Oxidative Stability and Antioxidant Activity of Selected Cold-Pressed Oils and Oils Mixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Material
2.2. Oils’ Initial Quality Assessment
2.3. Determination of the Fatty Acid Profile
2.4. Total Phenolic Content Determination
2.5. Determination of the Total Antioxidant Capacity by the Reduction in DPPH Free Radical
2.6. Determination of Total Antioxidant Capacity by ABTS Radical Cation Reduction Method
2.7. Determination of Oxidative Stability in the Rancimat Apparatus
2.8. Determination of the Parameters of Oxidation Kinetics Using the Rancimat Method
2.9. Statistical Analysis of the Results
3. Results and Discussion
3.1. Oils Oxidation Indices
3.2. Specific Extinction Coefficient under UV Light
3.3. Fatty Acid Composition of Analysed Cold-Pressed Oils
3.4. Total Phenolics Content of Analyzed Cold-Pressed Oils
3.5. Antioxidant Capacity Determined by the Reduction in DPPH Free Radicals and ABTS Cation Radicals
3.6. Oxidative Stability in the Rancimat Apparatus
3.7. Parameters of the Oxidation Kinetics of Analysed Oils
3.8. Influence of Selected Quality Parameters on the Oxidation Stability of Oil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wroniak, M.; Rękas, A.; Siger, A.; Janowicz, M. Microwave pretreatment effects on the changes in seeds microstructure, chemical composition and oxidative stability of rapeseed oil. LWT—Food Sci. Technol. 2016, 68, 634–641. [Google Scholar] [CrossRef]
- Boskou, D. Edible cold pressed oils and thier biologically active components. J. Exp. Food Chem. 2017, 3, 108–111. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.; Xiao, T.; Ni, X.; Wei, T.; Liu, X.; Deng, Z.Y.; Li, J. The comparative analysis of different oil extraction methods based on the quality of flaxseed oil. J. Food Compos. Anal. 2022, 107, 104373. [Google Scholar] [CrossRef]
- Symoniuk, E.; Ratusz, K.; Ostrowska-Ligęza, E.; Krygier, K. Impact of selected chemical characteristics of cold-pressed oils on their oxidative stability determined using the Rancimat and Pressure Differential Scanning Calorimetry method. Food Anal. Methods 2018, 11, 1095–1104. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, I.J.; Alexandre, E.M.; Saraiva, J.A.; Pintado, M. Green emerging extraction technologies to obtain high-quality vegetable oils from nuts: A review. Innov. Food Sci. Emerg. Technol. 2022, 76, 102931. [Google Scholar] [CrossRef]
- Choe, E.; Min, B. Chemistry and reactions of reactive oxygen species in foods. J. Food Sci. 2006, 70, 142–159. [Google Scholar] [CrossRef]
- Redondo-Cuevas, L.; Castellano, G.; Torrens, F.; Raikos, V. Revealing the relationship between vegetable oil composition and oxidative stability a multifactorial approach. J. Food Compos. Anal. 2018, 66, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Ciemniewska-Żytkiewicz, H.; Ratusz, K.; Bryś, J.; Reder, M.; Koczoń, P. Determination of the oxidative stability of hazelnut oils by PDSC and Rancimat methods. J. Therm. Anal. Calorim. 2014, 118, 875–881. [Google Scholar] [CrossRef] [Green Version]
- Mikołajczak, N.; Tańska, M. Effect of initial quality and bioactive compounds content in cold-pressed flaxseed oils on oxidative stability and oxidation products formation during one-month storage with light exposure. NFS J. 2022, 26, 10–21. [Google Scholar] [CrossRef]
- Neđeral, S.; Škevin, D.; Kraljić, K.; Obranović, M.; Papeša, S.; Bataljaku, A. Chemical composition and oxidative stability of roasted and cold pressed pumpkin seed oils. J. Am. Oil Chem. Soc. 2012, 89, 1763–1770. [Google Scholar] [CrossRef]
- EN ISO 3960:2012; Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Iodometric (Visual) Endpoint Determination. ISO: Geneva, Switzerland, 2012.
- EN ISO 6885:2008; Animal and Vegetable Fats and Oils—Determination of Anisidine Value. ISO: Geneva, Switzerland, 2008.
- EN ISO 3656:2011; Animal and Vegetable Fats and Oils—Determination of Ultraviolet Absorbance Expressed as Specific UV Extinction. ISO: Geneva, Switzerland, 2011.
- AOAC Official Method 996.06; Fat (Total, Saturated, and Unsaturated) in Foods; Hydrolytic Extraction Gas Chromatographic Method; Methods and Recommended Practices of the AOCS. AOCS International: Arlington, MA, USA, 2001.
- Mińkowski, K.; Zawada, K.; Ptasznik, S.; Kalinowski, A. Wpływ zawiązków fenolowych nasion na stabilność oksydacyjną i aktywność antyrodnikową wytłoczonych z nich olejów bogatych w PUFA n-3. Żywn. Nauka Technol. Jakość 2013, 4, 118–132. [Google Scholar]
- Espin, J.; Soler-Rivas, C.; Wichers, H.J. Characterisation of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2000, 48, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Szydłowska-Czerniak, A.; Łaszewska, A. Effect of refining process on antioxidant capacity, total phenolics and prooxidants contents in rapeseed oils. LWT—Food Sci. Technol. 2015, 64, 853–859. [Google Scholar] [CrossRef]
- EN ISO 6886:2009; Animal and Vegetable Fats and Oils—Determination of Oxidative Stability (Accelerated Oxidation Test). ISO: Geneva, Switzerland, 2009.
- Kowalski, B.; Ratusz, K.; Kowalska, D.; Bekas, W. Determination of the oxidative stability of vegetable oils by differential scanning calorimetry and Rancimat measurements. Eur. J. Lipid Sci. Technol. 2004, 106, 165–169. [Google Scholar] [CrossRef]
- ALINORM 09/32/17; FAO/WHO: Codex Standard for Named Vegetable Oils. Codex Stan 210. Codex Alimentarius Commission: Rome, Italy, 2009.
- Qian, Y.; Wojciechowska, P.; Siger, A.; Kaczmarek, A.; Rudzińska, M. Phytochemical content, oxidative stability, and nutritional properties of unconventional cold-pressed edible oils. J. Food Nutr. Res. 2018, 6, 476–485. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.H.; Alam, M.S.; Monir, M.M.; Ahmed, K. Comprehensive effects of black cumin (Nigella sativa) and synthetic antioxidant on sensory and physicochemical quality of beef patties during refrigerant storage. J. Agric. Food Inf. 2021, 4, 100145. [Google Scholar] [CrossRef]
- Mazaheri, Y.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. Effect of roasting and microwave pre-treatments of Nigella sativa L. seeds on lipase activity and the quality of the oil. Food Chem. 2019, 274, 480–486. [Google Scholar] [CrossRef]
- Kurzeja, E.; Kimsa-Dudek, M.; Synowiec-Wojtarowicz, A.; Ocytko, M.; Kuźmiak, M.; Pawłowska-Góral, K. Stabilność oksydacyjna i pojemność przeciwutleniająca wybranych olejów jadalnych. Bromatol. Chem. Toksyk. 2016, 49, 350–355. [Google Scholar]
- Choo, W.S.; Birch, J.; Dufour, J.P. Physicochemical and quality characteristics of cold-pressed flaxseed oils. J. Food Compos. Anal. 2007, 20, 202–211. [Google Scholar] [CrossRef]
- Bojanowska, M.; Lamorska, J. Evaluation of technological quality of selected rapeseed oils. Acta Agrophysica 2016, 23, 519–531. [Google Scholar]
- Kruszewski, B.; Fąfara, P.; Ratusz, K.; Obiedziński, M. Ocena pojemności przeciwutleniającej i stabilności oksydacyjnej wybranych olejów roślinnych. Zeszyty Problemowe Postępów Nauk Rolniczych 2013, 527, 43–52. [Google Scholar]
- Symoniuk, E.; Ratusz, K.; Krygier, K. Oxidative stability and the chemical composition of market cold-pressed linseed oil. Eur. J. Lipid Sci. Technol. 2017, 119, 10–19. [Google Scholar] [CrossRef]
- Rabiej-Kozioł, D.; Krzemiński, M.P.; Szydłowska-Czerniak, A. Steryl sinapate as a new antioxidant to improve rapeseed oil quality during accelerated shelf life. Materials 2021, 14, 3092. [Google Scholar] [CrossRef] [PubMed]
- The Commission of the European Communities. Commission Regulation (EEC). No. 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Eur. Union. 1991, L248, 1–102. [Google Scholar]
- Szterk, A.; Roszko, M.; Sosińska, E.; Derewiaka, D.; Lewicki, P.P. Chemical composition and oxidative stability of selected plant oils. J. Am. Oil Chem. Soc. 2010, 87, 637–645. [Google Scholar] [CrossRef]
- Wroniak, M.; Maszewska, M. Oliwa z oliwek w diecie śródziemnomorskiej. Żywność. Nauka. Technologia. Jakość 2011, 5, 26–36. [Google Scholar]
- Schmidt, S.; Pokorny, J. Potential application of oilseeds as sources of antioxidants for food lipids. Czech J. Food Sci. 2006, 23, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Vuolo, M.M.; Lima, V.S.; Maróstica, M.R., Jr. Phenolic compounds: Structure, classification, and antioxidant power. In Bioactive Compounds, 1st ed.; Campos, M.R.S., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 33–50. [Google Scholar]
- Sultan, M.T.; Butt, M.S.; Anjum, F.; Jamil, A.; Akhtar, S.; Nasir, M. Nutritional profile of indigenous cultivar of Black Cumin seeds and antioxidant potential of its fixed and essential oil. Pak. J. Bot. 2009, 41, 1321–1330. [Google Scholar]
- Haron, H.; Grace-Lynn, C.; Shahar, S. Comparison of physicochemical analysis and antioxidant activities of Nigella sativa seeds and oils from Yemen, Iran, Malaysia. Sains Malays. 2014, 43, 535–542. [Google Scholar]
- Padda, M.; Picha, D. Antioxidant activity and phenolic composition in beauregard sweetpotato are affected by root size and leaf age. J. Am. Soc. Hortic. Sci. 2007, 132, 447–451. [Google Scholar] [CrossRef] [Green Version]
- Franke, S.; Fröhlich, K.; Werner, S.; Böhm, V.; Schöne, F. Analysis of carotenoids and vitamin E in selected oilseeds, press cakes and oils. Eur. J. Lipid Sci. Technol. 2010, 112, 1122–1129. [Google Scholar] [CrossRef]
- Yu, L.L.; Zhou, K.K.; Parry, J. Antioxidant properties of cold-pressed black caraway, carrot, cranberry, and hemp seed oils. Food Chem. 2005, 91, 723–729. [Google Scholar] [CrossRef]
- Siger, A.; Nogala-Kałucka, M.; Lampart-Szczapa, E. The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J. Food Lipids 2008, 15, 137–149. [Google Scholar] [CrossRef]
- Prescha, A.; Grajzer, M.; Dedyk, M.; Grajeta, H. The antioxidant activity and oxidative stability of cold-pressed oils. J. Am. Oil Chem. Soc. 2014, 91, 1291–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abramovič, H.; Butinar, B.; Nikolic, V. Changes occurring in phenolic content, tocopherol composition and oxidative stability of Camelina sativa oil during storage. Food Chem. 2007, 104, 903–909. [Google Scholar] [CrossRef]
- Rubalya, S.; Neelamegam, P. Antioxidant potential in vegetable oil. Res. J. Chem. Environ. 2012, 16, 87–94. [Google Scholar]
- Górnaś, P.; Siger, A.; Nogala-Kałucka, M.; Polewski, K. Porównanie zmian oksydacyjnych i efektywności wiązania wolnych rodników w trakcie przechowywania olejów roślinnych tłoczonych na zimno oraz ich rafinowanych odpowiedników. Żywn. Nauka Technol. Jakość 2005, 2, 41–51. [Google Scholar]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Tułodziecka, A. Antioxidant capacity of rapeseed extracts obtained by conventional and ultrasound-assisted extraction. J. Am. Oil Chem. Soc. 2014, 91, 2011–2019. [Google Scholar] [CrossRef] [Green Version]
- Dabbour, I.R.; Al-Ismail, K.M.; Takruri, H.R.; Azzeh, F.S. Chemical characteristics and antioxidant content properties of cold pressed seed oil of wild milk thistle plant grown in Jordan. Pak. J. Nutr. 2014, 13, 67. [Google Scholar] [CrossRef] [Green Version]
- Zych, I.; Krzepiłko, A. Pomiar całkowitej zdolności antyoksydacyjnej wybranych antyoksydantów i naparów metodą redukcji rodnika DPPH. Chemia. Dydaktyka. Ekologia. Metrologia 2010, 15, 51–54. [Google Scholar]
- Pawłowska, A.; Kocur, A.; Siudem, P.; Paradowska, K. Badanie stabilności oleju lnianego i oleju z czarnuszki. Postępy Fitoterapii 2018, 19, 157–163. [Google Scholar] [CrossRef]
- Kiralan, M.; Bayrak, A.; Ozkaya, M.T. Oxidation stability of virgin olive oils from some important cultivars in East Mediterranean area in Turkey. J. Am. Oil Chem. Soc. 2009, 86, 247–252. [Google Scholar] [CrossRef]
- Guclu, K.; Altun, M.; Ozyurek, M.; Karademir, S.; Apak, R. Antioxidant capacity of fresh, sun- and sulphited-dried Malatya apricot (Prunus Armeniaca) assayed by CUPRAC, ABTS/TEAC and Folin methods. Int. J. Food Sci. 2006, 41, 76–85. [Google Scholar] [CrossRef]
- Marszałkiewicz, S.; Siger, A.; Radziejewska-Kubzdela, E.; Ratusz, K.; Rudzińska, M. Fizyczno-chemiczne właściwości olejów lniankowych tłoczonych na zimno. Nauka Przyroda Technologie 2017, 11, 235–244. [Google Scholar]
- Rękas, A.; Wroniak, M.; Ścibisz, I. Microwave radiation and conventional roasting in conjunction with hulling on the oxidative state and physicochemical properties of rapeseed oil. Eu.r J. Lipid Sci. Technol. 2017, 119, 1004–1014. [Google Scholar] [CrossRef]
- Monteleone, E.; Caporale, G.; Carlucci, A.; Pagliarini, E. Optimisation of extra virgin olive oil quality. J. Sci. Food Agric. 1998, 77, 31–3797. [Google Scholar] [CrossRef]
- Morelló, J.R.; Motilva, M.J.; Tovar, M.J.; Romero, M.P. Changes in commercial virgin olive oil during storage, with special emphasis on the phenolic fraction. Food Chem. 2004, 85, 357–364. [Google Scholar] [CrossRef]
- Gharby, S.; Harhar, H.; Guillaume, D.; Roudani, A.; Boulbaroud, S.; Ibrahimi, M.; Ahmad, M.; Sultana, S.; Hadda, T.B.; Chafchaouni-Moussaoui, I.; et al. Chemical investigation of Nagella sativa L. seed oil produced in Marocco. J. Saudi Soc. Agric. Sci. 2015, 14, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Ostrowska-Ligęza, E.; Bekas, W.; Kowalska, D.; Lobacz, M.; Wroniak, M.; Kowalski, B. Kinetics of commercial olive oil oxidation: Dynamic differential scanning calorimetry and Rancimat studies. Eur. J. Lipid Sci. Technol. 2010, 112, 268–274. [Google Scholar] [CrossRef]
- Gharby, S.; Harhar, H.; Mamouni, R.; Matthäus, B.; Addi, H.; Charrouf1, Z. Chemical characterisation and kinetic parameter determination under Rancimat test conditions of four monovarietal virgin olive oils grown in Morocco. OCL—Oilseeds Fats Crops Lipids 2016, 23, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Farhoosh, R. The effect of operational parameters of the Rancimat method on the determination of the oxidative stability measures and shelf-life prediction of soybean oil. J. Am. Oil Chem. Soc. 2007, 84, 205–209. [Google Scholar] [CrossRef]
- Ratusz, K.; Popis, E.; Ciemniewska-Żytkiewicz, H.; Wroniak, M. Oxidative stability of camelina (Camelina sativa L.) oil using pressure differential scanning calorimetry and Rancimat method. J. Therm. Anal. Calorim. 2016, 126, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Adhvaryu, A.; Erhan, S.Z.; Liu, Z.S.; Perez, J.M. Oxidation kinetic studies of oils derived from unmodified and genetically modified vegetables using pressurised differential scanning calorimetry and nuclear magnetic resonance spectroscopy. Thermochim. Acta 2000, 364, 87–97. [Google Scholar] [CrossRef]
- Kodali, D.R. Oxidative stability measurement of high stability oils by pressure differential scanning calorimeter (PDSC). J. Agric. Food Chem. 2005, 53, 7649–7653. [Google Scholar] [CrossRef]
- Kurpiewska, M. Analysis of Oxidative Stability of Linseed Oil; Warsaw University of Life Sciences: Warsaw, Poland, 2014. [Google Scholar]
- Farhoosh, R.; Niazmand, R.; Rezaei, M.; Sarabi, M. Kinetic parameter determination of vegetable oil oxidation under Rancimat test conditions. Eur. J. Lipid Sci. Technol. 2008, 110, 587–592. [Google Scholar] [CrossRef]
- Ratusz, K.; Symoniuk, E.; Wroniak, M.; Rudzińska, M. Bioactive compounds, nutritional quality and oxidative stability of cold-pressed camelina (Camelina sativa L.) oils. Oils. Appl. Sci. 2018, 8, 2606. [Google Scholar] [CrossRef] [Green Version]
- Papadimitriou, V.; Sotiroudis, T.G.; Xenakis, A.; Sofikiti, N.; Stavyiannoudaki, V.; Chaniotakis, N.A. Oxidative stability and free radical scavenging activity of extra virgin olive oils: An electron paramagnetic resonance spectroscopy study. Anal. Chim. Acta 2006, 573, 453–458. [Google Scholar] [CrossRef]

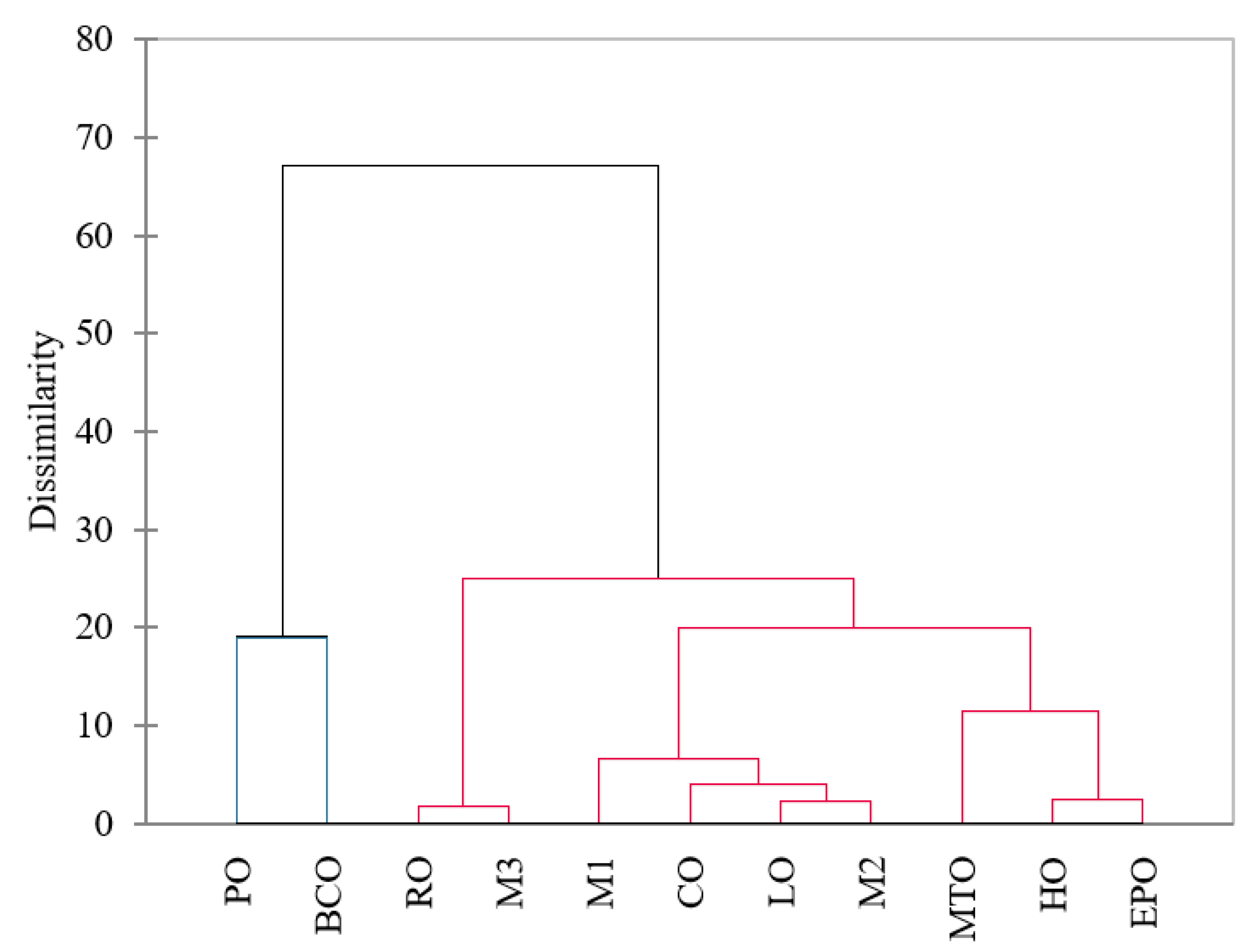
| Oil | PV | p-AnV | TOTOX | K232 | K268 |
|---|---|---|---|---|---|
| PO | 42.52 d | 7.14 g | 98.96 e | 4.57 h | 3.63 i |
| HO | 19.42 c | 2.68 d | 41.51 d | 2.10 e | 0.50 g |
| LO | 0.95 a | 0.49 a | 2.38 a | 1.86 cd | 0.31 e |
| CO | 4.91 b | 1.92 c | 11.74 bc | 1.75 c | 0.26 c,d |
| RO | 0.85 a | 3.55 e | 5.25 a | 1.45 b | 0.17 b |
| BCO | 81.93 e | 3.91 e | 167.76 f | 5.61 i | 2.20 h |
| MTO | 0.92 a | 1.14 b | 2.98 a | 0.26 a | 0.03 a |
| EPO | 4.70 b | 1.16 b | 10.56 b | 2.85 g | 0.28 d,e |
| M1 | 0.33 a | 5.51 f | 6.16 a | 2.49 f | 0.27 d,e |
| M2 | 5.83 b | 2.76 d | 14.42 c | 2.80 g | 0.41 f |
| M3 | 0.35 a | 2.59 d | 3.29 a | 1.94 d | 0.23 c |
| Fatty Acid | Oil | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PO | HO | LO | CO | RO | BCO | MTO | EPO | M1 | M2 | M3 | |
| C16:0 | 12.23 | 5.79 | 5.43 | 5.51 | 4.59 | 10.98 | 8.51 | 7.30 | 6.19 | 6.06 | 6.11 |
| C18:0 | 5.55 | 2.56 | 3.17 | 2.48 | 1.74 | 2.62 | 5.10 | 2.55 | 4.47 | 3.02 | 1.34 |
| C18:1 | 30.21 | 12.71 | 14.45 | 16.42 | 70.28 | 24.45 | 29.54 | 16.12 | 25.18 | 14.34 | 56.07 |
| C18:2 | 51.20 | 59.41 | 21.36 | 19.33 | 17.07 | 60.12 | 53.03 | 71.53 | 25.9 | 20.46 | 17.43 |
| C18:3 | 0.21 | 17.95 | 53.90 | 37.92 | 5.30 | 0.07 | 0.22 | 1.80 | 37.30 | 56.03 | 18.02 |
| C20:0 | 0.34 | 0.83 | - | 1.35 | - | 0.04 | 1.90 | - | 0.08 | - | - |
| C20:1 | 0.11 | 0.75 | 1.13 | 15.56 | 1.02 | 0.13 | - | 0.70 | 0.49 | 0.09 | 1.03 |
| C20:2 | - | - | 0.14 | 1.43 | - | 1.59 | - | - | - | - | - |
| C22:0 | 0.15 | - | 0.42 | - | - | - | 1.70 | - | 0.39 | - | - |
| ∑SFA | 18.27 | 9.18 | 9.02 | 9.34 | 6.33 | 13.64 | 17.21 | 9.85 | 11.13 | 9.08 | 7.45 |
| ∑MUFA | 30.32 | 13.46 | 15.58 | 31.98 | 71.30 | 24.58 | 29.54 | 16.82 | 25.67 | 14.43 | 57.10 |
| ∑PUFA | 51.41 | 77.36 | 75.40 | 58.68 | 22.37 | 61.78 | 53.25 | 73.33 | 63.2 | 76.49 | 35.45 |
| Oil | DPPH | ABTS | TPC [mg GAE/100 mg] | ||||
|---|---|---|---|---|---|---|---|
| AA [mM TEAC/kg] | % Inhibition | TEC50 [min] | AA [mM TEAC/kg] | % Inhibition | TEC50 [min] | ||
| PO | 2.54 a,b | 50.07 b | 57.5 g | 4.19 b | 38.83 b | - | 110.92 c |
| HO | 3.53 f,g | 83.62 c | 5.77 d | 5.49 d | 48.71 e | 12.99 c | 236.25 f |
| LO | 3.45 e,f | 82.83 c | 5.21 c | 8.43 h | 68.29 i | 0.92 a | 56.61 a |
| CO | 3.35 e | 77.46 i | 3.66 b | 9.46 i | 72.35 j | 2.48 a | 132.67 d |
| RO | 3.12 d | 69.86 h | 7.01 e | 7.53 g | 62.46 h | 1.00 a | 162.43 e |
| BCO | 2.41 a | 44.69 a | - | 11.60 j | 92.55 k | 0.05 a | 384.66 h |
| MTO | 2.59 b | 50.98 d | 49.77 f | 3.87 a | 36.53 a | - | 252.87 g |
| EPO | 3.65 g | 88.47 j | 1.85 a | 5.29 c | 47.86 d | 26.99 d | 128.77 d |
| M1 | 2.51 a,b | 49.93 b | - | 5.23 c | 45.85 c | - | 125.27 d |
| M2 | 2.91 c | 62.52 f | 7.07 e | 6.34 f | 54.01 g | 1.78 a | 78.26 b |
| M3 | 3.09 c,d | 68.81 g | 5.35 d | 5.73 e | 49.14 f | 7.82 b | 87.53 b |
| Oil | Induction Time [h] | ||||||
|---|---|---|---|---|---|---|---|
| 80 °C | 90 °C | 100 °C | 105 °C | 110 °C | 110 °C | 120 °C | |
| PO | - | - | 22.45 ± 0.05 | 17.04 ± 0.05 | 13.01 ± 0.16 | 8.62 ± 0.01 | 5.67 ± 0.05 |
| HO | 8.45 ± 0.08 | 6.52 ± 0.06 | 4.32 ± 0.02 | 3.65 ± 0.03 | 2.26 ± 0.06 | - | - |
| LO | 7.11 ± 0.02 | 5.73 ± 0.07 | 3.37 ± 0.14 | 2.66 ± 0.05 | 1.55 ± 0.01 | - | - |
| CO | - | - | 4.62 ± 0.05 | 3.57 ± 0.05 | 2.69 ± 0.06 | 1.89 ± 0.04 | 1.29 ± 0.03 |
| RO | - | - | 15.45 ± 0.06 | 10.99 ± 0.01 | 8.00 ± 0.10 | 5.77 ± 0.11 | 4.22 ± 0.04 |
| BCO | - | - | 38.34 ± 0.15 | 29.00 ± 0.06 | 18.98 ± 0.07 | 10.71 ± 0.12 | 7.12 ± 0.13 |
| MTO | - | - | 11.17 ± 0.02 | 8.21 ± 0.04 | 6.23 ± 0.05 | 4.23 ± 0.03 | 2.66 ± 0.02 |
| EPO | - | - | 7.20 ± 0.16 | 4.94 ± 0.18 | 3.65 ± 0.01 | 2.55 ± 0.12 | 1.85 ± 0.06 |
| M1 | 7.30 ± 0.01 | 5.99 ± 0.04 | 3.63 ± 0.09 | 2.94 ± 0.01 | 1.84 ± 0.04 | - | - |
| M2 | 7.14 ± 0.02 | 5.83 ± 0.07 | 3.47 ± 0.06 | 2.78 ± 0.01 | 1.68 ± 0.01 | - | - |
| M3 | - | - | 8.68 ± 0.01 | 6.91 ± 0.05 | 5.02 ± 0.04 | 4.67 ± 0.04 | 3.68 ± 0.11 |
| Oil | Z | Kinetic Parameter | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| k [h−1] | Ea [kJ/mol] | ΔH [kJ/mol] | ΔS [J/mol K] | ||||||||
| 80 °C | 90 °C | 100 °C | 105 °C | 110 °C | 115 °C | 120 °C | |||||
| PO | 2.07 × 1010 | - | - | 0.11 | 0.16 | 0.23 | 0.31 | 0.43 | 80.40 | 77.22 | −126.02 |
| HO | 7.16 × 109 | 0.29 | 0.40 | 0.55 | 0.75 | 1.01 | - | - | 72.23 | 69.12 | −132.93 |
| LO | 6.55 × 1011 | 0.39 | 0.57 | 0.83 | 1.19 | 1.70 | - | - | 84.99 | 81.87 | −95.06 |
| CO | 1.18 × 1010 | - | - | 0.53 | 0.72 | 0.98 | 1.32 | 1.77 | 73.94 | 70.76 | −130.56 |
| RO | 7.99 × 109 | - | - | 0.17 | 0.24 | 0.32 | 0.44 | 0.59 | 76.23 | 73.05 | −133.82 |
| BCO | 1.65 × 1013 | - | - | 0.09 | 0.13 | 0.20 | 0.31 | 0.46 | 102.02 | 98.84 | −70.33 |
| MTO | 1.04 × 1011 | - | - | 0.24 | 0.34 | 0.48 | 0.68 | 0.94 | 83.11 | 79.93 | −119.71 |
| EPO | 4.26 × 1010 | - | - | 0.38 | 0.53 | 0.74 | 1.01 | 1.38 | 78.94 | 75.76 | −123.41 |
| M1 | 4.61 × 1010 | 0.35 | 0.49 | 0.69 | 0.96 | 1.32 | - | - | 77.32 | 74.20 | −123.97 |
| M2 | 1.36 × 1011 | 0.37 | 0.53 | 0.76 | 1.06 | 1.48 | - | - | 80.39 | 77.28 | −104.30 |
| M3 | 5.83 × 109 | - | - | 0.18 | 0.25 | 0.34 | 0.47 | 0.62 | 75.03 | 71.84 | −119.24 |
| Nr | Parameter | IT at 100 °C [h] |
|---|---|---|
| 1 | PV | 0.89 |
| 2 | p-AnV | 0.43 |
| 3 | TOTOX | 0.89 |
| 4 | K232 | 0.71 |
| 5 | K268 | 0.72 |
| 6 | MUFA | 0.19 |
| 7 | PUFA | −0.30 |
| 8 | SFA | 0.48 |
| 9 | C18:2 | 0.40 |
| 10 | C18:3 | −0.64 |
| 11 | TPC | 0.69 |
| 12 | DPPH | −0.58 |
| 13 | ABTS | 0.42 |
| 14 | Ea | 0.77 |
| 15 | k at 100 °C | −0.75 |
| Parameter | PC1 | PC2 |
|---|---|---|
| PV | 0.89 | 0.05 |
| AnV | 0.30 | 0.07 |
| TOTOX | 0.90 | 0.04 |
| K232 | 0.63 | 0.09 |
| K268 | 0.68 | 0.00 |
| MUFA | 0.01 | 0.90 |
| PUFA | 0.01 | 0.95 |
| SFA | 0.42 | 0.01 |
| C18:2 | 0.31 | 0.10 |
| C18:3 | 0.40 | 0.23 |
| TPC | 0.49 | 0.00 |
| DPPH | 0.41 | 0.04 |
| ABTS | 0.07 | 0.06 |
| Ea | 0.53 | 0.10 |
| k at 100 °C | 0.45 | 0.43 |
| IT at 100 °C | 0.90 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Symoniuk, E.; Wroniak, M.; Napiórkowska, K.; Brzezińska, R.; Ratusz, K. Oxidative Stability and Antioxidant Activity of Selected Cold-Pressed Oils and Oils Mixtures. Foods 2022, 11, 1597. https://doi.org/10.3390/foods11111597
Symoniuk E, Wroniak M, Napiórkowska K, Brzezińska R, Ratusz K. Oxidative Stability and Antioxidant Activity of Selected Cold-Pressed Oils and Oils Mixtures. Foods. 2022; 11(11):1597. https://doi.org/10.3390/foods11111597
Chicago/Turabian StyleSymoniuk, Edyta, Małgorzata Wroniak, Katarzyna Napiórkowska, Rita Brzezińska, and Katarzyna Ratusz. 2022. "Oxidative Stability and Antioxidant Activity of Selected Cold-Pressed Oils and Oils Mixtures" Foods 11, no. 11: 1597. https://doi.org/10.3390/foods11111597
APA StyleSymoniuk, E., Wroniak, M., Napiórkowska, K., Brzezińska, R., & Ratusz, K. (2022). Oxidative Stability and Antioxidant Activity of Selected Cold-Pressed Oils and Oils Mixtures. Foods, 11(11), 1597. https://doi.org/10.3390/foods11111597








