Effects of Xanthan Gum, Lambda-Carrageenan and Psyllium Husk on the Physical Characteristics and Glycaemic Potency of White Bread
Abstract
1. Introduction
2. Materials and Methods
2.1. Bread Formulation
2.2. Loaf Specific Volume Measurement
2.3. Visual Observation of Crumb Appearance
2.4. Moisture Analysis of Bread Crumb
2.5. Bread Firmness
2.6. In Vitro Method for Determination of Glycaemic Potency of Bread Samples
2.6.1. In Vitro Digestion
2.6.2. Quantification of Glucose Released
2.6.3. Derivation of Glycaemic Potency of Bread Samples
2.7. Determination of NSP Leaching
2.8. Statistical Analysis
3. Results and Discussion
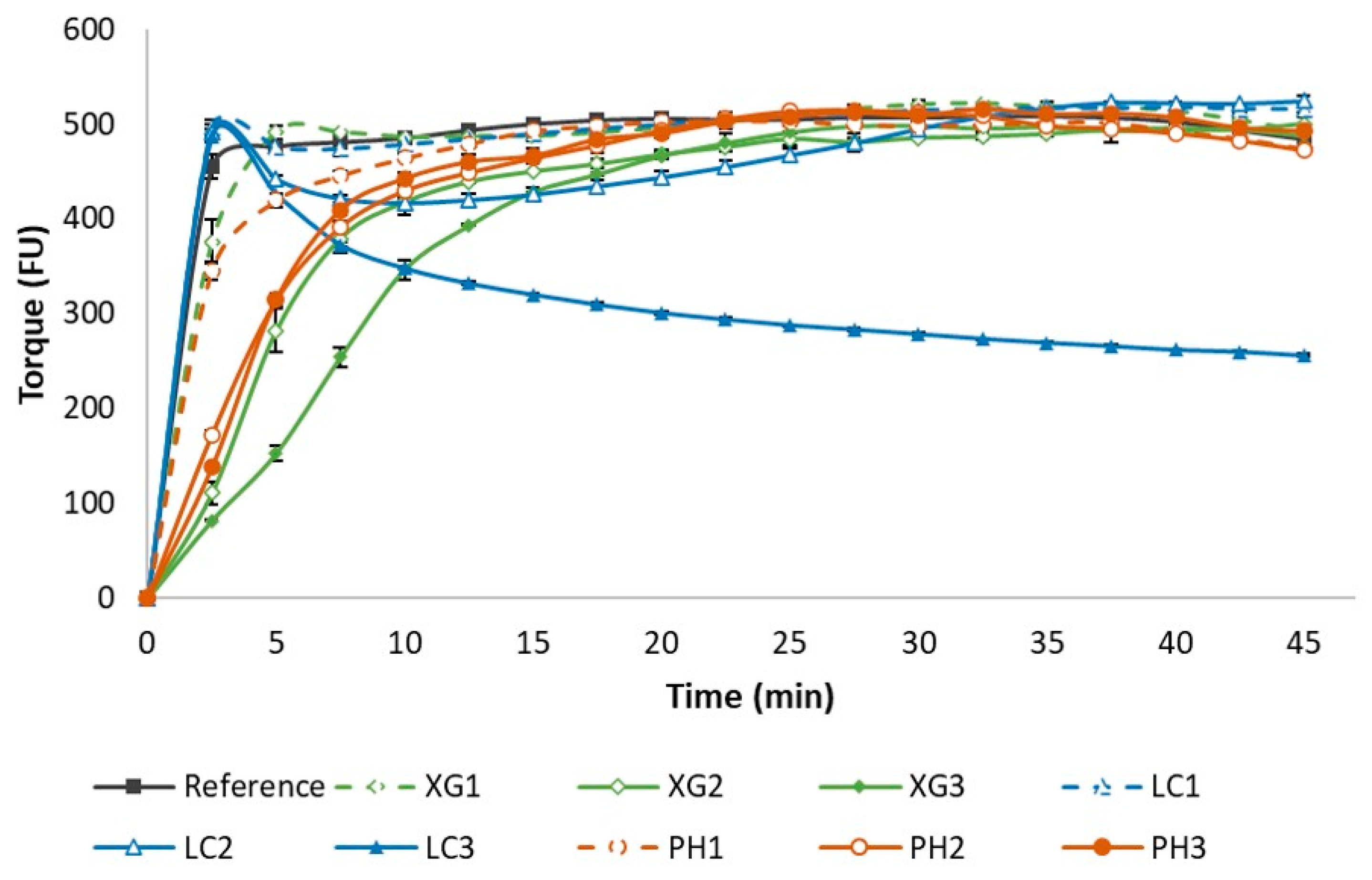
3.1. Rheological Behaviour of Dough
3.2. Loaf Specific Volume
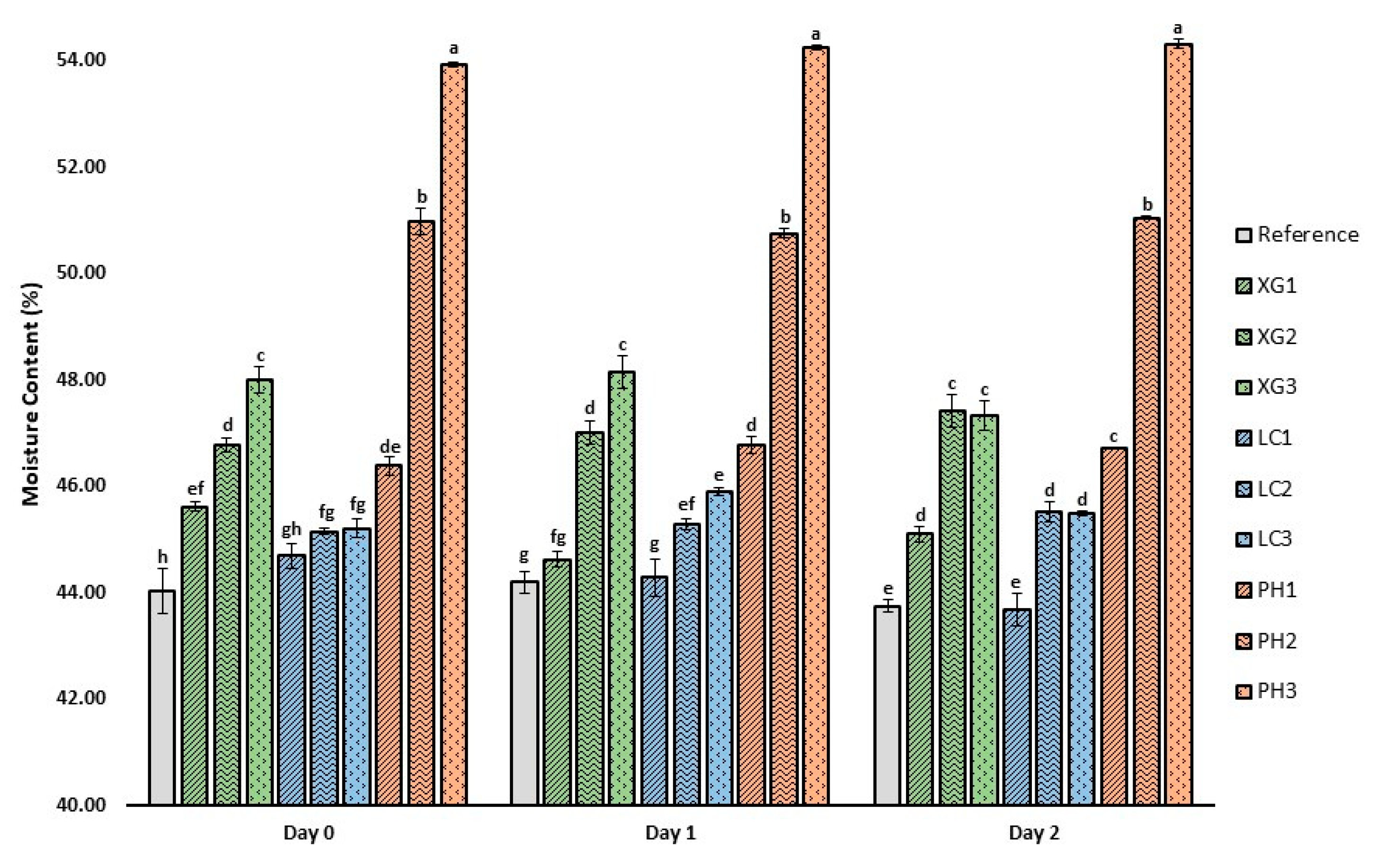
3.3. Moisture Content of Bread Crumb
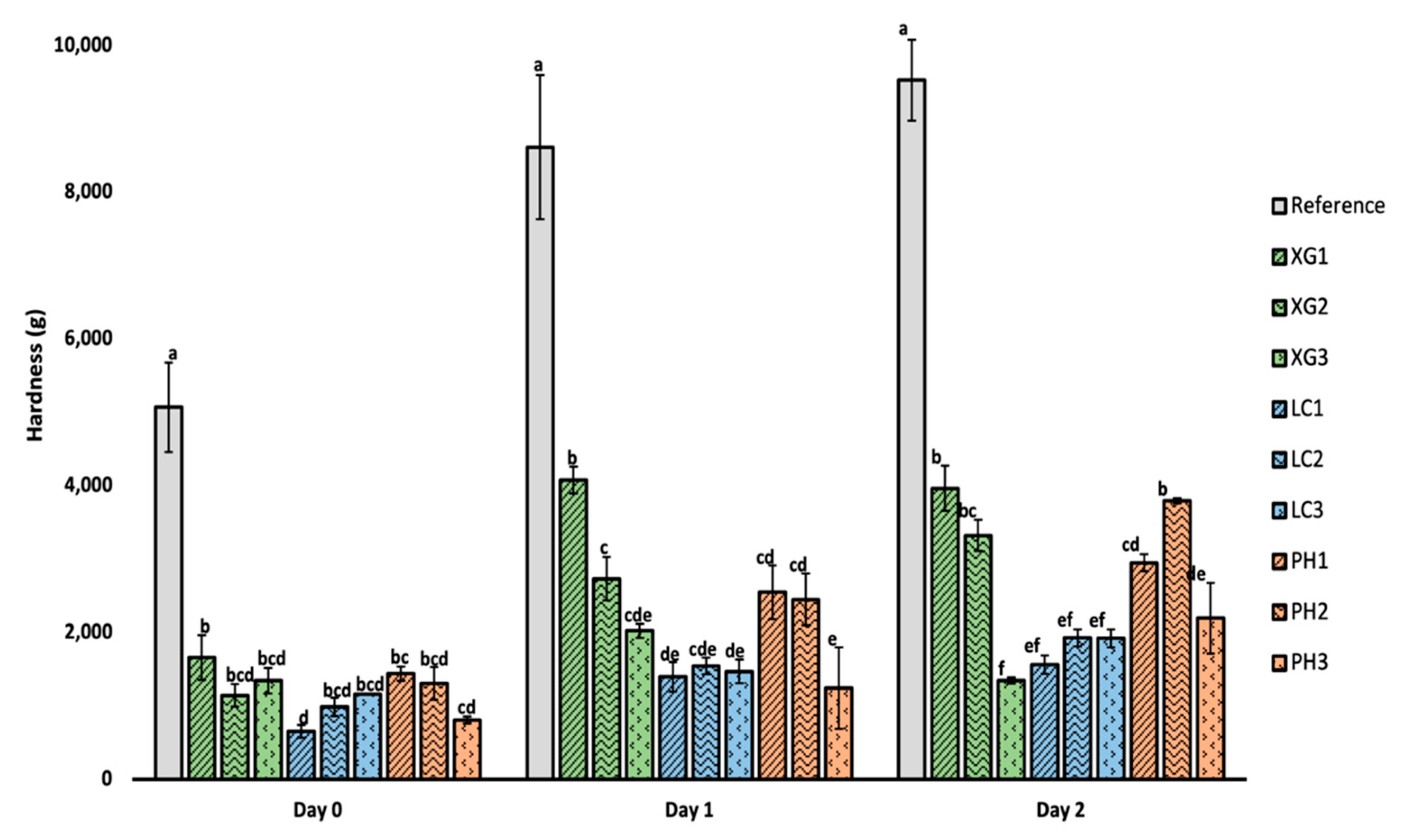
3.4. Firmness of Bread
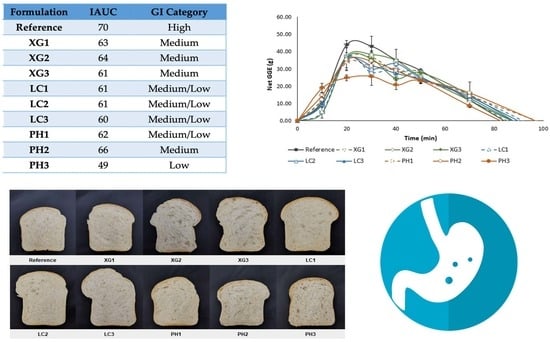
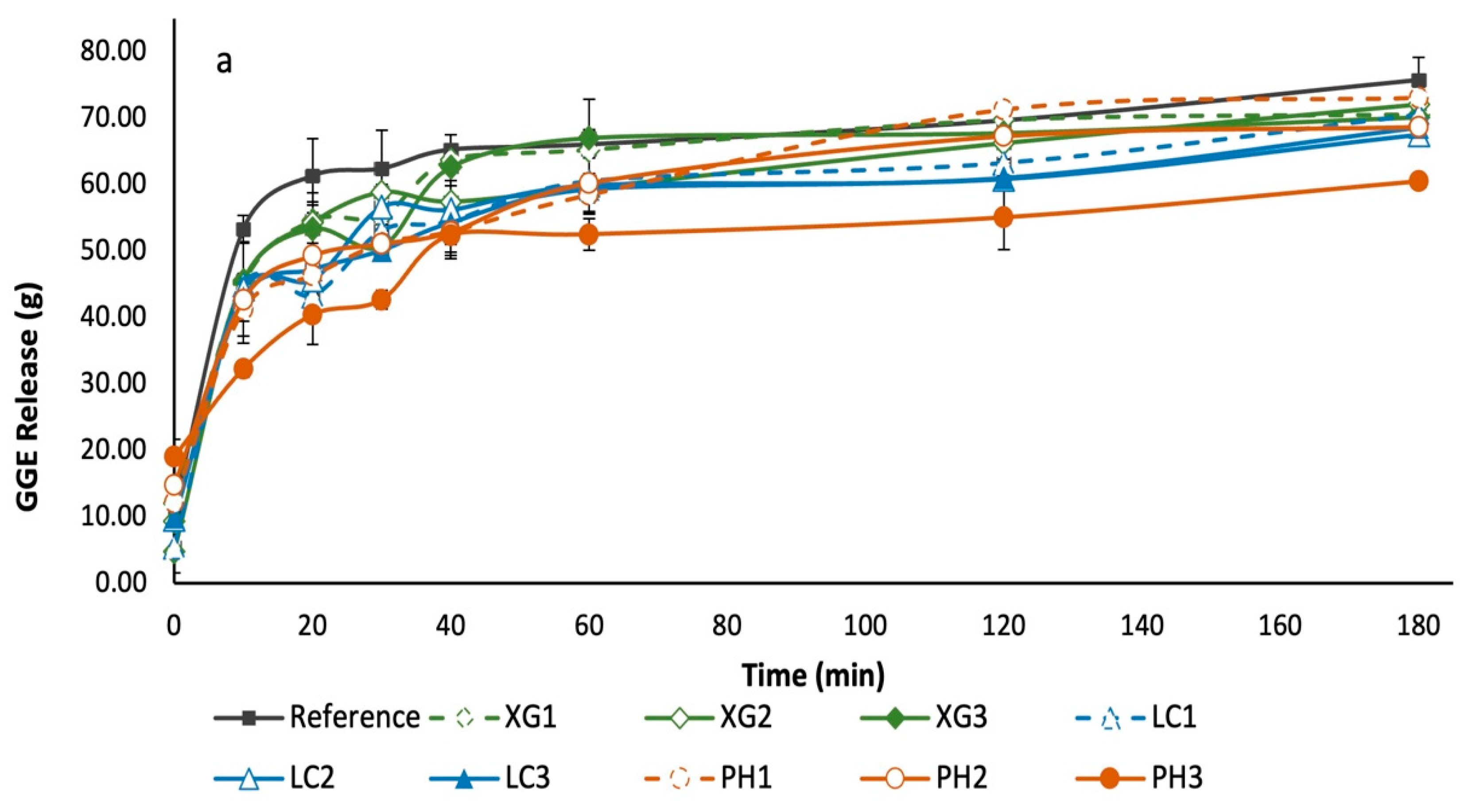
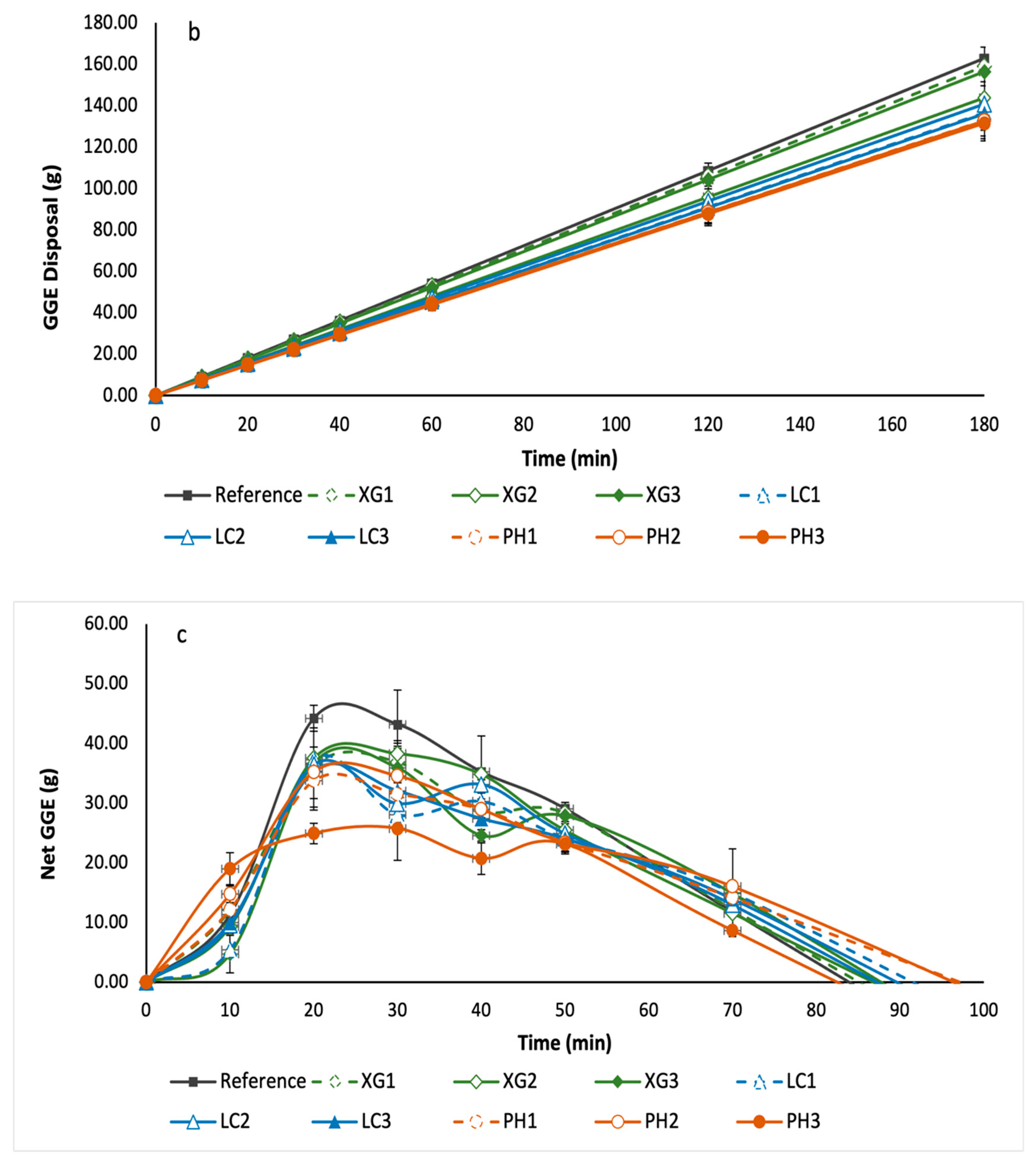
3.5. Glycaemic Potency of Bread Samples
3.6. Leaching of NSP from Bread
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, A.K.; Gupta, R.; Ghosh, A.; Misra, A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Akila, S.R.V.; Mishra, S.; Hardacre, A.; Matia-Merino, L.; Goh, K.; Warren, F.; Monro, J. Kernel structure in breads reduces in vitro starch digestion rate and estimated glycaemic potency only at high grain inclusion rates. Food Struct.-Neth. 2019, 21, 100109. [Google Scholar] [CrossRef]
- Sadler, M. Food, Glycaemic Response and Health; ILSI Europe: Bruxelles, Belgium, 2011; p. 40. [Google Scholar]
- Bloksma, A. Dough structure, dough rheology, and baking quality. Cereal Foods World 1990, 35, 237–244. [Google Scholar]
- Horstmann, S.W.; Lynch, K.M.; Arendt, E.K. Starch characteristics linked to gluten-free products. Foods 2017, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Roman, L.; Martinez, M.M. Structural basis of resistant starch (RS) in bread: Natural and commercial alternatives. Foods 2019, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.; Lundquist, I.; Björck, I.; Eliasson, A.C.; Asp, N.G. Degree of starch gelatinization, digestion rate of starch in vitro, and metabolic response in rats. Am. J. Clin. Nutr. 1988, 47, 1010–1016. [Google Scholar] [CrossRef]
- Burton, P.M.; Monro, J.A.; Alvarez, L.; Gallagher, E. Glycemic impact and health: New horizons in white bread formulations. Crit. Rev. Food Sci. Nutr. 2011, 51, 965–982. [Google Scholar] [CrossRef]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.S.; de Boeck, G.; Becker, K. Dietary Roles of Non-Starch Polysachharides in Human Nutrition: A Review. Crit. Rev. Food Sci. Nutr. 2012, 52, 899–935. [Google Scholar] [CrossRef]
- Wood, P.J.; Braaten, J.T.; Scott, F.W.; Riedel, K.D.; Wolynetz, M.S.; Collins, M.W. Effect of dose and modification of viscous properties of oat gum on plasma glucose and insulin following an oral glucose load. Br. J. Nutr. 1994, 72, 731–743. [Google Scholar] [CrossRef]
- Wursch, P.; PiSunyer, F.X. The role of viscous soluble fiber in the metabolic control of diabetes—A review with special emphasis on cereals rich in beta-glucan. Diabetes Care 1997, 20, 1774–1780. [Google Scholar] [CrossRef]
- Ho, I.H.H.; Matia-Merino, L.; Huffman, L.M. Use of viscous fibres in beverages for appetite control: A review of studies. Int. J. Food Sci. Nutr. 2015, 66, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Wolever, T.M.S.; Leeds, A.R.; Gassull, M.A.; Haisman, P.; Dilawari, J.; Goff, D.V.; Metz, G.L.; Alberti, K. Dietary fibres, fibre analogues, and glucose tolerance: Importance of viscosity. Br. Med. J. 1978, 1, 1392–1394. [Google Scholar] [CrossRef] [PubMed]
- Adiotomre, J.; Eastwood, M.A.; Edwards, C.A.; Brydon, W.G. Dietary fiber: In vitro methods that anticipate nutrition and metabolic activity in humans. Am. J. Clin. Nutr. 1990, 52, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, N.A.; Redfern, J.S.; Jarjis, H.; Holgate, A.M.; Hanning, I.; Scarpello, J.H.B.; Johnson, I.T.; Read, N.W. The mechanism of action of guar gum in improving glucose tolerance in man. Clin. Sci. 1984, 66, 329–336. [Google Scholar] [CrossRef]
- Lentle, R.G.; Janssen, P.W.M. Physical characteristics of digesta and their influence on flow and mixing in the mammalian intestine: A review. J. Comp. Physiol. B 2008, 178, 673–690. [Google Scholar] [CrossRef]
- Flourie, B.; Vidon, N.; Florent, C.; Bernier, J.J. Effect of pectin on jejunal glucose absorption and unstirred layer thickness in normal man. Gut 1984, 25, 936–941. [Google Scholar] [CrossRef]
- Johnson, I.T.; Gee, J.M. Effect of gel-forming gums on the intestinal unstirred layer and sugar transport in vitro. Gut 1981, 22, 398–403. [Google Scholar] [CrossRef]
- Brennan, C.S.; Blake, D.E.; Ellis, P.R.; Schofield, J.D. Effects of guar galactomannan on wheat bread microstructure and on the in vitro and in vivo digestibility of starch in bread. J. Cereal Sci. 1996, 24, 151–160. [Google Scholar] [CrossRef]
- Hardacre, A.K.; Yap, S.-Y.; Lentle, R.G.; Monro, J.A. The effect of fibre and gelatinised starch type on amylolysis and apparent viscosity during in vitro digestion at a physiological shear rate. Carbohydr. Polym. 2015, 123, 80–88. [Google Scholar] [CrossRef]
- Monro, J.A.; Mishra, S.; Venn, B. Baselines representing blood glucose clearance improve in vitro prediction of the glycaemic impact of customarily consumed food quantities. Br. J. Nutr. 2010, 103, 295–305. [Google Scholar] [CrossRef]
- Monro, J.A.; Mishra, S. Glycemic impact as a property of foods is accurately measured by an available carbohydrate method that mimics the glycemic response. J. Nutr. 2010, 140, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Foster-Powell, K.; Holt, S.H.; Brand-Miller, J.C. International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr. 2002, 76, 5–56. [Google Scholar] [CrossRef] [PubMed]
- Englyst, K.N.; Englyst, H.N.; Hudson, G.J.; Cole, T.J.; Cummings, J.H. Rapidly available glucose in foods: An in vitro measurement that reflects the glycemic response. Am. J. Clin. Nutr. 1999, 69, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Belorio, M.; Gomez, M. Effect of Hydration on Gluten-Free Breads Made with Hydroxypropyl Methylcellulose in Comparison with Psyllium and Xanthan Gum. Foods 2020, 9, 1548. [Google Scholar] [CrossRef]
- Culetu, A.; Duta, D.E.; Papageorgiou, M.; Varzakas, T. The Role of Hydrocolloids in Gluten-Free Bread and Pasta; Rheology, Characteristics, Staling and Glycemic Index. Foods 2021, 10, 3121. [Google Scholar] [CrossRef] [PubMed]
- Leon, A.E.; Ribotta, P.D.; Ausar, S.F.; Fernandez, C.; Landa, C.A.; Beltramo, D.M. Interactions of different carrageenan isoforms and flour components in breadmaking. J. Agric. Food Chem. 2000, 48, 2634–2638. [Google Scholar] [CrossRef]
- Shyama Prasad Rao, R.; Sai Manohar, R.; Muralikrishna, G. Functional properties of water-soluble non-starch polysaccharides from rice and ragi: Effect on dough characteristics and baking quality. LWT Food Sci. Technol. 2007, 40, 1678–1686. [Google Scholar] [CrossRef]
- Man, S.; Paucean, A.; Muste, S.; Pop, A.; Muresan, E.A. Influence of psyllium husk (Plantago ovata) on bread quality. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca-Food Sci. Technol. 2017, 74, 33–34. [Google Scholar] [CrossRef][Green Version]
- Mironeasa, S.; Codina, G.G. Optimization of Bread Quality of Wheat Flour with Psyllium Addition by Using Response Surface Methodology. J. Culin. Sci. Technol. 2021, 1–16. [Google Scholar] [CrossRef]
- Rathnayake, H.; Navaratne, S.; Navaratne, C. Porous crumb structure of leavened baked products. Int. J. Food Sci. 2018, 8187318. [Google Scholar] [CrossRef]
- Guarda, A.; Rosell, C.M.; Benedito, C.; Galotto, M.J. Different hydrocolloids as bread improvers and antistaling agents. Food Hydrocoll. 2004, 18, 241–247. [Google Scholar] [CrossRef]
- Pawar, H.; Varkhade, C. Isolation, characterization and investigation of Plantago ovata husk polysaccharide as superdisintegrant. Int. J. Biol. Macromol. 2014, 69, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Chong, R.W.W.; Ball, M.; McRae, C.; Packer, N.H. Comparing the chemical composition of dietary fibres prepared from sugarcane, psyllium husk and wheat dextrin. Food Chem. 2019, 298, 125032. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.; Ronda, F.; Blanco, C.A.; Caballero, P.A.; Apesteguía, A. Effect of dietary fibre on dough rheology and bread quality. Eur. Food Res. Technol. 2003, 216, 51–56. [Google Scholar] [CrossRef]
- Kosseva, M.; Webb, C. Food Industry Wastes: Assessment and Recuperation of Commodities; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Mandala, I.G. Viscoelastic properties of starch and non-starch thickeners in simple mixtures or model food. In Viscoelasticity: From Theory to Biological Applications; Intech: London, UK, 2012; pp. 217–236. [Google Scholar]
- Abdullah, M.M.; Aldughpassi, A.D.H.; Sidhu, J.S.; Al-Foudari, M.Y.; Al-Othman, A.R.A. Effect of psyllium husk addition on the instrumental texture and consumer acceptability of high-fiber wheat pan bread and buns. Ann. Agric. Sci. 2021, 66, 75–80. [Google Scholar] [CrossRef]
- Fabek, H.; Messerschmidt, S.; Brulport, V.; Goff, H.D. The effect of in vitro digestive processes on the viscosity of dietary fibres and their influence on glucose diffusion. Food Hydrocoll. 2014, 35, 718–726. [Google Scholar] [CrossRef]
- von Borries-Medrano, E.; Jaime-Fonseca, M.R.; Aguilar-Méndez, M.Á. Starch-galactomannans mixtures: Rheological and viscosity behavior in aqueous systems for food modeling. In Solubility of Polysaccharides; Intech: London, UK, 2017; pp. 65–87. [Google Scholar]
- Ellis, P.R.; Dawoud, F.M.; Morris, E.R. Blood glucose, plasma insulin and sensory responses to guar-containing wheat breads: Effects of molecular weight and particle size of guar gum. Br. J. Nutr. 1991, 66, 363–379. [Google Scholar] [CrossRef]
- Matia-Merino, L.; Prieto, M.; Roman, L.; Gómez, M. The impact of basil seed gum on native and pregelatinized corn flour and starch gel properties. Food Hydrocoll. 2019, 89, 122–130. [Google Scholar] [CrossRef]
- Slaughter, S.L.; Ellis, P.R.; Jackson, E.C.; Butterworth, P.J. The effect of guar galactomannan and water availability during hydrothermal processing on the hydrolysis of starch catalysed by pancreatic α-amylase. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2002, 1571, 55–63. [Google Scholar] [CrossRef]


| Formulation | Ingredients | Flour | Water | Yeast | Salt | NSP | Total |
|---|---|---|---|---|---|---|---|
| Reference | g | 600 | 387.4 | 7.2 | 12 | 0 | 1006.6 |
| %w/w | 59.6 | 38.5 | 0.7 | 1.2 | 0.0 | 100.0 | |
| XG1 | g | 600 | 415.2 | 7.2 | 12 | 6 | 1040.4 |
| %w/w | 57.7 | 39.9 | 0.7 | 1.2 | 0.6 | 100.0 | |
| XG2 | g | 600 | 480.6 | 7.2 | 12 | 18 | 1117.8 |
| %w/w | 53.7 | 43.0 | 0.6 | 1.1 | 1.6 | 100.0 | |
| XG3 | g | 600 | 525.3 | 7.2 | 12 | 30 | 1174.5 |
| %w/w | 51.1 | 44.7 | 0.6 | 1.0 | 2.6 | 100.0 | |
| LC1 | g | 600 | 390.3 | 7.2 | 12 | 6 | 1015.5 |
| %w/w | 59.1 | 38.4 | 0.7 | 1.2 | 0.6 | 100.0 | |
| LC2 | g | 600 | 414 | 7.2 | 12 | 18 | 1051.2 |
| %w/w | 57.1 | 39.4 | 0.7 | 1.1 | 1.7 | 100.0 | |
| LC3 | g | 600 | 435.3 | 7.2 | 12 | 30 | 1084.5 |
| %w/w | 55.3 | 40.1 | 0.7 | 1.1 | 2.8 | 100.0 | |
| PH1 | g | 600 | 426 | 7.2 | 12 | 6 | 1051.2 |
| %w/w | 57.1 | 40.5 | 0.7 | 1.1 | 0.6 | 100.0 | |
| PH2 | g | 600 | 528.9 | 7.2 | 12 | 18 | 1166.1 |
| %w/w | 51.5 | 45.4 | 0.6 | 1.0 | 1.5 | 100.0 | |
| PH3 | g | 600 | 634.2 | 7.2 | 12 | 30 | 1283.4 |
| %w/w | 46.8 | 49.4 | 0.6 | 0.9 | 2.3 | 100.0 |
| Formulation | IAUC (g.min) | RGP (GGE/100g dry wt) | RDS (%) |
|---|---|---|---|
| Reference | 1978.42 ± 186.79 a | 48.75 ± 1.20 a | 61.32 ± 5.57 a |
| XG1 | 1781.81 ± 225.49 ab | 40.45 ± 5.12 ab | 54.58 ± 4.22 ab |
| XG2 | 1796.87 ± 24.45 ab | 40.79 ± 0.55 ab | 54.31 ± 3.07 ab |
| XG3 | 1716.59 ± 167.89 ab | 38.97 ± 3.81 ab | 53.30 ± 3.67 ab |
| LC1 | 1713.33 ± 264.11 ab | 38.89 ± 6.00 ab | 43.50 ± 0.00 b |
| LC2 | 1710.49 ± 12.00 ab | 38.83 ± 0.27 ab | 45.63 ± 1.48 b |
| LC3 | 1686.67 ± 134.73 ab | 38.29 ± 3.06 ab | 47.22 ± 6.51 ab |
| PH1 | 1756.26 ± 24.56 ab | 39.87 ± 0.56 ab | 46.31 ± 2.99 ab |
| PH2 | 1855.75 ± 43.61 ab | 42.12 ± 0.99 ab | 49.31 ± 3.01 ab |
| PH3 | 1394.10 ± 50.28 b | 31.65 ± 1.14 b | 40.40 ± 4.49 b |
| Formulation | Estimated GI | GI Category | ||
|---|---|---|---|---|
| IAUC | RGP | RDS | ||
| Reference | 70 | 70 | 70 | High |
| XG1 | 63 | 58 | 62 | Medium |
| XG2 | 64 | 59 | 62 | Medium |
| XG3 | 61 | 56 | 61 | Medium |
| LC1 | 61 | 56 | 50 | Medium/Low |
| LC2 | 61 | 56 | 52 | Medium/Low |
| LC3 | 60 | 55 | 54 | Medium/Low |
| PH1 | 62 | 57 | 53 | Medium/Low |
| PH2 | 66 | 60 | 56 | Medium |
| PH3 | 49 | 45 | 46 | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yassin, Z.; Tan, Y.L.; SRV, A.; Monro, J.; Matia-Merino, L.; Lim, K.; Hardacre, A.; Mishra, S.; Goh, K.K.T. Effects of Xanthan Gum, Lambda-Carrageenan and Psyllium Husk on the Physical Characteristics and Glycaemic Potency of White Bread. Foods 2022, 11, 1513. https://doi.org/10.3390/foods11101513
Yassin Z, Tan YL, SRV A, Monro J, Matia-Merino L, Lim K, Hardacre A, Mishra S, Goh KKT. Effects of Xanthan Gum, Lambda-Carrageenan and Psyllium Husk on the Physical Characteristics and Glycaemic Potency of White Bread. Foods. 2022; 11(10):1513. https://doi.org/10.3390/foods11101513
Chicago/Turabian StyleYassin, Zawanah, Yin Li Tan, Akila SRV, John Monro, Lara Matia-Merino, Kaiyang Lim, Allan Hardacre, Suman Mishra, and Kelvin Kim Tha Goh. 2022. "Effects of Xanthan Gum, Lambda-Carrageenan and Psyllium Husk on the Physical Characteristics and Glycaemic Potency of White Bread" Foods 11, no. 10: 1513. https://doi.org/10.3390/foods11101513
APA StyleYassin, Z., Tan, Y. L., SRV, A., Monro, J., Matia-Merino, L., Lim, K., Hardacre, A., Mishra, S., & Goh, K. K. T. (2022). Effects of Xanthan Gum, Lambda-Carrageenan and Psyllium Husk on the Physical Characteristics and Glycaemic Potency of White Bread. Foods, 11(10), 1513. https://doi.org/10.3390/foods11101513