Variability of Meat and Carcass Quality from Worldwide Native Chicken Breeds
Abstract
1. Introduction
2. Material and Methods
2.1. Data Collection
2.2. Data Analysis
2.2.1. Multicollinearity Preliminary Testing
2.2.2. Data-Mining CHAID Decision Tree
3. Results
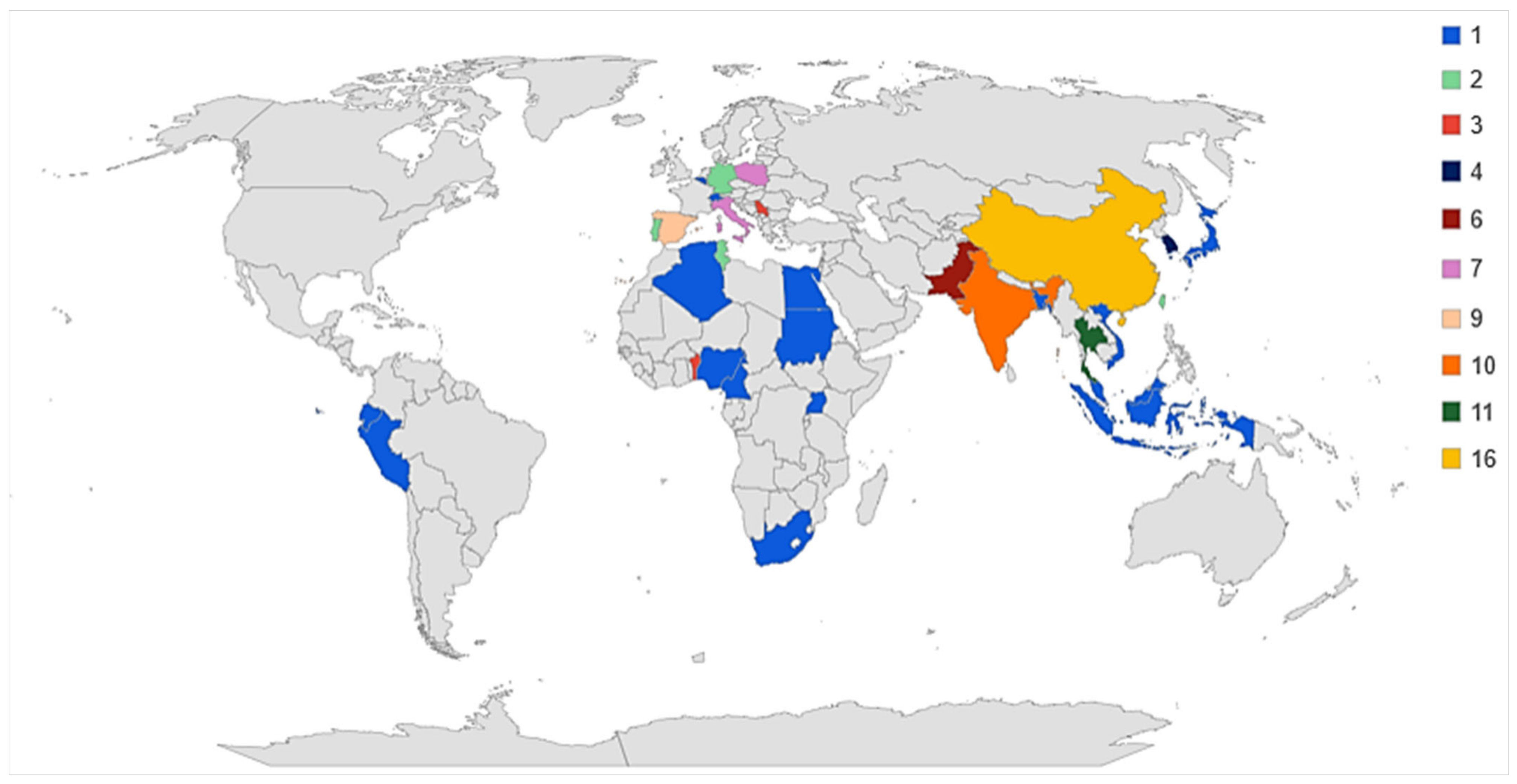
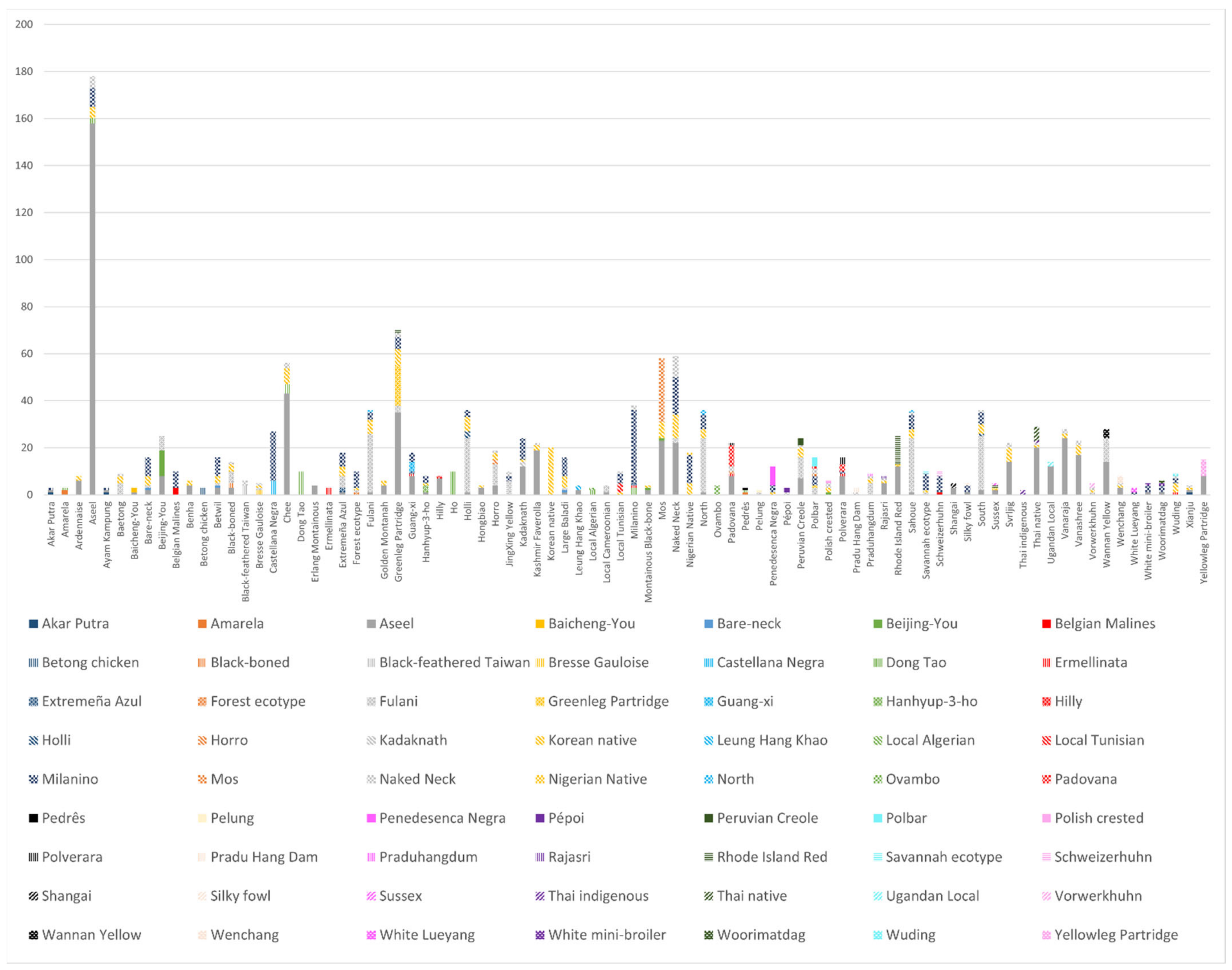
3.1. Study Georeferencing
3.2. Analysis of Model Reliability
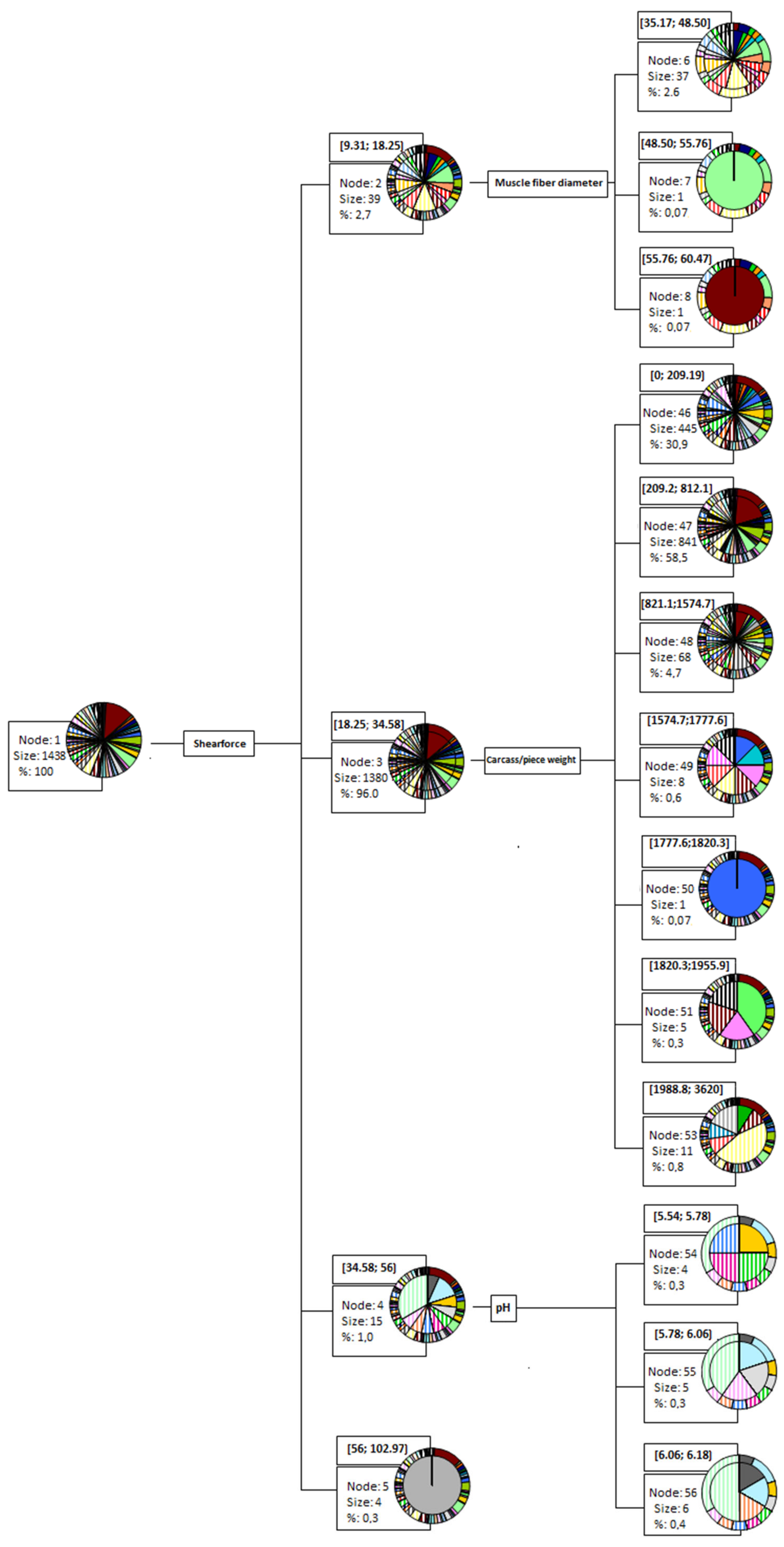
3.3. Data-Mining CHAID Decision Tree
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pym, R. Poultry Genetics and Breeding in Developing Countries. Poultry Development Review; FAO: Rome, Italy, 2013; pp. 80–83. [Google Scholar]
- Di Rosa, A.R.; Chiofalo, B.; Lo Presti, V.; Chiofalo, V.; Liotta, L. Egg Quality from Siciliana and Livorno Italian Autochthonous Chicken Breeds Reared in Organic System. Animals 2020, 10, 864. [Google Scholar] [CrossRef] [PubMed]
- Badruzzaman, A.; Rahman, M.M.; Hasan, M.; Hossain, M.K.; Husna, A.; Hossain, F.M.A.; Giasuddin, M.; Uddin, M.J.; Islam, M.R.; Alam, J. Semi-Scavenging Poultry as Carriers of Avian Influenza Genes. Life 2022, 12, 320. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Sørensen, P.; Howlider, M. Egg production performances of a breed and three crossbreeds under semi-scavenging system of management. Livest. Res. Rural 2018, 16, 1–12. [Google Scholar]
- Jahan, S.S.; Islam, M.S.; Hossain, K.; Islam, M.; Islam, M.; Kabir, A.; Alim, M. Comparative study of growth performance of Deshi, Fayoumi, RIR and Sonali chicken reared under farm and semi scavenging condition. J. Agric. Food Environ. 2021, 2, 30–36. [Google Scholar] [CrossRef]
- Hantanirina, H.; Rabearimisa, R.; Andrianantenaina, N.; Rakotozandriny, J. Indigenous Race of Hen: Egg Physical Characteristics and Laying Performance-Case of a Family Poultry Farm in Madagascar. Poult. Sci. J. 2019, 7, 171–181. [Google Scholar]
- Hannah, W.; Astatkie, T.; Rathgeber, B. Hatch rate of laying hen strains provided a photoperiod during incubation. Animal 2020, 14, 353–359. [Google Scholar] [CrossRef]
- González Ariza, A.; Arando Arbulu, A.; León Jurado, J.M.; Navas González, F.J.; Delgado Bermejo, J.V.; Camacho Vallejo, M.E. Discriminant Canonical Tool for Differential Biometric Characterization of Multivariety Endangered Hen Breeds. Animals 2021, 11, 2211. [Google Scholar] [CrossRef]
- Toalombo Vargas, P.A.; Navas González, F.J.; Landi, V.; León Jurado, J.M.; Delgado Bermejo, J.V. Sexual dimorphism and breed characterization of Creole hens through biometric canonical discriminant analysis across Ecuadorian agroecological areas. Animals 2020, 10, 32. [Google Scholar] [CrossRef]
- Niknafs, S.; Abdi, H.; Fatemi, S.; Zandi, M.; Baneh, H. Genetic trend and inbreeding coefficients effects for growth and reproductive traits in Mazandaran indigenous chicken. J. Biol. 2013, 3, 25–31. [Google Scholar]
- Ogbaje, C.; Agbo, E.; Ajanusi, O. Prevalence of Ascaridia galli, Heterakis gallinarum and Tapeworm infections in birds slaughtered in Makurdi township. Int. J. Poult. Sci. 2012, 11, 103–107. [Google Scholar] [CrossRef][Green Version]
- González Ariza, A.; Arando Arbulu, A.; Navas González, F.J.; Delgado Bermejo, J.V.; Camacho Vallejo, M.E. Discriminant Canonical Analysis as a Validation Tool for Multivariety Native Breed Egg Commercial Quality Classification. Foods 2021, 10, 632. [Google Scholar] [CrossRef] [PubMed]
- González Ariza, A.; Arando Arbulu, A.; Navas González, F.J.; Ruíz Morales, F.D.A.; León Jurado, J.M.; Barba Capote, C.J.; Camacho Vallejo, M.E. Sensory Preference and Professional Profile Affinity Definition of Endangered Native Breed Eggs Compared to Commercial Laying Lineages’ Eggs. Animals 2019, 9, 920. [Google Scholar] [CrossRef] [PubMed]
- González Ariza, A.; Navas González, F.J.; Arando Arbulu, A.; León Jurado, J.M.; Barba Capote, C.J.; Camacho Vallejo, M.E. Non-Parametrical Canonical Analysis of Quality-Related Characteristics of Eggs of Different Varieties of Native Hens Compared to Laying Lineage. Animals 2019, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Muchadeyi, F.; Eding, H.; Wollny, C.; Groeneveld, E.; Makuza, S.; Shamseldin, R.; Simianer, H.; Weigend, S. Absence of population substructuring in Zimbabwe chicken ecotypes inferred using microsatellite analysis. Anim. Genet. 2007, 38, 332–339. [Google Scholar] [CrossRef]
- FAO. Domestic Animal Diversity Information System (DAD-IS): Risk Status of Animal Genetic Resources; FAO: Rome, Italy, 2022. [Google Scholar]
- FAO. Sustainable Development Goals: Goals; FAO: Rome, Italy, 2022. [Google Scholar]
- Babić, J.; Milićević, D.; Vranić, D.; Lukić, M.; Petrović, Z. The effect of season of transportation on the welfare of broilers and selected parameters of broiler meat quality. Tehnol. Mesa 2014, 55, 46–53. [Google Scholar] [CrossRef]
- Gomez, D.L.; Kòsa, G.; Hansen, L.D.; Mydland, L.T.; Passoth, V. Production and characterization of yeasts grown on media composed of spruce-derived sugars and protein hydrolysates from chicken by-products. Microb. Cell Factories 2020, 19, 19. [Google Scholar]
- Nguyen Van, D.; Moula, N.; Moyse, E.; Do Duc, L.; Vu Dinh, T.; Farnir, F. Productive Performance and Egg and Meat Quality of Two Indigenous Poultry Breeds in Vietnam, Ho and Dong Tao, Fed on Commercial Feed. Animals 2020, 10, 408. [Google Scholar] [CrossRef]
- Puspita, U.E.; Saragih, H.; Hartatik, T.; Daryono, B.S. Body Weight Gain and Carcass Quality of the Hybrid Chicken Derived from the Crossing between Female F1 Kampung Super and Male F1 Kampung-Broiler. JTBB 2021, 6, 60934. [Google Scholar] [CrossRef]
- Nollet, L.M.; Toldrá, F. Handbook of Processed Meats and Poultry Analysis; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Schreuders, F.K.; Schlangen, M.; Kyriakopoulou, K.; Boom, R.M.; van der Goot, A.J. Texture methods for evaluating meat and meat analogue structures: A review. Food Control 2021, 127, 108103. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, H.; Mu, T.; Wang, W.; Yu, B.; Guo, J.; Wang, Y.; Zhou, Z.; Gu, Y.; Huang, Z. Correlation analysis between AK1 mRNA expression and inosine monophosphate deposition in Jingyuan chickens. Animals 2020, 10, 439. [Google Scholar] [CrossRef]
- Bennato, F.; Di Luca, A.; Martino, C.; Ianni, A.; Marone, E.; Grotta, L.; Ramazzotti, S.; Cichelli, A.; Martino, G. Influence of grape pomace intake on nutritional value, lipid oxidation and volatile profile of poultry meat. Foods 2020, 9, 508. [Google Scholar] [CrossRef] [PubMed]
- Sanden, K.W.; Böcker, U.; Ofstad, R.; Pedersen, M.E.; Høst, V.; Afseth, N.K.; Rønning, S.B.; Pleshko, N. Characterization of collagen structure in normal, wooden breast and spaghetti meat chicken fillets by FTIR microspectroscopy and histology. Foods 2021, 10, 548. [Google Scholar] [CrossRef] [PubMed]
- Purslow, P.P.; Warner, R.D.; Clarke, F.M.; Hughes, J.M. Variations in meat colour due to factors other than myoglobin chemistry; a synthesis of recent findings (invited review). Meat Sci. 2020, 159, 107941. [Google Scholar] [CrossRef] [PubMed]
- González Ariza, A.; Arando Arbulu, A.; Navas González, F.J.; Nogales Baena, S.; Delgado Bermejo, J.V.; Camacho Vallejo, M.E. The Study of Growth and Performance in Local Chicken Breeds and Varieties: A Review of Methods and Scientific Transference. Animals 2021, 11, 2492. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.K.; Gonzalez, F.J.N. Can scientists influence donkey welfare? Historical perspective and a contemporary view. J. Equine Vet. Sci. 2018, 65, 25–32. [Google Scholar] [CrossRef]
- Iglesias Pastrana, C.; Navas González, F.J.; Ciani, E.; Barba Capote, C.J.; Delgado Bermejo, J.V. Effect of research impact on emerging camel husbandry, welfare and social-related awareness. Animals 2020, 10, 780. [Google Scholar] [CrossRef]
- Gehanno, J.-F.; Rollin, L.; Darmoni, S. Is the coverage of Google Scholar enough to be used alone for systematic reviews. BMC Med. Inform. Decis. Mak. 2013, 13, 7. [Google Scholar] [CrossRef]
- Schlosser, R.W.; Wendt, O.; Bhavnani, S.; Nail-Chiwetalu, B. Use of information-seeking strategies for developing systematic reviews and engaging in evidence-based practice: The application of traditional and comprehensive Pearl Growing. A review. Int. J. Lang. Commun. 2006, 41, 567–582. [Google Scholar] [CrossRef]
- FAO. Domestic Animal Diversity Information System (DAD-IS): Browse by Species and Country; FAO: Rome, Italy, 2022. [Google Scholar]
- Choo, Y.; Kwon, H.; Oh, S.; Um, J.; Kim, B.; Kang, C.; Lee, S.; An, B. Comparison of growth performance, carcass characteristics and meat quality of Korean local chickens and silky fowl. Asian-Australas. J. Anim. Sci. 2014, 27, 398. [Google Scholar] [CrossRef]
- Zidane, A.; Ababou, A.; Metlef, S.; Niar, A.; Bouderoua, K. Growth and meat quality of three free-range chickens and commercial broiler under the same breeding conditions. Acta Sci. Anim. Sci. 2018, 40, 39663. [Google Scholar] [CrossRef]
- Jaspal, M.H.; Ali, S.; Rajput, N.; Naeem, M.; Talpur, F.N.; Rehman, I. Fatty acid profiling and comparative evaluation of carcass cut up yield, meat quality traits of Cobb Sasso, commercial broiler and native aseel chicken. Pure Appl. Biol. 2020, 9, 56–65. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.; He, T.; Xiong, F.; Chen, X.; Chen, X.; Jin, S.; Geng, Z. Association of residual feed intake with growth performance, carcass traits, meat quality, and blood variables in native chickens. J. Anim. Sci. 2020, 98, skaa121. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Rois, D.; Vázquez, J.A.; Purriños, L.; González, R.; Lorenzo, J.M. Breed effect between Mos rooster (Galician indigenous breed) and Sasso T-44 line and finishing feed effect of commercial fodder or corn. Poult. Sci. 2012, 91, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Koomkrong, N.; Theerawatanasirikul, S.; Boonkaewwan, C.; Jaturasitha, S.; Kayan, A. Breed-related number and size of muscle fibres and their response to carcass quality in chickens. Ital. J. Anim. Sci. 2015, 14, 4145. [Google Scholar] [CrossRef]
- Zanetti, E.; De Marchi, M.; Dalvit, C.; Molette, C.; Rémignon, H.; Cassandro, M. Carcase characteristics and qualitative meat traits of three Italian local chicken breeds. Br. Poult. Sci. 2010, 51, 629–634. [Google Scholar] [CrossRef][Green Version]
- Iqbal, S.; Pampori, Z.; Hasin, D. Carcass and egg characteristics of indigenous chicken of Kashmir (Kashmir favorella). Indian J. Anim. Res. 2009, 43, 194–196. [Google Scholar]
- Jaturasitha, S.; Kayan, A.; Wicke, M. Carcass and meat characteristics of male chickens between Thai indigenous compared with improved layer breeds and their crossbred. Arch. Anim. Breed 2008, 51, 283–294. [Google Scholar] [CrossRef]
- Motsepe, R.; Mabelebele, M.; Norris, D.; Brown, D.; Ngambi, J.; Ginindza, M. Carcass and meat quality characteristics of South African indigenous chickens. Indian J. Anim. Res. 2016, 50, 580–587. [Google Scholar] [CrossRef]
- Devatkal, S.K.; Vishnuraj, M.R.; Kulkarni, V.V.; Kotaiah, T. Carcass and meat quality characterization of indigenous and improved variety of chicken genotypes. Poult. Sci. 2018, 97, 2947–2956. [Google Scholar] [CrossRef]
- Kaewkot, C.; Ruangsuriya, J.; Kreuzer, M.; Jaturasitha, S. Carcass and meat quality of crossbreds of Thai indigenous chickens and Rhode Island Red layer chickens as compared with the purebreds and with broilers. Anim. Prod. Sci. 2019, 60, 454–463. [Google Scholar] [CrossRef]
- Mueller, S.; Kreuzer, M.; Siegrist, M.; Mannale, K.; Messikommer, R.E.; Gangnat, I.D. Carcass and meat quality of dual-purpose chickens (Lohmann Dual, Belgian Malines, Schweizerhuhn) in comparison to broiler and layer chicken types. Poult. Sci. 2018, 97, 3325–3336. [Google Scholar] [CrossRef] [PubMed]
- Haunshi, S.; Sunitha, R.; Shanmugam, M.; Padhi, M.; Niranjan, M. Carcass characteristics and chemical composition of breast and thigh muscles of native chicken breeds. Indian J. Poult. Sci. 2013, 48, 219–222. [Google Scholar]
- Pripwai, N.; Pattanawong, W.; Punyatong, M.; Teltathum, T. Carcass characteristics and meat quality of Thai inheritance chickens. J. Agric. Sci. 2014, 6, 182. [Google Scholar] [CrossRef]
- Cassandro, M.; De Marchi, M.; Penasa, M.; Rizzi, C. Carcass characteristics and meat quality traits of the Padovana chicken breed, a commercial line, and their cross. Ital. J. Anim. Sci. 2015, 14, 3848. [Google Scholar] [CrossRef]
- Jatoi, A.; Iqbal, M.; Sahota, A.; Akram, M.; Javed, K.; Mehmood, S.; Hussain, J.; Ishaq, H. Carcass characteristics and organ development in four different varieties of native Aseel chicken of Pakistan. Pak. J. Sci. 2015, 67, 127–132. [Google Scholar]
- Liu, F.; Niu, Z. Carcass quality of different meat-typed chickens when achieve a common physiological body weight. Int. J. Poult. Sci. 2008, 7, 319–322. [Google Scholar] [CrossRef]
- Jung, S.; Bae, Y.S.; Kim, H.J.; Jayasena, D.D.; Lee, J.H.; Park, H.B.; Heo, K.N.; Jo, C. Carnosine, anserine, creatine, and inosine 5′-monophosphate contents in breast and thigh meats from 5 lines of Korean native chicken. Poult. Sci. 2013, 92, 3275–3282. [Google Scholar] [CrossRef]
- Nematbakhsh, S.; Selamat, J.; Idris, L.H.; Abdull Razis, A.F. Chicken Authentication and Discrimination via Live Weight, Body Size, Carcass Traits, and Breast Muscle Fat Content Clustering as Affected by Breed and Sex Varieties in Malaysia. Foods 2021, 10, 1575. [Google Scholar] [CrossRef]
- Rajkumar, U.; Muthukumar, M.; Haunshi, S.; Niranjan, M.; Raju, M.; Rama Rao, S.; Chatterjee, R. Comparative evaluation of carcass traits and meat quality in native Aseel chickens and commercial broilers. Br. Poult. Sci. 2016, 57, 339–347. [Google Scholar] [CrossRef]
- Biazen, A.; Mengistu, U.; Negassi, A.; Getenet, A.; Solomon, A.; Tadelle, D. Comparative Growth Performance, Carcass Characteristics and Meat Quality of Local Horro and Exotic Cockerels of Tropical Origin Fed Growers Diet. Open J. Anim. Sci. 2021, 11, 62–83. [Google Scholar] [CrossRef]
- Jaturasitha, S.; Leangwunta, V.; Leotaragul, A.; Phongphaew, A.; Apichartsrungkoon, T.; Simasathitkul, N.; Vearasilp, T.; Worachai, L.; Meulen, U.T. A comparative study of Thai native chicken and broiler on productive performance, carcass and meat quality. Dtsch. Trop. 2002, 146, 1–9. [Google Scholar]
- Khan, U.; Hussain, J.; Mahmud, A.; Khalique, A.; Mehmood, S.; Badar, I.; Usman, M.; Jaspal, M.; Ahmad, S. Comparative study on carcass traits, meat quality and taste in broiler, broiler breeder and aseel chickens. Braz. J. Poult. Sci. 2019, 21, 770. [Google Scholar] [CrossRef]
- Promket, D.; Ruangwittayanusorn, K. The comparatives of growth and carcass performance of the Thai native chicken between economic selection (Chee KKU12) and natural selection (Chee N). Vet. Integr. Sci. 2021, 19, 247–257. [Google Scholar] [CrossRef]
- Tor, M.; Estany, J.; Villalba, D.; Molina, E.; Cubiló, D. Comparison of carcass composition by parts and tissues between cocks and capons. Anim. Res. 2002, 51, 421–431. [Google Scholar] [CrossRef]
- Sarsenbek, A.; Wang, T.; Zhao, J.; Jiang, W. Comparison of carcass yields and meat quality between Baicheng-You chickens and Arbor Acres broilers. Poult. Sci. 2013, 92, 2776–2782. [Google Scholar] [CrossRef]
- Franco, D.; Rois, D.; Vázquez, J.A.; Lorenzo, J. Comparison of growth performance, carcass components, and meat quality between Mos rooster (Galician indigenous breed) and Sasso T-44 line slaughtered at 10 months. Poult. Sci. 2012, 91, 1227–1239. [Google Scholar] [CrossRef]
- Wattanachant, S.; Benjakul, S.; Ledward, D.A. Composition, color, and texture of Thai indigenous and broiler chicken muscles. Poult. Sci. 2004, 83, 123–128. [Google Scholar] [CrossRef]
- Jaturasitha, S.; Srikanchai, T.; Kreuzer, M.; Wicke, M. Differences in carcass and meat characteristics between chicken indigenous to northern Thailand (Black-boned and Thai native) and imported extensive breeds (Bresse and Rhode Island Red). Poult. Sci. 2008, 87, 160–169. [Google Scholar] [CrossRef]
- Kwiecień, M.; Kasperek, K.; Tomaszewska, E.; Muszyński, S.; Jeżewska-Witkowska, G.; Winiarska-Mieczan, A.; Grela, E.; Kamińska, E. Effect of breed and caponisation on the growth performance, carcass composition, and fatty acid profile in the muscles of Greenleg Partridge and Polbar breeds. Braz. J. Poult. Sci. 2018, 20, 583–594. [Google Scholar] [CrossRef]
- Pateiro, M.; Rois, D.; Lorenzo, J.M.; Vázquez, J.A.; Franco, D. Effect of breed and finishing diet on growth performance, carcass and meat quality characteristics of Mos young hens. Span. J. Agric. Res. 2018, 16, e0402. [Google Scholar] [CrossRef]
- Puchała, M.; Krawczyk, J.; Sokołowicz, Z.; Utnik-Banaś, K. Effect of breed and production system on physicochemical characteristics of meat from multi-purpose hens. Ann. Anim. Sci. 2015, 15, 247–261. [Google Scholar] [CrossRef]
- Tougan, P.; Dahouda, M.; Ahounou, G.; Salifou, C.; Kpodekon, M.; Mensah, G.; Kossou, D.; Amenou, C.; Kogbeto, C.; Thewis, A. Effect of breeding mode, type of muscle and slaughter age on technological meat quality of local poultry population of Gallus gallus species of Benin. Int. J. Biosci. 2013, 3, 81–97. [Google Scholar]
- Miguel, J.; Ciria, J.; Asenjo, B.; Calvo, J. Effect of caponisation on growth and on carcass and meat characteristics in Castellana Negra native Spanish chickens. Animal 2008, 2, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Calik, J.; Poltowicz, K.; Swiatkiewicz, S.; Krawczyk, J.; Nowak, J. Effect of caponization on meat quality of Greenleg Partridge cockerels. Ann. Anim. Sci. 2015, 15, 541. [Google Scholar] [CrossRef]
- Durán, A.M. The effect of caponization on production indices and carcass and meat characteristics in free-range Extremeña Azul chickens. Span. J. Agric. Res. 2004, 2, 211–216. [Google Scholar] [CrossRef]
- Jiang, R.; Zhao, G.; Chen, J.; Zheng, M.; Zhao, J.; Li, P.; Hu, J.; Wen, J. Effect of dietary supplemental nicotinic acid on growth performance, carcass characteristics and meat quality in three genotypes of chicken. J. Anim. Physiol. Anim. Nutr. 2011, 95, 137–145. [Google Scholar] [CrossRef]
- Sosnówka-Czajka, E.; Skomorucha, I.; Muchacka, R. Effect of organic production system on the performance and meat quality of two purebred slow-growing chicken breeds. Ann. Anim. Sci. 2017, 17, 1197. [Google Scholar] [CrossRef]
- Jiang, M.; Fan, W.; Xing, S.; Wang, J.; Li, P.; Liu, R.; Li, Q.; Zheng, M.; Cui, H.; Wen, J. Effects of balanced selection for intramuscular fat and abdominal fat percentage and estimates of genetic parameters. Poult. Sci. 2017, 96, 282–287. [Google Scholar] [CrossRef]
- Franco, D.; Pateiro, M.; Rois, D.; Vázquez, J.A.; Lorenzo, J.M.; Rodriguez, J. Effects of caponization on growth performance, carcass and meat quality of Mos breed capons reared in free-range production system. Ann. Anim. Sci. 2016, 16, 909–929. [Google Scholar] [CrossRef]
- Guo, X.; Nan, H.; Shi, D.; Zhou, J.; Wan, Y.; Zhou, B.; Geng, Z.; Chen, X.; Jiang, R. Effects of caponization on growth, carcass, and meat characteristics and the mRNA expression of genes related to lipid metabolism in roosters of a Chinese indigenous breed. Czech J. Anim. Sci. 2015, 60, 327–333. [Google Scholar] [CrossRef]
- Wang, D.; Huang, H.; Zhou, L.; Li, W.; Zhou, H.; Hou, G.; Liu, J.; Hu, L. Effects of dietary supplementation with turmeric rhizome extract on growth performance, carcass characteristics, antioxidant capability, and meat quality of Wenchang broiler chickens. Ital. J. Anim. Sci. 2015, 14, 3870. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, G.; Jiang, R.; Zheng, M.; Chen, J.; Liu, R.; Wen, J. Effects of diet-induced differences in growth rate on metabolic, histological, and meat-quality properties of 2 muscles in male chickens of 2 distinct broiler breeds. Poult. Sci. 2012, 91, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Khatun, H.; Faruqe, S.; Mostafa, M.G. Effects of different dietary energy and protein levels on the performance and carcass characteristics of native hilly chicken during growing phase in confinement. Asian Australas. J. Biosci. Biotechnol. 2021, 6, 1–9. [Google Scholar] [CrossRef]
- Cheng, F.-Y.; Huang, C.; Wan, T.-C.; Liu, Y.-T.; Lin, L.; Lou Chyr, C.-Y. Effects of free-range farming on carcass and meat qualities of black-feathered Taiwan native chicken. Asian-Australas. J. Anim. Sci. 2008, 21, 1201–1206. [Google Scholar] [CrossRef]
- Bughio, E.; Hussain, J.; Mahmud, A.; Khalique, A. Effects of production system and feeding regimen on carcass and meat quality traits of Naked Neck chicken. S. Afr. J. Anim. Sci. 2021, 51, 250–261. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, G.; Zheng, M.; Wen, J.; Yang, N. Estimation of genetic parameters for contents of intramuscular fat and inosine-5′-monophosphate and carcass traits in Chinese Beijing-You chickens. Poult. Sci. 2008, 87, 1098–1104. [Google Scholar] [CrossRef]
- Yousif, I.; Binda, B.; Elamin, K.; Malik, H.; Babiker, M. Evaluation of carcass characteristics and meat quality of indigenous fowl ecotypes and exotic broiler strains raised under hot climate. Glob. J. Anim. Sci. 2014, 2, 365–371. [Google Scholar]
- Rajkumar, U.; Prince, L.; Haunshi, S.; Paswan, C.; Reddy, B. Evaluation of Vanaraja female line chicken for growth, production, carcass and egg quality traits. Indian J. Anim. Sci. 2020, 90, 603–609. [Google Scholar]
- Cerolini, S.; Vasconi, M.; Sayed, A.A.; Iaffaldano, N.; Mangiagalli, M.G.; Pastorelli, G.; Moretti, V.M.; Zaniboni, L.; Mosca, F. Free-range rearing density for male and female Milanino chickens: Carcass yield and qualitative meat traits. J. Appl. Poult. Res. 2019, 28, 1349–1358. [Google Scholar] [CrossRef]
- Molee, A.; Kuadsantia, P.; Kaewnakian, P. Gene effects on body weight, carcass yield, and meat quality of Thai indigenous chicken. J. Poult. Sci. 2018, 55, 94–102. [Google Scholar] [CrossRef]
- Bungsrisawat, P.; Tumwasorn, S.; Loongyai, W.; Nakthong, S.; Sopannarath, P. Genetic parameters of some carcass and meat quality traits in Betong chicken (KU line). Agric. Nat. Resour. 2018, 52, 274–279. [Google Scholar] [CrossRef]
- Peters, S.O.; Idowu, O.M.; Agaviezor, B.O.; Egbede, R.O.; Fafiolu, A.O. Genotype and sex effect on gastrointestinal nutrient content, microflora and carcass traits in Nigerian native chickens. Int. J. Poult. Sci. 2010, 9, 731–737. [Google Scholar] [CrossRef][Green Version]
- Franco, D.; Rois, D.; Vázquez, J.A.; Lorenzo, J. Growth performance, carcass morphology and meat quality of meat from roosters slaughtered at eight months affected by genotype and finishing feeding. Span. J. Agric. Res. 2013, 11, 382–393. [Google Scholar] [CrossRef]
- Nolte, T.; Jansen, S.; Weigend, S.; Moerlein, D.; Halle, I.; Link, W.; Hummel, J.; Simianer, H.; Sharifi, A.R. Growth performance of local chicken breeds, a high-performance genotype and their crosses fed with regional faba beans to replace soy. Animals 2020, 10, 702. [Google Scholar] [CrossRef] [PubMed]
- Keambou, T.; Mboumba, S.; Touko, B.; Bembide, C.; Mezui, T.; Tedongmo, A.; Manjeli, Y. Growth performances, carcass and egg charac-teristics of the local chicken and its first generation reciprocal crossbreds with an exotic strain in Cameroon. Adv. Anim. Vet. Sci 2015, 3, 507–513. [Google Scholar] [CrossRef]
- Paredes, M.; Vásquez, B. Growth, carcass characteristics, weight of internal organs and meat proximate composition of six genotypes in chickens reared in Andean region of northern Peruvian. Sci. Agropecu. 2020, 11, 365–374. [Google Scholar] [CrossRef]
- Tsudzuki, M.; Onitsuka, S.; Akiyama, R.; Iwamizu, M.; Goto, N.; Nishibori, M.; Takahashi, H.; Ishikawa, A. Identification of quantitative trait loci affecting shank length, body weight and carcass weight from the Japanese cockfighting chicken breed, Oh-Shamo (Japanese Large Game). Cytogenet. Genome Res. 2007, 117, 288–295. [Google Scholar] [CrossRef]
- Magala, H.; Kugonza, D.; Kwizera, H.; Kyarisiima, C. Influence of management system on growth and carcass characteristics of Ugandan local chickens. J. Anim. Sci. Adv. 2012, 2, 558–567. [Google Scholar]
- Kasperek, K.; Drabik, K.; Miachalak, K.; Pietras-Ożga, D.; Winiarczyk, S.; Zięba, G.; Batkowska, J. The Influence of Sex on the Slaughter Parameters and Selected Blood Indices of Greenleg Partridge, Polish Native Breed of Hens. Animals 2021, 11, 517. [Google Scholar] [CrossRef]
- Haunshi, S.; Paswan, C.; Prince, L.; Chatterjee, R. Inheritance of growth traits and impact of selection on carcass and egg quality traits in Vanashree, an improved indigenous chicken. Trop. Anim. Health Prod. 2021, 53, 128. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Zhao, G.; Zheng, M.; Jiang, R.; Wen, J. Live performance, carcass composition, and blood metabolite responses to dietary nutrient density in two distinct broiler breeds of male chickens. Poult. Sci. 2009, 88, 2575–2584. [Google Scholar] [CrossRef] [PubMed]
- Obrzut, J.; Krawczyk, J.; Calik, J.; Świątkiewicz, S.; Pietras, M.; Utnik-Banaś, K. Meat quality of poulards obtained from three conserved breeds of hens. Ann. Anim. Sci. 2018, 18, 261. [Google Scholar] [CrossRef]
- Liu, L.; Dou, T.; Li, Q.; Rong, H.; Tong, H.; Xu, Z.; Huang, Y.; Gu, D.; Chen, X.; Ge, C. Myostatin mRNA expression and its association with body weight and carcass traits in Yunnan Wuding chicken. Genet. Mol. Res. 2016, 15, gmr15048967. [Google Scholar] [CrossRef]
- Pavlovski, Z.; Škrbić, Z.; Lukić, M.; Vitorović, D.; Petričević, V.; Milošević, N. Naked Neck chicken of Serbian and foreign origin: Carcass characteristic. Biotechnol. Anim. Husb. 2009, 25, 1023–1032. [Google Scholar] [CrossRef]
- Pavlovski, Z.; Škrbić, Z.; Lukić, M.; Vitorović, D.; Petričević, V. Naked neck: Autochthonous breed of chicken in Serbia: Carcass characteristics. Biotechnol. Anim. Husb. 2009, 25, 1–10. [Google Scholar] [CrossRef]
- Lariviere, J.; Farnir, F.; Detilleux, J.; Michaux, C.; Verleyen, V.; Leroy, P. Performance, breast morphological and carcass traits in the Ardennaise chicken breed. Int. J. Poult. Sci. 2009, 8, 452–456. [Google Scholar] [CrossRef][Green Version]
- Raach-Moujahed, A.; Haddad, B. Performance, livability, carcass yield and meat quality of Tunisian local poultry and fast-growing genotype (Arbor Acres) fed standard diet and raised outdoor access. J. Anim. Prod. Adv. 2013, 3, 75–85. [Google Scholar] [CrossRef]
- Amorim, A.; Rodrigues, S.; Pereira, E.; Teixeira, A. Physicochemical composition and sensory quality evaluation of capon and rooster meat. Poult. Sci. 2016, 95, 1211–1219. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Q.; Liu, Y.; Gilbert, E.R.; Li, D.; Yin, H.; Wang, Y.; Yang, Z.; Wang, Z.; Yuan, Y. Polymorphisms in the perilipin gene may affect carcass traits of Chinese meat-type chickens. Asian-Australas. J. Anim. Sci. 2015, 28, 763. [Google Scholar] [CrossRef]
- Tasoniero, G.; Cullere, M.; Baldan, G.; Dalle Zotte, A. Productive performances and carcase quality of male and female Italian Padovana and Polverara slow-growing chicken breeds. Ital. J. Anim. Sci. 2018, 17, 530–539. [Google Scholar] [CrossRef]
- Dalle Zotte, A.; Gleeson, E.; Franco, D.; Cullere, M.; Lorenzo, J.M. Proximate composition, amino acid profile, and oxidative stability of slow-growing indigenous chickens compared with commercial broiler chickens. Foods 2020, 9, 546. [Google Scholar] [CrossRef] [PubMed]
- Mosca, F.; Zaniboni, L.; Stella, S.; Kuster, C.; Iaffaldano, N.; Cerolini, S. Slaughter performance and meat quality of Milanino chickens reared according to a specific free-range program. Poult. Sci. 2018, 97, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Węglarz, A.; Andres, K.; Wojtysiak, D. Slaughter value and meat quality in two strains of polish crested cockerels. Ital. J. Anim. Sci. 2020, 19, 813–821. [Google Scholar] [CrossRef]
- Shakila, S.; GV, B.R.; Amaravathi, P. Studies on carcass and meat quality characteristics of Rajasri chicken. J. Entomol. Zool. Stud. 2020, 8, 1345–1349. [Google Scholar]
- Pathak, P.; Dubey, P.; Dash, S.; Chaudhary, M. Studies on growth and carcass traits of Aseel and Kadaknath chicken. Indian J. Poult. Sci. 2015, 50, 327–328. [Google Scholar]
- Hussain, M.; Mahmud, A.; Hussain, J.; Qaisrani, S.; Mehmood, S.; Rehman, A. Subsequent effect of dietary lysine regimens fed in the starter phase on the growth performance, carcass traits and meat chemical composition of aseel chicken in the grower phase. Braz. J. Poult. Sci. 2018, 20, 455–462. [Google Scholar] [CrossRef]
- Tougan, U.; Dahouda, M.; Salifou, C.; Ahounou, S.; Kpodekon, M.; Mensah, G.; Kossou, D.; Amenou, C.; Kogbeto, C.; Thewis, A. Variability of carcass traits of local poultry populations of gallus gallus species of Benin. Int. J. Poult. Sci. 2013, 12, 473. [Google Scholar] [CrossRef]
- Tang, H.; Gong, Y.; Wu, C.; Jiang, J.; Wang, Y.; Li, K. Variation of meat quality traits among five genotypes of chicken. Poult. Sci. 2009, 88, 2212–2218. [Google Scholar] [CrossRef]
- Toalombo, P.; Villafuerte, A.; Fiallos, L.; Andino, P.; Damián, P.; Duchi, N.; Trujillo, V.; Hidalgo, L. Polyphenols of Thyme (Thymus vulgaris) and ginger (Zingiber officinale) in the feeding of local hens. Actas Iberoam. Conserv. Anim. 2017, 10, 88–93. [Google Scholar]
- Zhao, G.; Cui, H.; Liu, R.; Zheng, M.; Chen, J.; Wen, J. Comparison of breast muscle meat quality in 2 broiler breeds. Poult. Sci. 2011, 90, 2355–2359. [Google Scholar] [CrossRef]
- Rajkumar, U.; Haunshi, S.; Paswan, C.; Raju, M.; Rao, S.R.; Chatterjee, R. Characterization of indigenous Aseel chicken breed for morphological, growth, production, and meat composition traits from India. Poult. Sci. 2017, 96, 2120–2126. [Google Scholar] [CrossRef] [PubMed]
- Chuaynukool, K.; Wattanachant, S.; Siripongvutikorn, S.; Yai, H. Chemical and physical properties of raw and cooked spent hen, broiler and Thai indigenous chicken muscles in mixed herbs acidified soup (Tom Yum). J. Food Technol. 2007, 5, 180–186. [Google Scholar]
- El-Attrouny, M.M.; Iraqi, M.M.; Sabike, I.I.; Abdelatty, A.M.; Moustafa, M.M.; Badr, O.A. Comparative evaluation of growth performance, carcass characteristics and timed series gene expression profile of GH and IGF-1 in two Egyptian indigenous chicken breeds versus Rhode Island Red. J. Anim. Breed. Genet. 2021, 138, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Youssao, I.; Alkoiret, I.; Dahouda, M.; Assogba, M.; Idrissou, N.; Kayang, B.; Yapi-Gnaoré, V.; Assogba, H.; Houinsou, A.; Ahounou, S. Comparison of growth performance, carcass characteristics and meat quality of Benin indigenous chickens and Label Rouge (T55× SA51). Afr. J. Biotechnol. 2012, 11, 15569–15579. [Google Scholar]
- Elkhazen, A.; LARBI, M.; M’hamdi, N.; Haddad, B. Comparison of meat quality of local poultry and Arbors acres reared in two farming systems in Tunisia. J. New Sci. 2016, 34, 1922–1929. [Google Scholar]
- Jung, Y.-K.; Jeon, H.-J.; Jung, S.; Choe, J.-H.; Lee, J.-H.; Heo, K.-N.; Kang, B.-S.; Jo, C.-R. Comparison of quality traits of thigh meat from Korean native chickens and broilers. Food Sci. Anim. 2011, 31, 684–692. [Google Scholar] [CrossRef]
- Amorim, A.; Rodrigues, S.; Pereira, E.; Valentim, R.; Teixeira, A. Effect of caponisation on physicochemical and sensory characteristics of chickens. Animal 2016, 10, 978–986. [Google Scholar] [CrossRef]
- Batool, T.; Farooq, S.; Roohi, N.; Mahmud, A.; Usman, M.; Ghayas, A.; Ahmad, S. Effect of different dietary lysine regimens on meat quality attributes in varieties of indigenous Aseel chicken. Kafkas Univ. Vet. Fak. Derg. 2018, 24, 639–645. [Google Scholar]
- Ramella, M.V.; Rodríguez, J.M.L.; Losada, D.R.; Arias, A.; Justo, J.R.; Moure, M.P.; Pedrouso, M.D.L.; Chico, D. Effect of finishing diet on carcass characteristics and meat quality of Mos cockerel. Span. J. Agric. Res. 2021, 19, 601. [Google Scholar]
- Jin, S.; Yang, L.; Zang, H.; Xu, Y.; Chen, X.; Chen, X.; Liu, P.; Geng, Z. Influence of free-range days on growth performance, carcass traits, meat quality, lymphoid organ indices, and blood biochemistry of Wannan Yellow chickens. Poult. Sci. 2019, 98, 6602–6610. [Google Scholar] [CrossRef]
- Escobedo del Bosque, C.I.; Altmann, B.A.; Ciulu, M.; Halle, I.; Jansen, S.; Nolte, T.; Weigend, S.; Mörlein, D. Meat quality parameters and sensory properties of one high-performing and two local chicken breeds fed with Vicia faba. Foods 2020, 9, 1052. [Google Scholar] [CrossRef] [PubMed]
- Gnanaraj, P.T.; Sundaram, A.S.; Rajkumar, K.; Babu, R.N. Proximate composition and meat quality of three indian native chicken breeds. Indian J. Anim. Res. 2020, 54, 1584–1589. [Google Scholar] [CrossRef]
- Jeong, H.S.; Utama, D.T.; Kim, J.; Barido, F.H.; Lee, S.K. Quality comparison of retorted Samgyetang made from white semi-broilers, commercial broilers, Korean native chickens, and old laying hens. Asian-Australas. J. Anim. Sci. 2020, 33, 139. [Google Scholar] [CrossRef]
- Chumngoen, W.; Tan, F.-J. Relationships between descriptive sensory attributes and physicochemical analysis of broiler and Taiwan native chicken breast meat. Asian-Australas. J. Anim. Sci. 2015, 28, 1028. [Google Scholar] [CrossRef] [PubMed]
- Mosca, F.; Kuster, C.; Stella, S.; Farina, G.; Madeddu, M.; Zaniboni, L.; Cerolini, S. Growth performance, carcass characteristics and meat composition of Milanino chickens fed on diets with different protein concentrations. Br. Poult. Sci. 2016, 57, 531–537. [Google Scholar] [CrossRef]
- Tunim, S.; Phasuk, Y.; Aggrey, S.E.; Duangjinda, M. Gene expression of fatty acid binding protein genes and its relationship with fat deposition of Thai native crossbreed chickens. Anim. Biosci. 2021, 34, 751–758. [Google Scholar] [CrossRef]
- Marín Navas, C.; Delgado, J.V.; McLean, A.; Jurado, J.; Torres, A.; Navas González, F. Discriminant Canonical Analysis of the Contribution of Spanish and Arabian Purebred Horses to the Genetic Diversity and Population Structure of Hispano-Arabian Horses. Animals 2021, 11, 269. [Google Scholar] [CrossRef]
- Rogerson, P.A. Data Reduction: Factor Analysis and Cluster Analysis; Sage: London, UK, 2001; pp. 192–197. [Google Scholar]
- Nanda, M.A.; Seminar, K.B.; Nandika, D.; Maddu, A. Discriminant analysis as a tool for detecting the acoustic signals of termites Coptotermes curvignathus (Isoptera: Rhinotermitidae). Int. J. Technol. 2018, 9, 840–851. [Google Scholar] [CrossRef]
- Ceylan, Z.; Gürsev, S.; Bulkan, S. An application of data mining in individual pension savings and investment system. EJOSAT 2018, 1, 7–11. [Google Scholar]
- Leroy, G.; Baumung, R.; Notter, D.; Verrier, E.; Wurzinger, M.; Scherf, B. Stakeholder involvement and the management of animal genetic resources across the world. Livest. Sci. 2017, 198, 120–128. [Google Scholar] [CrossRef]
- Toalombo Vargas, P.A.; León, J.M.; Fiallos Ortega, L.R.; Martinez, A.; Villafuerte Gavilanes, A.A.; Delgado, J.V.; Landi, V. Deciphering the patterns of genetic admixture and diversity in the Ecuadorian Creole chicken. Animals 2019, 9, 670. [Google Scholar] [CrossRef] [PubMed]
- Araújo de Carvalho, D.; Martínez Martínez, A.; Carolino, I.; Barros, M.C.; Camacho Vallejo, M.E.; Santos-Silva, F.; de Oliveira Almeida, M.J.; Carolino, N.; Delgado Bermejo, J.V.; Sarmento, J.L.R. Diversity and Genetic Relationship of Free-Range Chickens from the Northeast Region of Brazil. Animals 2020, 10, 1857. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ou, J.H.; Zan, Y.; Wang, Y.; Li, H.; Zhu, C.; Chen, K.; Zhou, X.; Hu, X.; Carlborg, Ö. Researching on the fine structure and admixture of the worldwide chicken population reveal connections between populations and important events in breeding history. Evol. Appl. 2021, 15, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Petracci, M.; Baéza, E. Harmonization of methodologies for the assessment of poultry meat quality features. Worlds Poult. Sci. J. 2011, 67, 137–151. [Google Scholar] [CrossRef]
- Froning, G. Color of poultry meat. Poult. Avian Biol. Rev. 1995, 6, 83–93. [Google Scholar]
- Rondelli, S.; Martinez, O.; Garcia, P. Sex effect on productive parameters, carcass and body fat composition of two commercial broilers lines. Braz. J. Poult. Sci. 2003, 5, 169–173. [Google Scholar] [CrossRef]
- Lyon, C.; Lyon, B.; Davis, C.; Townsend, W. Texture profile analysis of patties made from mixed and flake-cut mechanically deboned poultry meat. Poult. Sci. 1980, 59, 69–76. [Google Scholar] [CrossRef]
- Fitzgerald, E.; Buckley, J. Effect of total and partial substitution of sodium chloride on the quality of Cheddar cheese. J. Dairy Sci. 1985, 68, 3127–3134. [Google Scholar] [CrossRef]
- Hashizawa, Y.; Kubota, M.; Kadowaki, M.; Fujimura, S. Effect of dietary vitamin E on broiler meat qualities, color, water-holding capacity and shear force value, under heat stress conditions. Anim. Sci. J. 2013, 84, 732–736. [Google Scholar] [CrossRef]
- Essèn-Gustavsson, B. Muscle-Fiber Characteristics in Pigs and Relationships to Meat-Quality Paramertaers-Review. Pork Quality: Genetics and Metabolic Factors; CABI: Wallingford, UK, 1993; pp. 140–159. [Google Scholar]
- Smith, D.; Fletcher, D. Chicken breast muscle fiber type and diameter as influenced by age and intramuscular location. Poult. Sci. 1988, 67, 908–913. [Google Scholar] [CrossRef]
- Herring, H.; Cassens, R.; Rriskey, E. Further studies on bovine muscle tenderness as influenced by carcass position, sarcomere length, and fiber diameter. J. Food Sci. 1965, 30, 1049–1054. [Google Scholar] [CrossRef]
- Ashmore, C.; Doerr, L. Postnatal development of fiber types in normal and dystrophic skeletal muscle of the chick. Exp. Neurol. 1971, 30, 431–446. [Google Scholar] [CrossRef]
- Muller, E.; Galavazi, G.; Szirmai, J. Effect of castration and testosterone treatment on fiber width of the flexor carpi radialis muscle in the male frog (Rana temporaria L.). Gen. Comp. Endocrinol. 1969, 13, 275–284. [Google Scholar] [CrossRef]
- Venable, J.H. Morphology of the cells of normal, testosterone-deprived and testosterone-stimulated levator ani muscles. Am. J. Anat. 1966, 119, 271–301. [Google Scholar] [CrossRef] [PubMed]
- Uhlířová, L.; Tůmová, E.; Chodová, D.; Vlčková, J.; Ketta, M.; Volek, Z.; Skřivanová, V. The effect of age, genotype and sex on carcass traits, meat quality and sensory attributes of geese. Asian-Australas. J. Anim. Sci. 2018, 31, 421–428. [Google Scholar] [CrossRef]
- Jing, Z.; Wang, X.; Cheng, Y.; Wei, C.; Hou, D.; Li, T.; Li, W.; Han, R.; Li, H.; Sun, G. Detection of CNV in the SH3RF2 gene and its effects on growth and carcass traits in chickens. BMC Genet. 2020, 21, 22. [Google Scholar] [CrossRef]
- Tilki, M.; Saatci, M.; Kirmizibayrak, T.; Aksoy, A. Effect of age on growth and carcass composition of Native Turkish Geese. Arch. Geflügelkd. 2005, 69, 77–83. [Google Scholar]
- Kirmizibayrak, T.; Önk, K.; Ekiz, B.; Yalçintan, H.; Yilmaz, A.; Yazici, K.; Altinel, A. Effects of age and sex on meat quality of Turkish native geese raised under a free-range system. Kafkas Univ. Vet. Fak. Derg. 2011, 17, 817–823. [Google Scholar]
- Hertanto, B.; Nurmalasari, C.; Nuhriawangsa, A.; Cahyadi, M.; Kartikasari, L. The physical and microbiological quality of chicken meat in the different type of enterprise poultry slaughterhouse: A case study in Karanganyar District. IOP Conf. Ser. Earth Environ. Sci. 2018, 102, 012051. [Google Scholar] [CrossRef]
- Andrés-Bello, A.; Barreto-Palacios, V.; García-Segovia, P.; Mir-Bel, J.; Martínez-Monzó, J. Effect of pH on color and texture of food products. Food Eng. Rev. 2013, 5, 158–170. [Google Scholar] [CrossRef]
- Pérez, M.L.; Escalona, H.; Guerrero, I. Effect of calcium chloride marination on calpain and quality characteristics of meat from chicken, horse, cattle and rabbit. Meat Sci. 1998, 48, 125–134. [Google Scholar] [CrossRef]
- Purchas, R.; Yan, X.; Hartley, D. The influence of a period of ageing on the relationship between ultimate pH and shear values of beef M. longissimus thoracis. Meat Sci. 1999, 51, 135–141. [Google Scholar] [CrossRef]
- Watanabe, A.; Daly, C.; Devine, C. The effects of the ultimate pH of meat on tenderness changes during ageing. Meat Sci. 1996, 42, 67–78. [Google Scholar] [CrossRef]
- Swatland, H.J. How pH causes paleness or darkness in chicken breast meat. Meat Sci. 2008, 80, 396–400. [Google Scholar] [CrossRef]
- Calkins, C.R.; Hodgen, J.M. A fresh look at meat flavor. Meat Sci. 2007, 77, 63–80. [Google Scholar] [CrossRef]
- Mir, N.A.; Rafiq, A.; Kumar, F.; Singh, V.; Shukla, V. Determinants of broiler chicken meat quality and factors affecting them: A review. J. Food Sci. Technol. 2017, 54, 2997–3009. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Feng, X. Exergy analysis for a freeze-drying process. Appl. Therm. Eng. 2008, 28, 675–690. [Google Scholar] [CrossRef]
| Cluster | References | Trait | Units |
|---|---|---|---|
| Weight-related traits | [21,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114] | Carcass/piece weight (g) | g |
| Carcass yield (%) | % | ||
| Cold weight | g | ||
| Histological properties | [39,44,60,69,77,108,109,115] | Muscle fiber density | Fibers/mm2 |
| Muscle fiber diameter | μm | ||
| pH | [21,34,35,36,37,38,40,42,43,44,45,46,49,51,54,56,57,61,62,63,65,66,67,68,69,71,72,74,75,76,77,78,79,80,86,88,91,93,97,102,103,105,107,108,109,116,117,118,119,120,121,122,123,124,125,126,127,128,129] | pH | |
| pH 24 h post mortem | |||
| pH 72 h post mortem | |||
| Color-related traits | [34,36,37,38,40,42,43,44,45,46,48,49,51,54,56,57,61,62,63,65,66,67,68,69,72,74,75,77,78,79,80,84,86,88,97,102,103,105,107,108,117,118,120,121,124,125,126,127,129] | L* meat | |
| a* meat | |||
| b* meat | |||
| L* meat 72 h post mortem | |||
| a* meat 72 h post mortem | |||
| b* meat 72 h post mortem | |||
| L* skin | |||
| a* skin | |||
| b* skin | |||
| Water-holding capacity | [34,36,37,40,42,44,45,46,48,49,56,57,60,61,62,63,65,66,67,69,71,72,74,75,76,77,78,79,80,85,86,88,93,97,107,108,109,112,113,117,118,121,122,124,125,126,127,128,129,130] | Drip loss | % |
| Water-holding capacity | % | ||
| Cooking loss | % | ||
| Texture-related traits | [34,37,38,40,42,43,44,45,46,48,49,54,56,57,60,61,62,63,66,67,69,71,74,79,80,85,86,88,97,107,108,109,113,117,118,120,121,122,124,125,126,127,128,129] | Firmness | kg s−1 |
| Total work | kg mm | ||
| Shear force | N | ||
| Hardness | N | ||
| Springiness | mm | ||
| Cohesiveness | N | ||
| Gumminess | N | ||
| Chewiness | kg mm | ||
| Content of flavor-related nucleotides | [45,52,60,81,113,121,126] | IMP | mg/g |
| AMP | mg/100 g | ||
| Inosine | mg/100 g | ||
| Gross nutrients | [20,21,35,38,40,42,43,44,45,46,47,51,53,56,59,60,62,63,64,65,68,69,71,72,74,76,79,81,82,84,87,88,91,93,96,97,103,104,106,107,108,109,111,113,116,117,118,119,121,122,124,127,128,129,130,131] | Moisture | % |
| Protein | % | ||
| Fat | % | ||
| Ash | % | ||
| Collagen | % | ||
| Cholesterol | mg/100 g |
| Statistics/Traits | Tolerance (1 − R2) | VIF 1 |
|---|---|---|
| Chewiness | 0.2468 | 4.0515 |
| Gumminess | 0.3126 | 3.1989 |
| Hardness | 0.4300 | 2.3258 |
| Shear force | 0.4867 | 2.0546 |
| a* meat | 0.5302 | 1.8862 |
| b* skin | 0.5635 | 1.7745 |
| a* skin | 0.5867 | 1.7044 |
| Muscle fiber diameter | 0.6164 | 1.6223 |
| Cooking loss | 0.6172 | 1.6202 |
| L* skin | 0.6191 | 1.6152 |
| L* meat | 0.6285 | 1.5910 |
| Water-holding capacity | 0.6418 | 1.5580 |
| pH | 0.7088 | 1.4108 |
| Drip loss | 0.7201 | 1.3886 |
| pH 24 h post mortem | 0.7415 | 1.3486 |
| Moisture | 0.7428 | 1.3462 |
| b* meat | 0.7458 | 1.3408 |
| Total work | 0.7875 | 1.2699 |
| IMP | 0.7978 | 1.2534 |
| Springiness | 0.8208 | 1.2183 |
| Cholesterol | 0.8264 | 1.2101 |
| Cohesiveness | 0.8981 | 1.1135 |
| Collagen | 0.8985 | 1.1130 |
| Inosine | 0.9044 | 1.1058 |
| Carcass/piece weight | 0.9133 | 1.0949 |
| Carcass yield | 0.9176 | 1.0898 |
| Protein | 0.9293 | 1.0761 |
| AMP | 0.9315 | 1.0735 |
| Ash | 0.9558 | 1.0463 |
| Muscle fiber density | 0.9692 | 1.0317 |
| Cold canal weight | 0.9732 | 1.0275 |
| Average age | 0.9740 | 1.0267 |
| Fat | 0.9792 | 1.0213 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González Ariza, A.; Navas González, F.J.; Arando Arbulu, A.; León Jurado, J.M.; Delgado Bermejo, J.V.; Camacho Vallejo, M.E. Variability of Meat and Carcass Quality from Worldwide Native Chicken Breeds. Foods 2022, 11, 1700. https://doi.org/10.3390/foods11121700
González Ariza A, Navas González FJ, Arando Arbulu A, León Jurado JM, Delgado Bermejo JV, Camacho Vallejo ME. Variability of Meat and Carcass Quality from Worldwide Native Chicken Breeds. Foods. 2022; 11(12):1700. https://doi.org/10.3390/foods11121700
Chicago/Turabian StyleGonzález Ariza, Antonio, Francisco Javier Navas González, Ander Arando Arbulu, José Manuel León Jurado, Juan Vicente Delgado Bermejo, and María Esperanza Camacho Vallejo. 2022. "Variability of Meat and Carcass Quality from Worldwide Native Chicken Breeds" Foods 11, no. 12: 1700. https://doi.org/10.3390/foods11121700
APA StyleGonzález Ariza, A., Navas González, F. J., Arando Arbulu, A., León Jurado, J. M., Delgado Bermejo, J. V., & Camacho Vallejo, M. E. (2022). Variability of Meat and Carcass Quality from Worldwide Native Chicken Breeds. Foods, 11(12), 1700. https://doi.org/10.3390/foods11121700






