Development of Healthy Vegan Bonbons Enriched with Lyophilized Peach Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bonbons
2.3. Size and Weight Measurements
2.4. Ash Content
2.5. Moisture Content
2.6. Nutritional Data
2.7. Color
2.8. Texture Profile Analysis (TPA)
2.9. Determination of Total Polyphenolic Content (TPC)
2.10. Determination of Total Flavonoid Content (TFC)
2.11. Determination of Antioxidant Activity (AOA)
2.11.1. DPPH• Radical Scavenging Assay
2.11.2. ABTS•+ Radical Scavenging Assay
2.11.3. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.11.4. Cupric-Ion-Reducing Antioxidant Capacity (CUPRAC) Assay
2.12. Water Activity
2.13. Microbial Count—Product Shelf Life
2.14. Microscopic Imaging
2.15. Statistical Analysis
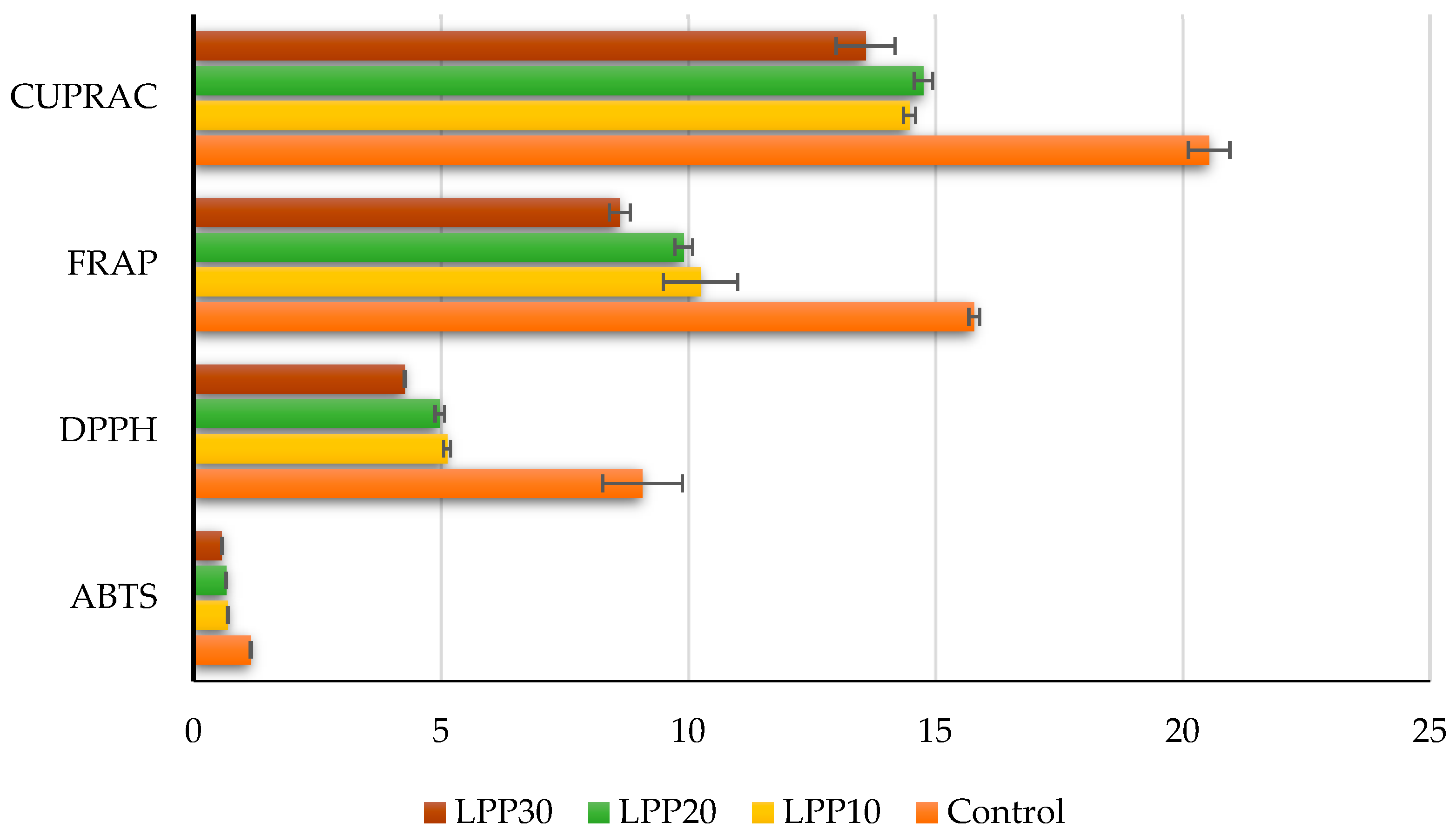
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rothman, A.J.; Sheeran, P.; Wood, W. Reflective and Automatic Processes in the Initiation and Maintenance of Dietary Change. Ann. Behav. Med. 2009, 38, 4–17. [Google Scholar] [CrossRef] [PubMed]
- González-Monroy, C.; Gómez-Gómez, I.; Olarte-Sánchez, C.M.; Motrico, E. Eating Behaviour Changes during the COVID-19 Pandemic: A Systematic Review of Longitudinal Studies. Int. J. Environ. Res. Public Health 2021, 18, 11130. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J.; Mangels, A.R.; Fresán, U.; Marsh, K.; Miles, F.L.; Saunders, A.V.; Haddad, E.H.; Heskey, C.E.; Johnston, P.; Larson-Meyer, E.; et al. The Safe and Effective Use of Plant-Based Diets with Guidelines for Health Professionals. Nutrients 2021, 13, 4144. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization European Office for the Prevention and Control of Noncommunicable Diseases. Plant-Based Diets and Their Impact on Health, Sustainability and the Environment: A Review of the Evidence: WHO European Office for the Prevention and Control of Noncommunicable Diseases; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2021. [Google Scholar]
- Kamiński, M.; Skonieczna-Żydecka, K.; Nowak, J.K.; Stachowska, E. Global and local diet popularity rankings, their secular trends, and seasonal variation in Google Trends data. Nutrition 2020, 79–80, 110759. [Google Scholar] [CrossRef] [PubMed]
- Amao, I. Health Benefits of Fruits and Vegetables: Review from Sub-Saharan Africa. In Vegetables: Importance of Quality Vegetables to Human Health; IntechOpen: Rijeka, Croatia, 2018; pp. 33–53, Chapter 3; ISBN 978-1-78923-507-4. [Google Scholar]
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. Int. Rev. J. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Mead, M.L.; Hill, A.; Carter, S.; Coates, A. The Effect of Nut Consumption on Diet Quality, Cardiometabolic and Gastrointestinal Health in Children: A Systematic Review of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2021, 18, 454. [Google Scholar] [CrossRef]
- Pradhan, C. Nuts as Dietary Source of Fatty Acids and Micro Nutrients in Human Health. In Nuts and Nut Products in Human Health and Nutrition; Peter, N., Ed.; IntechOpen: Rijeka, Croatia, 2021; Chapter 3; ISBN 978-1-78985-511-1. [Google Scholar]
- Vadivel, V.; Kunyanga, C.N.; Biesalski, H.K. Health benefits of nut consumption with special reference to body weight control. Nutrition 2012, 28, 1089–1097. [Google Scholar] [CrossRef]
- Mihaylova, D.; Popova, A.; Desseva, I.; Manolov, I.; Petkova, N.; Vrancheva, R.; Peltekov, A.; Slavov, A.; Zhivondov, A. Comprehensive Evaluation of Late Season Peach Varieties (Prunus persica L.): Fruit Nutritional Quality and Phytochemicals. Molecules 2021, 26, 2818. [Google Scholar] [CrossRef]
- Mihaylova, D.; Desseva, I.; Popova, A.; Dincheva, I.; Vrancheva, R.; Lante, A.; Krastanov, A. GC-MS Metabolic Profile and α-Glucosidase-, α-Amylase-, Lipase-, and Acetylcholinesterase-Inhibitory Activities of Eight Peach Varieties. Molecules 2021, 26, 4183. [Google Scholar] [CrossRef]
- Potter, M.; Vlassopoulos, A.; Lehmann, U. Snacking Recommendations Worldwide: A Scoping Review. Adv. Nutr. Int. Rev. J. 2018, 9, 86–98. [Google Scholar] [CrossRef]
- Barnes, T.L.; French, S.A.; Harnack, L.J.; Mitchell, N.R.; Wolfson, J. Snacking Behaviors, Diet Quality, and Body Mass Index in a Community Sample of Working Adults. J. Acad. Nutr. Diet. 2015, 115, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Dos Prazeres, I.C.; Domingues, A.F.N.; Campos, A.P.R.; Carvalho, A.V. Elaboration and characterization of snack bars made with ingredients from the Amazon. Acta Amaz. 2017, 47, 103–110. [Google Scholar] [CrossRef]
- Ahmad, S.; Vashney, A.; Srivasta, P. Quality Attributes of Fruit Bar Made from Papaya and Tomato by Incorporating Hydrocolloids. Int. J. Food Prop. 2005, 8, 89–99. [Google Scholar] [CrossRef]
- Abedelmaksoud, T.G.; Smuda, S.S.; Altemimi, A.B.; Mohamed, R.M.; Pratap-Singh, A.; Ali, M.R. Sunroot snack bar: Optimization, characterization, consumer perception, and storage stability assessment. Food Sci. Nutr. 2021, 9, 4394–4407. [Google Scholar] [CrossRef]
- Szydłowska, A.; Zielińska, D.; Łepecka, A.; Trząskowska, M.; Neffe-Skocińska, K.; Kołożyn-Krajewska, D. Development of Functional High-Protein Organic Bars with the Addition of Whey Protein Concentrate and Bioactive Ingredients. Agriculture 2020, 10, 390. [Google Scholar] [CrossRef]
- Orrego, C.; Salgado, N.; Botero, C.A. Developments and Trends in Fruit Bar Production and Characterization. Crit. Rev. Food Sci. Nutr. 2013, 54, 84–97. [Google Scholar] [CrossRef]
- Cunniff, P.; Washington, D. Official methods of analysis of aoac international. J. AOAC Int. 1997, 80, 127A. [Google Scholar]
- Analysis, A.A.M. of AACC Method 44-15.02. Moisture—Air-Oven Methods. Available online: http://methods.aaccnet.org/summaries/44-15-02.aspx. (accessed on 20 February 2022).
- Habiba, U.; Robin, A.; Hasan, M.; Toma, M.A.; Akhter, D.; Mazumder, A.R. Nutritional, textural, and sensory quality of bars enriched with banana flour and pumpkin seed flour. Foods Raw Mater. 2021, 9, 282–289. [Google Scholar] [CrossRef]
- Kujala, T.S.; Loponen, J.M.; Klika, K.D.; Pihlaja, K. Phenolics and Betacyanins in Red Beetroot (Beta vulgaris) Root: Distribution and Effect of Cold Storage on the Content of Total Phenolics and Three Individual Compounds. J. Agric. Food Chem. 2000, 48, 5338–5342. [Google Scholar] [CrossRef]
- Kivrak, I.; Duru, M.E.; Öztürk, M.; Mercan, N.; Harmandar, M.; Topçu, G. Antioxidant, anticholinesterase and antimicrobial constituents from the essential oil and ethanol extract of Salvia potentillifolia. Food Chem. 2009, 116, 470–479. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Mihaylova, D.; Lante, A.; Krastanov, A. Total phenolic content, antioxidant and antimicrobial activity ofHaberlea rhodopensisextracts obtained by pressurized liquid extraction. Acta Aliment. 2015, 44, 326–332. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J.B.T.-M. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 15–27. ISBN 0076-6879. [Google Scholar]
- Apak, R.; Özyürek, M.; Karademir Çelik, S.; Güçlü, K. CUPRAC Method 2004. JAFC 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- ISO 4833-1:2013; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Microorganisms—Colony-Count Technique at 30 °C. International Organization for Standardization: London, UK, 2013.
- Assaad, H.I.; Zhou, L.; Carroll, R.J.; Wu, G. Rapid publication-ready MS-Word tables for one-way ANOVA. SpringerPlus 2014, 3, 474. [Google Scholar] [CrossRef]
- Memon, N.N. Nutritional Characteristics (Fatty Acid Profile, Proximate Composition and Dietary Feature) of Selected Nuts Available in Local Market. Pak. J. Anal. Environ. Chem. 2019, 20, 39–46. [Google Scholar] [CrossRef]
- Constantin, O.E. Functional Properties of Snack Bars. In Functional Foods; Lagouri, D.I.I.E.-V., Ed.; IntechOpen: Rijeka, Croatia, 2019; pp. 1–14, Chapter 4; ISBN 978-1-83881-150-1. [Google Scholar]
- EUR-Lex—32006R1924—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32006R1924 (accessed on 21 February 2022).
- Jabeen, S.; Huma, N.; Sameen, A.; Zia, M.A. Formulation and characterization of protein-energy bars prepared by using dates, apricots, cheese and whey protein isolate. Food Sci. Technol. 2021, 41, 197–207. [Google Scholar] [CrossRef]
- Arocas, A.; Varela, P.; González-Miret, M.L.; Salvador, A.; Heredia, F.J.; Fiszman, S.M. Differences in Colour Gamut Obtained with Three Synthetic Red Food Colourants Compared with Three Natural Ones: pH and Heat Stability. Int. J. Food Prop. 2013, 16, 766–777. [Google Scholar] [CrossRef]
- Srebernich, S.M.; Gonçalves, G.M.S.; De Cássia Salvucci Celeste Ormenese, R.; Ruffi, C.R.G. Physico-chemical, sensory and nutritional characteristics of cereal bars with addition of acacia gum, inulin and sorbitol. Food Sci. Technol. 2016, 36, 555–562. [Google Scholar] [CrossRef]
- Blumberg, J.B.; Camesano, T.A.; Cassidy, A.; Kris-Etherton, P.; Howell, A.; Manach, C.; Ostertag, L.M.; Sies, H.; Skulas-Ray, A.; Vita, J.A. Cranberries and Their Bioactive Constituents in Human Health. Adv. Nutr. 2013, 4, 618–632. [Google Scholar] [CrossRef]
- Schuster, M.J.; Wang, X.; Hawkins, T.; Painter, J.E. A Comprehensive review of raisins and raisin components and their relationship to human health. J. Nutr. Health 2017, 50, 203–216. [Google Scholar] [CrossRef]
- Średnicka-Tober, D.; Kazimierczak, R.; Ponder, A.; Hallmann, E. Biologically Active Compounds in Selected Organic and Conventionally Produced Dried Fruits. Foods 2020, 9, 1005. [Google Scholar] [CrossRef] [PubMed]
- Dobrinas, S.; Soceanu, A.; Popescu, V.; Popovici, I.C.; Jitariu, D. Relationship between Total Phenolic Content, Antioxidant Capacity, Fe and Cu Content from Tea Plant Samples at Different Brewing Times. Processes 2021, 9, 1311. [Google Scholar] [CrossRef]
- Osman, M.O.; Mahmoud, G.I.; Shoman, S. Correlation Between Total Phenols Content, Antioxidant Power and Cytotoxicity. Biointerface Res. Appl. Chem. 2020, 11, 10640–10653. [Google Scholar] [CrossRef]
- Ly, H.T.; Le, V.K.T.; Le, V.M.; Phan, C.S.; Vo, C.T.; Nguyen, M.L.; Phan, T.A.D. Phytochemical analysis and correlation of total polyphenol content and antioxidant properties of Symplocos cochinchinensis leaves. Minist. Sci. Technol. Vietnam 2022, 64, 43–48. [Google Scholar] [CrossRef]
- Kilicgun, H.; Altıner, D.; Kılıçgün, H. Correlation between antioxidant effect mechanisms and polyphenol content of Rosa canina. Pharmacogn. Mag. 2010, 6, 238–241. [Google Scholar] [CrossRef]
- Szczesniak, A.S. Texture is a sensory property. Food Qual. Prefer. 2002, 13, 215–225. [Google Scholar] [CrossRef]
- Ribeiro, A. Elaboração de uma barra de cereal de quinoa e suas propriedades sensoriais e nutricionais. Aliment. E Nutr. Araraquara 2011, 22, 63–69. [Google Scholar]
- Samakradhamrongthai, R.S.; Jannu, T.; Renaldi, G. Physicochemical properties and sensory evaluation of high energy cereal bar and its consumer acceptability. Heliyon 2021, 7, e07776. [Google Scholar] [CrossRef]
- Rao, Q.; Kamdar, A.K.; Labuza, T.P. Storage Stability of Food Protein Hydrolysates—A Review. Crit. Rev. Food Sci. Nutr. 2013, 56, 1169–1192. [Google Scholar] [CrossRef]
- das Neves, J.S.C. Physical Stability of High Protein Bars during Shelf-Life. 2016. Available online: https://fenix.tecnico.ulisboa.pt/downloadFile/1689244997256129/Physical%20Stability%20of%20High%20Protein%20Bars%20during%20Shelf-life.pdf (accessed on 25 May 2022).
- Da Silva, E.P.; Siqueira, H.H.; Damiani, C.; Boas, E.V.D.B.V. Physicochemical and sensory characteristics of snack bars added of jerivá flour (Syagrus romanzoffiana). Food Sci. Technol. 2016, 36, 421–425. [Google Scholar] [CrossRef]
- Loveday, S.M.; Hindmarsh, J.P.; Creamer, L.K.; Singh, H. Physicochemical changes in intermediate-moisture protein bars made with whey protein or calcium caseinate. Food Res. Int. 2010, 43, 1321–1328. [Google Scholar] [CrossRef]
- Agbaje, R.; Hassan, C.Z.; Arifin, N.; Abdul Rahman, A.; Faujan, N.H. Development and physico-chemical analysis of granola formulated with puffed glutinous rice and selected dried Sunnah foods. Int. Food Res. J. 2016, 23, 498–506. [Google Scholar]
- Pintado, C.M.; Veloso, A.; Maria, Z.; Silveira, A.; Beato, H.; de Andrade, L.P.; Delgado, F. New flavour bars with cherry, almond and honey. Emir. J. Food Agric. 2020, 32, 857–863. [Google Scholar] [CrossRef]
- Kang, J.; Tang, S.; Liu, R.H.; Wiedmann, M.; Boor, K.; Bergholz, T.; Wang, S. Effect of Curing Method and Freeze-Thawing on Subsequent Growth of Listeria monocytogenes on Cold-Smoked Salmon. J. Food Prot. 2012, 75, 1619–1626. [Google Scholar] [CrossRef]
- Rahman, M.S. pH in Food Preservation. In Handbook of Food Preservation; Rahman, M.S., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 289–295. ISBN 9780429191084. [Google Scholar]
- Mihaylova, D.; Popova, A.; Desseva, I.; Petkova, N.; Stoyanova, M.; Vrancheva, R.; Slavov, A.; Slavchev, A.; Lante, A. Comparative Study of Early- and Mid-Ripening Peach (Prunus persica L.) Varieties: Biological Activity, Macro-, and Micro- Nutrient Profile. Foods 2021, 10, 164. [Google Scholar] [CrossRef]
- Mohd-Zaki, Z.; Bastidas-Oyanedel, J.R.; Lu, Y.; Hoelzle, R.; Pratt, S.; Slater, F.R.; Batstone, D.J. Influence of pH Regulation Mode in Glucose Fermentation on Product Selection and Process Stability. Microorganisms 2016, 4, 2. [Google Scholar] [CrossRef]



| Type of Bonbon | Walnut, % | Almond, % | Raisin, % | Cranberry, % | Cocoa Butter, % | LPP, % |
|---|---|---|---|---|---|---|
| Control | 18 | 18 | 18 | 18 | 28 | - |
| LPP10 | 15 | 15 | 15 | 15 | 30 | 10 |
| LPP20 | 12 | 12 | 12 | 12 | 32 | 20 |
| LPP30 | 9 | 9 | 9 | 9 | 34 | 30 |
| Bonbon Formulations | Weight, g | Diameter, mm | Ash Content, % | Moisture Content, % |
|---|---|---|---|---|
| Control | 8.87 ± 0.60 a | 25.15 ± 0.39 a | 1.20 ± 0.31 a | 7.51 ± 0.03 a |
| LPP10 | 8.31 ± 0.54 a | 25.12 ± 0.67 a | 1.44 ± 0.34 a | 5.05 ± 0.05 d |
| LPP20 | 7.92 ± 0.69 a | 24.61 ± 0.88 a | 1.41 ± 0.08 a | 7.07 ± 0.09 b |
| LPP30 | 7.86 ± 0.79 a | 24.70 ± 0.80 a | 1.47 ± 0.00 a | 6.45 ± 0.06 c |
| Bonbon Formulations, 100 g | Proteins, g | Carbohydrates, g | Sugars, g | Fiber, g | Fat, g | Monosaturated Fats, g | ώ 3, g | Energy, kcal |
|---|---|---|---|---|---|---|---|---|
| Control sample | 7.77 | 29.16 | 25.70 | 4.96 | 50.54 | 18.96 | 1.73 | 612.36 |
| LPP10 | 6.48 | 25.50 | 21.89 | 4.38 | 48.76 | 19.80 | 1.44 | 574.99 |
| LPP20 | 5.18 | 21.87 | 18.09 | 3.80 | 46.98 | 20.64 | 1.15 | 537.63 |
| LPP30 | 3.89 | 18.22 | 14.27 | 3.22 | 45.21 | 21.48 | 0.86 | 500.27 |
| Bonbon Formulations | L | a | b | c | h |
|---|---|---|---|---|---|
| Control sample | 47.74 ± 4.26 a | 9.46 ± 1.97 a | 15.66 ± 2.33 b | 18.44 ± 1.63 b | 58.63 ± 8.13 a |
| LPP10 | 50.88 ± 2.13 a | 11.38 ± 1.31 a | 21.75 ± 1.19 ab | 24.56 ± 1.57 ab | 62.44 ± 1.89 a |
| LPP20 | 54.96 ± 2.65 a | 13.12 ± 1.62 a | 28.04 ± 2.01 a | 30.96 ± 2.44 a | 64.98 ± 1.55 a |
| LPP30 | 53.85 ± 2.50 a | 12.42 ± 1.67 a | 26.57 ± 1.83 a | 29.39 ± 1.21 a | 64.86 ± 4.22 a |
| Bonbon Formulations | Total Flavonoid Content, μgQE/g fw | Total Phenolic Content, mgGAE/g dw |
|---|---|---|
| Control sample | 84.64 ± 1.69 c | 1.89 ± 0.03 a |
| LPP10 | 78.13 ± 1.36 d | 1.33 ± 0.00 c |
| LPP20 | 117.63 ± 1.37 a | 1.40 ± 0.04 b |
| LPP30 | 100.29 ± 2.55 b | 1.21 ± 0.01 d |
| Bonbon Formulations | Hardness/MCF, N | Fracturability, N | Adhesiveness, J | |||
|---|---|---|---|---|---|---|
| Day 1 | Day 5 | Day 1 | Day 5 | Day 1 | Day 5 | |
| Control | 31.14 ± 1.96 a | 38.03 ± 3.11 a | 16.10 ± 2.26 a | 18.02 ± 0.51 a | 0.18 ± 0.05 a | 0.36 ± 0.09 a |
| LPP10 | 33.89 ± 4.79 b | 42.80 ± 2.80 b | 19.88 ± 1.92 a | 19.46 ± 1.28 a | 0.32 ± 0.03 b | 0.73 ± 0.07 b |
| LPP20 | 41.90 ± 2.93 c | 46.81 ± 0.62 c | 22.25 ± 1.22 ab | 28.42 ± 6.47 b | 0.35 ± 0.04 b | 1.14 ± 0.63 c |
| LPP30 | 48.94 ± 1.79 d | 54.02 ± 2.94 d | 24.91 ± 2.27 c | 30.50 ± 1.39 b | 0.38 ± 0.03 c | 0.98 ± 0.15 d |
| Bonbon Formulations | Water Activity, aw | |
|---|---|---|
| Day 1 | Day 5 | |
| Control sample | 0.559 ± 0.007 c | 0.546 ± 0.06 c |
| LPP10 | 0.503 ± 0.009 b | 0.496 ± 0.06 b |
| LPP20 | 0.492 ± 0.003 a | 0.482 ± 0.003 b |
| LPP30 | 0.468 ± 0.013 a | 0.458 ± 0.013 a |
| Bonbon Formulations | YM, CFU/mL | AMM, CFU/mL | ||||
|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 5 | Day 1 | Day 3 | Day 5 | |
| Control sample | 1500 | 2100 | 3600 | 1050 | 3400 | 25,000 |
| LPP10 | 1150 | 2200 | 1450 | 400 | 1000 | 2250 |
| LPP20 | 500 | 700 | 600 | 100 | 950 | 1000 |
| LPP30 | 1300 | 1000 | 500 | 600 | 200 | 1100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihaylova, D.; Popova, A.; Goranova, Z.; Doykina, P. Development of Healthy Vegan Bonbons Enriched with Lyophilized Peach Powder. Foods 2022, 11, 1580. https://doi.org/10.3390/foods11111580
Mihaylova D, Popova A, Goranova Z, Doykina P. Development of Healthy Vegan Bonbons Enriched with Lyophilized Peach Powder. Foods. 2022; 11(11):1580. https://doi.org/10.3390/foods11111580
Chicago/Turabian StyleMihaylova, Dasha, Aneta Popova, Zhivka Goranova, and Pavlina Doykina. 2022. "Development of Healthy Vegan Bonbons Enriched with Lyophilized Peach Powder" Foods 11, no. 11: 1580. https://doi.org/10.3390/foods11111580
APA StyleMihaylova, D., Popova, A., Goranova, Z., & Doykina, P. (2022). Development of Healthy Vegan Bonbons Enriched with Lyophilized Peach Powder. Foods, 11(11), 1580. https://doi.org/10.3390/foods11111580








