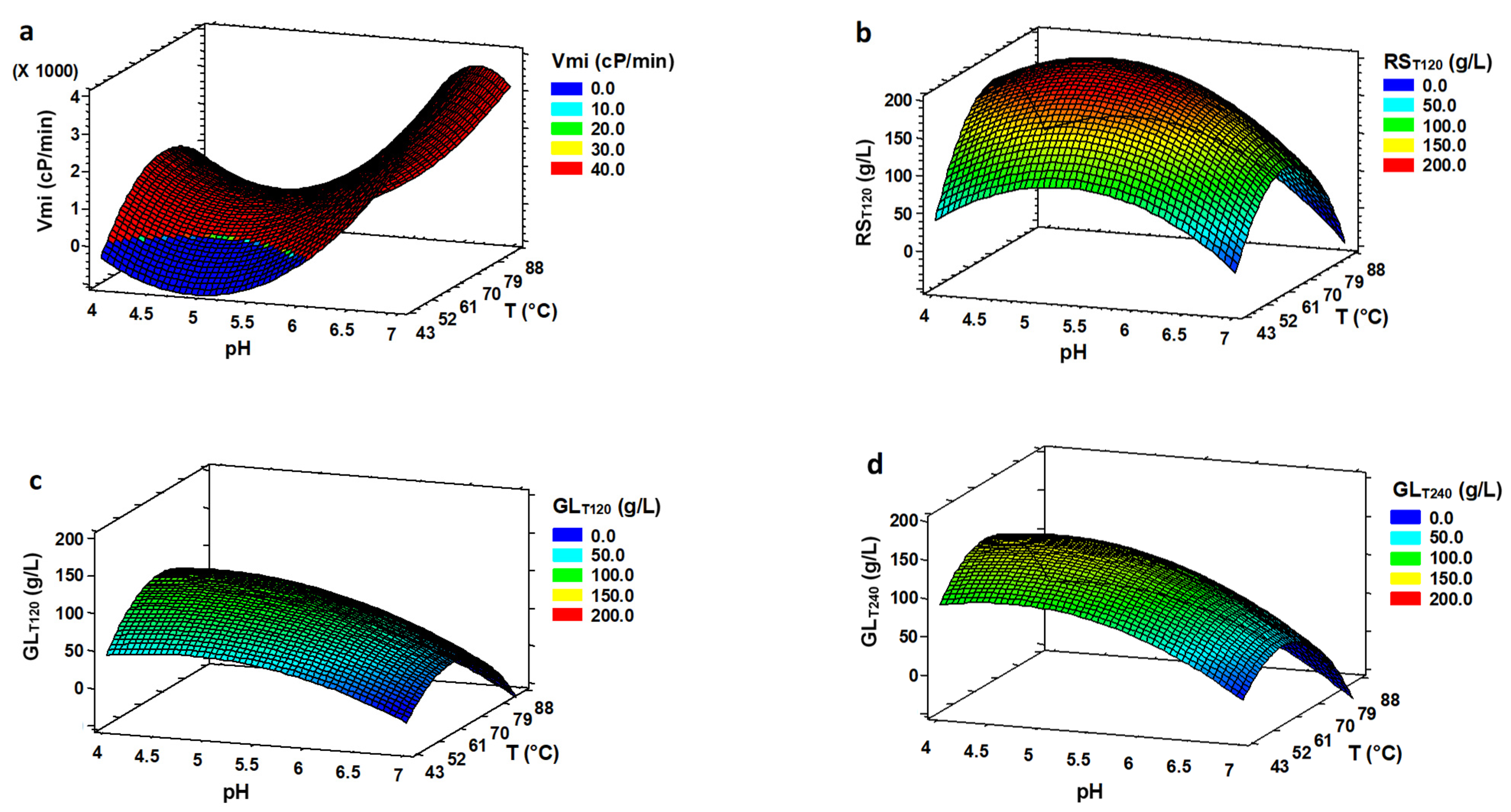
3.3. Optimization of Simultaneous Hydrolysis (SH)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO Food Wastage Footprint: Impacts on Natural Resources—Summary Report. Available online: http://www.fao.org/3/a-i3347e.pdf (accessed on 22 April 2021).
- Melikoglu, M.; Webb, C. Use of waste bread to produce fermentation products. In Food Industry Wastes; Maria Kosseva, C.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 63–76. ISBN 9780123919212. [Google Scholar]
- European Comission Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives (Text with EEA Relevance). Available online: http://data.europa.eu/eli/dir/2008/98/oj (accessed on 17 May 2021).
- Gélinas, P.; McKinnon, C.M.; Pelletier, M. Sourdough-type bread from waste bread crumb. Food Microbiol. 1999, 16, 37–43. [Google Scholar] [CrossRef]
- Luo, S.; Koksel, F. Physical and technofunctional properties of yellow pea flour and bread crumb mixtures processed with low moisture extrusion cooking. J. Food Sci. 2020, 85, 2688–2698. [Google Scholar] [CrossRef]
- Samray, M.N.; Masatcioglu, T.M.; Koksel, H. Bread crumbs extrudates: A new approach for reducing bread waste. J. Cereal Sci. 2019, 85, 130–136. [Google Scholar] [CrossRef]
- Martínez, M.M.; Gómez, M. Rheological and microstructural evolution of the most common gluten-free flours and starches during bread fermentation and baking. J. Food Eng. 2017, 197, 78–86. [Google Scholar] [CrossRef]
- Dura, A.; Rosell, C.M. Enzymes in Baking. Microb. Enzym. Technol. Food Appl. 2017, 295–314. [Google Scholar] [CrossRef]
- Immonen, M.; Maina, N.H.; Wang, Y.; Coda, R.; Katina, K. Waste bread recycling as a baking ingredient by tailored lactic acid fermentation. Int. J. Food Microbiol. 2020, 327, 108652. [Google Scholar] [CrossRef]
- Rosa-Sibakov, N.; Sorsamäki, L.; Immonen, M.; Nihtilä, H.; Maina, N.H.; Siika-aho, M.; Katina, K.; Nordlund, E. Functionality and economic feasibility of enzymatically hydrolyzed waste bread as a sugar replacer in wheat bread making. J. Food Process. Preserv. 2022, 46, e16378. [Google Scholar] [CrossRef]
- Verni, M.; Minisci, A.; Convertino, S.; Nionelli, L.; Rizzello, C.G. Wasted bread as substrate for the cultivation of starters for the food industry. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [Green Version]
- Nionelli, L.; Wang, Y.; Pontonio, E.; Immonen, M.; Rizzello, C.G.; Maina, H.N.; Katina, K.; Coda, R. Antifungal effect of bioprocessed surplus bread as ingredient for bread-making: Identification of active compounds and impact on shelf-life. Food Control 2020, 118. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Khanahmadi, M.; Roodpeyma, S.; Taherzadeh, M. Ethanol production from bread residues. Biomass Bioenergy 2008, 32, 333–337. [Google Scholar] [CrossRef]
- Torabi, S.; Satari, B.; Hassan-Beygi, S.R. Process optimization for dilute acid and enzymatic hydrolysis of waste wheat bread and its effect on aflatoxin fate and ethanol production. Biomass Conv. Bioref. 2020, 11, 2617–2625. [Google Scholar] [CrossRef]
- Sükrü Demirci, A.; Palabıyık, I.; Gümüs, T.; Özalp, Ş. Waste bread as a biomass source: Optimization of enzymatic hydrolysis and relation between rheological behavior and glucose yield. Waste Biomass Valorization 2017, 8, 775–782. [Google Scholar] [CrossRef]
- Hudečková, H.; Šupinová, P.; Babák, L. Optimization of enzymatic hydrolysis of waste bread before fermentation. Acta Univ. Agric. Silvic. Mendel. Brun. 2017, 65, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Sarbatly, R. The simultineous enzymatic hydrolysis of tapioca starch for instant formation of glucose. J. Appl. Sci. 2007, 15, 2057–2062. [Google Scholar] [CrossRef]
- Słomińska, L.; Zielonka, R.; Jarosławski, L. The unconventional single stage hydrolysis of potato starch. Pol. J. Chem. Technol. 2013, 15, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Legin, E.; Copinet, A.; Duchiron, F. A single step high temperature hydrolysis of wheat starch. Starch Stärke 1998, 50, 84–89. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Montesinos, T.; Navarro, J.M. Production of alcohol from raw wheat flour by amyloglucosidase and Saccharomyces cerevisiae. Enzyme Microb. Technol. 2000, 27, 362–370. [Google Scholar] [CrossRef]
- Zhang, C.; Kang, X.; Wang, F.; Tian, Y.; Liu, T.; Su, Y.; Qian, T.; Zhang, Y. Valorization of food waste for cost-effective reducing sugar recovery in a two-stage enzymatic hydrolysis platform. Energy 2020, 208, 118379. [Google Scholar] [CrossRef]
- Leman, P.; Goesaert, H.; Delcour, J.A. Residual amylopectin structures of amylase-treated wheat starch slurries reflect amylase mode of action. Food Hydrocoll. 2009, 23, 153–164. [Google Scholar] [CrossRef]
- Martinez, M.M.; Gómez, M. Insight of the α-amylase family of enzymes. Microb. Enzym. Technol. Food Appl. 2020, 21–45. [Google Scholar] [CrossRef]
- Robyt, J.F. Enzymes and Their Action on Starch. In Starch; BeMiller, J., Whistler, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 237–292. ISBN 9780127462752. [Google Scholar]
- Sirohi, R.; Pandey, J.P.; Goel, R.; Singh, A.; Lohani, U.C.; Kumar, A. Two-stage enzymatic hydrolysis for fermentable sugars production from damaged wheat grain starch with sequential process optimization and reaction kinetics. Starch Stärke 2021, 73, 2000082. [Google Scholar] [CrossRef]
- Fernández-Peláez, J.; Guerra, P.; Gallego, C.; Gomez, M. Physical properties of flours obtained from wasted bread crusts and crumbs. Foods 2021, 10, 282. [Google Scholar] [CrossRef]
- Martínez, M.M.; Román, L.; Gómez, M. Implications of hydration depletion in the in vitro starch digestibility of white bread crumb and crust. Food Chem. 2018, 239, 295–303. [Google Scholar] [CrossRef]
- Primo-Martín, C.; van Nieuwenhuijzen, N.H.; Hamer, R.J.; van Vliet, T. Crystallinity changes in wheat starch during the bread-making process: Starch crystallinity in the bread crust. J. Cereal Sci. 2007, 45, 219–226. [Google Scholar] [CrossRef]
- Han, W.; Liu, W.-X.; Yu, C.-M.; Huang, J.-G.; Tang, J.-H.; Li, Y.-F. BioH2 production from waste bread using a two-stage process of enzymatic hydrolysis and dark fermentation. Int. J. Hydrogen Energy 2017, 42, 29929–29934. [Google Scholar] [CrossRef]
- Govindasamy, S.; Campanella, O.H.; Oates, C.G. Influence of extrusion variables on subsequent saccharification behaviour of sago starch. Food Chem. 1995, 54, 289–296. [Google Scholar] [CrossRef]
- Rendón-Villalobos, J.R.; Solorza-Feria, J.; Aguilar-Sandoval, A. Optimisation of conditions for glucose syrup production from banana (Musa paradisiaca L.) pulp using response surface methodology. Int. J. Food Sci. Technol. 2011, 46, 739–745. [Google Scholar] [CrossRef]
- Amaral-Fonseca, M.; Morellon-Sterling, R.; Fernández-Lafuente, R.; Tardioli, P.W. Optimization of simultaneous saccharification and isomerization of dextrin to high fructose syrup using a mixture of immobilized amyloglucosidase and glucose isomerase. Catal. Today 2021, 362, 175–183. [Google Scholar] [CrossRef]
- Brandam, C.; Meyer, X.M.; Proth, J.; Strehaiano, P.; Pingaud, H. An original kinetic model for the enzymatic hydrolysis of starch during mashing. Biochem. Eng. J. 2003, 13, 43–52. [Google Scholar] [CrossRef]
- Fujii, M.; Homma, T.; Taniguchi, M. Synergism of α-amylase and glucoamylase on hydrolysis of native starch granules. Biotechnol. Bioeng. 1988, 32, 910–915. [Google Scholar] [CrossRef]
- Guzmán-Maldonado, H.; Paredes-López, O.; Biliaderis, C.G.; Maldonado-Guzmán, H.; López-Paredes, O.; Biliaderis, G.C. Amylolytic enzymes and products derived from starch: A review. Crit. Rev. Food Sci. Nutr. 1995, 35, 373–403. [Google Scholar] [CrossRef]
- Wong, D.W.S.; Robertson, G.H.; Lee, C.C.; Wagschal, K. Synergistic action of recombinant α-amylase and glucoamylase on the hydrolysis of starch granules. Protein J. 2007, 26, 159–164. [Google Scholar] [CrossRef]
- Zhang, B.; Dhital, S.; Gidley, M.J. Synergistic and antagonistic effects of α-amylase and amyloglucosidase on starch digestion. Biomacromolecules 2013, 14, 1945–1954. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, H.; Xu, B.; Tan, J. Development of kinetic model structures for glutinous rice saccharification by different enzymes. J. Food Process Eng. 2014, 37, 317–328. [Google Scholar] [CrossRef]
| Run | Experimental Factors | Responses | |||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | T | Liquefaction | Saccharification | ||||||
| RSE120 | RST120 | RSmi | Vmi | GLE120 | GLT120 | GLmi | |||
| 1 | 4.50 | 50.0 | 76.34 | 72.67 | 0.89 | 122.65 | 81.80 | 83.06 | 0.81 |
| 2 | 6.50 | 50.0 | 62.74 | 62.19 | 0.67 | 249.75 | 57.02 | 56.72 | 0.48 |
| 3 | 4.50 | 80.0 | 98.58 | 98.20 | 1.08 | 937.05 | 89.72 | 88.34 | 2.69 |
| 4 | 6.50 | 80.0 | 91.33 | 85.95 | 1.70 | 626.06 | 35.76 | 37.47 | 0.58 |
| 5 | 4.09 | 65.0 | 64.68 | 64.40 | 0.67 | 314.21 | 141.20 | 137.30 | 1.57 |
| 6 | 6.91 | 65.0 | 88.62 | 88.97 | 1.03 | 1783.97 | 41.99 | 38.59 | 0.56 |
| 7 | 5.50 | 43.8 | 56.66 | 56.15 | 0.48 | 151.14 | 63.16 | 63.00 | 0.53 |
| 8 | 5.50 | 86.2 | 102.52 | 99.69 | 1.34 | 515.48 | 29.37 | 30.26 | 0.47 |
| 9 | 5.50 | 65.0 | 98.48 | 95.03 | 1.24 | 364.82 | 108.95 | 104.94 | 1.08 |
| 10 | 5.50 | 65.0 | 84.05 | 85.27 | 0.96 | 340.56 | 124.75 | 121.64 | 1.10 |
| 11 | 5.50 | 65.0 | 88.09 | 86.79 | 1.07 | 395.04 | 114.62 | 113.37 | 1.32 |
| 12 | 5.50 | 65.0 | 82.86 | 80.32 | 0.94 | 609.25 | 109.72 | 105.55 | 1.26 |
| 13 | 5.50 | 65.0 | 84.50 | 81.29 | 1.00 | 515.81 | 104.76 | 101.15 | 1.11 |
| 14 | 5.50 | 65.0 | 85.28 | 80.55 | 0.94 | 346.10 | 126.72 | 123.03 | 1.05 |
| 15 | 5.50 | 65.0 | 88.72 | 84.72 | 1.08 | 577.47 | 114.35 | 112.26 | 1.38 |
| 16 | 5.50 | 65.0 | 89.08 | 85.56 | 1.21 | 368.40 | 116.24 | 112.50 | 1.25 |
| Run | Experimental Factors | Responses | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | T | Simultaneous Hydrolysis | |||||||||
| RSE120 | RST120 | RSmi | GLE120 | GLT120 | GLmi | GLE240 | GLT240 | Vmi | |||
| 1 | 4.50 | 50.0 | 127.85 | 125.46 | 1.33 | 80.66 | 80.74 | 1.13 | 138.19 | 127.27 | 140.77 |
| 2 | 6.50 | 50.0 | 106.37 | 105.97 | 1.16 | 40.77 | 39.80 | 0.46 | 70.01 | 69.28 | 157.54 |
| 3 | 4.50 | 80.0 | 143.49 | 135.89 | 3.01 | 78.28 | 79.11 | 1.61 | 90.42 | 85.45 | 827.42 |
| 4 | 6.50 | 80.0 | 86.59 | 85.30 | 1.17 | 16.19 | 15.94 | 0.22 | 15.65 | 15.43 | 634.58 |
| 5 | 4.09 | 65.0 | 204.58 | 197.17 | 2.00 | 115.46 | 113.18 | 1.40 | 154.77 | 145.68 | 621.11 |
| 6 | 6.91 | 65.0 | 113.87 | 113.78 | 1.46 | 30.93 | 30.22 | 0.30 | 35.29 | 34.95 | 6173.47 |
| 7 | 5.50 | 43.8 | 110.20 | 108.77 | 1.30 | 47.69 | 49.32 | 0.62 | 87.58 | 85.88 | 53.15 |
| 8 | 5.50 | 86.2 | 122.89 | 118.45 | 2.05 | 27.43 | 28.02 | 0.42 | 37.53 | 35.49 | 597.52 |
| 9 | 5.50 | 65.0 | 219.88 | 213.48 | 2.64 | 96.06 | 93.30 | 1.31 | 135.71 | 126.86 | 1.59 × 106 |
| 10 | 5.50 | 65.0 | 226.33 | 220.43 | 2.50 | 115.97 | 108.44 | 1.31 | 132.97 | 127.05 | 2246.65 |
| 11 | 5.50 | 65.0 | 225.44 | 227.54 | 2.46 | 101.51 | 97.73 | 1.17 | 130.87 | 129.83 | 391.60 |
| 12 | 5.50 | 65.0 | 206.92 | 204.93 | 2.82 | 100.92 | 96.12 | 1.33 | 143.48 | 127.58 | 862.77 |
| 13 | 5.50 | 65.0 | 206.11 | 205.13 | 2.58 | 96.74 | 92.04 | 1.46 | 148.40 | 130.38 | 489.84 |
| 14 | 5.50 | 65.0 | 200.12 | 203.61 | 2.32 | 108.73 | 105.86 | 1.54 | 133.27 | 126.59 | 370.87 |
| 15 | 5.50 | 65.0 | 201.16 | 198.65 | 2.30 | 84.24 | 93.28 | 1.18 | 138.72 | 132.11 | 146,197.85 |
| 16 | 5.50 | 65.0 | 228.73 | 227.13 | 2.44 | 99.54 | 95.10 | 1.53 | 140.49 | 121.89 | 724.20 |
| Responses | Optimal Conditions | Maximum Theoretical Response | ||
|---|---|---|---|---|
| pH | T | R2 | ||
| Liquefaction | ||||
| RSE120 | 5.49 | 86.2 | 88.75 | 103.00 |
| RST120 | 5.24 | 86.2 | 85.33 | 99.73 |
| RSmi | 6.91 | 86.2 | 85.12 | 1.92 |
| Vmi | 6.91 | 69.2 | 62.73 | 1288.46 |
| Saccharification | ||||
| GLE120 | 4.39 | 65.1 | 94.48 | 130.10 |
| GLT120 | 4.38 | 64.8 | 93.97 | 126.92 |
| GLmi | 4.09 | 86.2 | 78.09 | 2.63 |
| Simultaneous hydrolysis | ||||
| RSE120 | 5.15 | 65.4 | 94.23 | 219.52 |
| RST120 | 5.10 | 65.7 | 94.61 | 216.73 |
| RSmi | 4.63 | 77.5 | 92.30 | 2.80 |
| GLE120 | 4.47 | 64.7 | 96.16 | 114.19 |
| GLT120 | 4.51 | 64.7 | 98.13 | 112.11 |
| GLmi | 4.28 | 69.3 | 92.51 | 1.63 |
| GLE240 | 4.59 | 61.0 | 98.55 | 158.32 |
| GLT240 | 4.61 | 60.9 | 99.05 | 146.84 |
| Vmi | 6.91 | 67.04 | 54.56 | 4055.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sigüenza-Andrés, T.; Pando, V.; Gómez, M.; Rodríguez-Nogales, J.M. Optimization of a Simultaneous Enzymatic Hydrolysis to Obtain a High-Glucose Slurry from Bread Waste. Foods 2022, 11, 1793. https://doi.org/10.3390/foods11121793
Sigüenza-Andrés T, Pando V, Gómez M, Rodríguez-Nogales JM. Optimization of a Simultaneous Enzymatic Hydrolysis to Obtain a High-Glucose Slurry from Bread Waste. Foods. 2022; 11(12):1793. https://doi.org/10.3390/foods11121793
Chicago/Turabian StyleSigüenza-Andrés, Teresa, Valentín Pando, Manuel Gómez, and José M. Rodríguez-Nogales. 2022. "Optimization of a Simultaneous Enzymatic Hydrolysis to Obtain a High-Glucose Slurry from Bread Waste" Foods 11, no. 12: 1793. https://doi.org/10.3390/foods11121793
APA StyleSigüenza-Andrés, T., Pando, V., Gómez, M., & Rodríguez-Nogales, J. M. (2022). Optimization of a Simultaneous Enzymatic Hydrolysis to Obtain a High-Glucose Slurry from Bread Waste. Foods, 11(12), 1793. https://doi.org/10.3390/foods11121793







