Encapsulation of Catechin into β-Glucan Matrix Using Wet Milling and Ultrasonication as a Coupled Approach: Characterization and Bioactivity Retention
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. β-Glucan Extraction
2.3. Preparation of Nanoparticles
2.4. Nanoencapsulation of Catechin
2.5. Characterisation of Nanoparticles
2.5.1. Particle Size, Polydispersity Index and Zeta Potential
2.5.2. Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.5.3. Scanning Electron Microscopy (SEM)
2.6. Encapsulation Efficiency
2.7. Swelling Behaviour
2.8. In Vitro Release Behaviour of Catechin
2.9. Retention of Antioxidant Activity after Gastrointestinal Digestion
2.9.1. In Vitro Anti-Obesity Activity
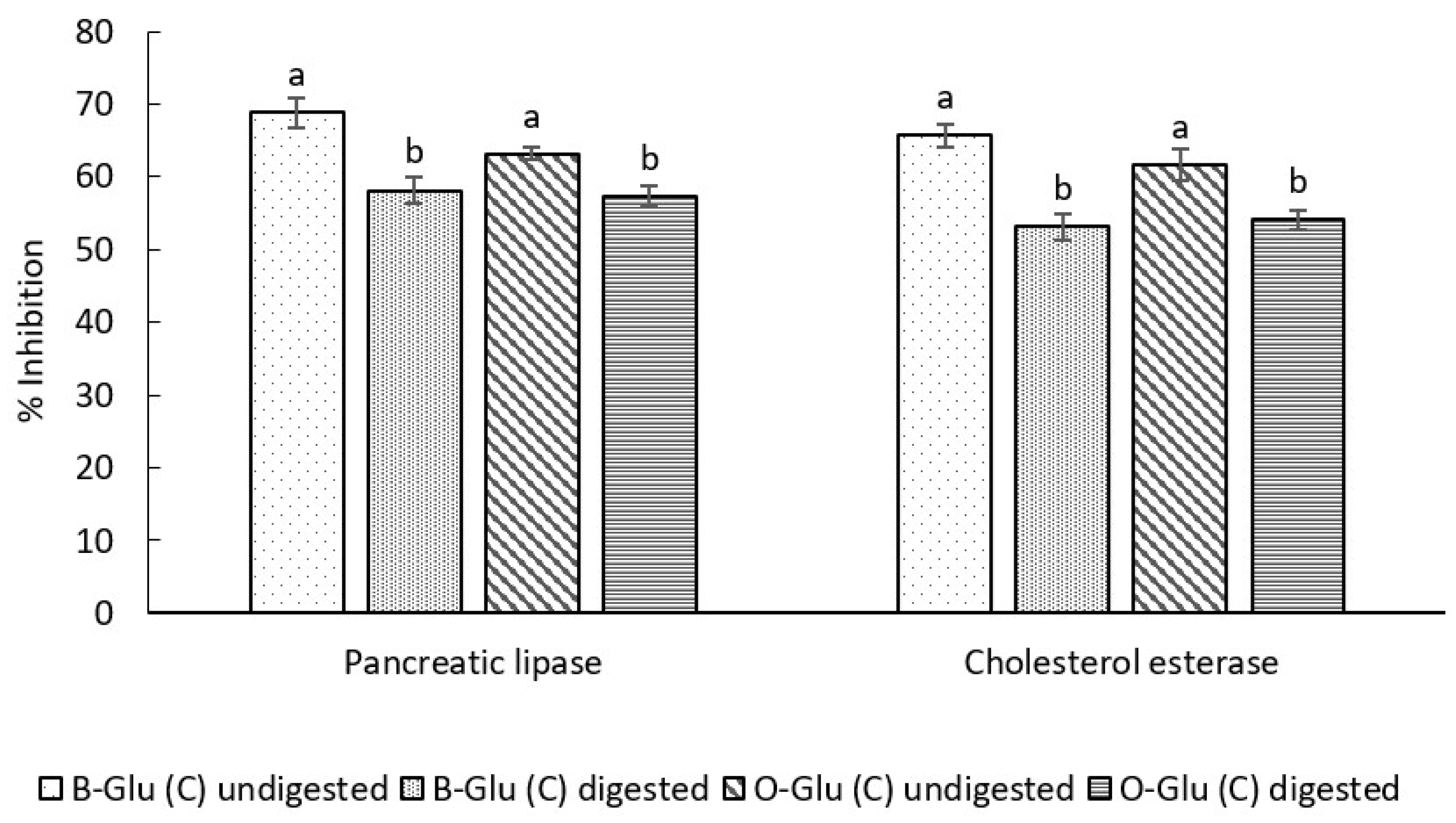
Pancreatic Lipase Inhibition Activity
Cholesterol Esterase (CE) Inhibition Assay
2.9.2. In Vitro Antioxidant Assay
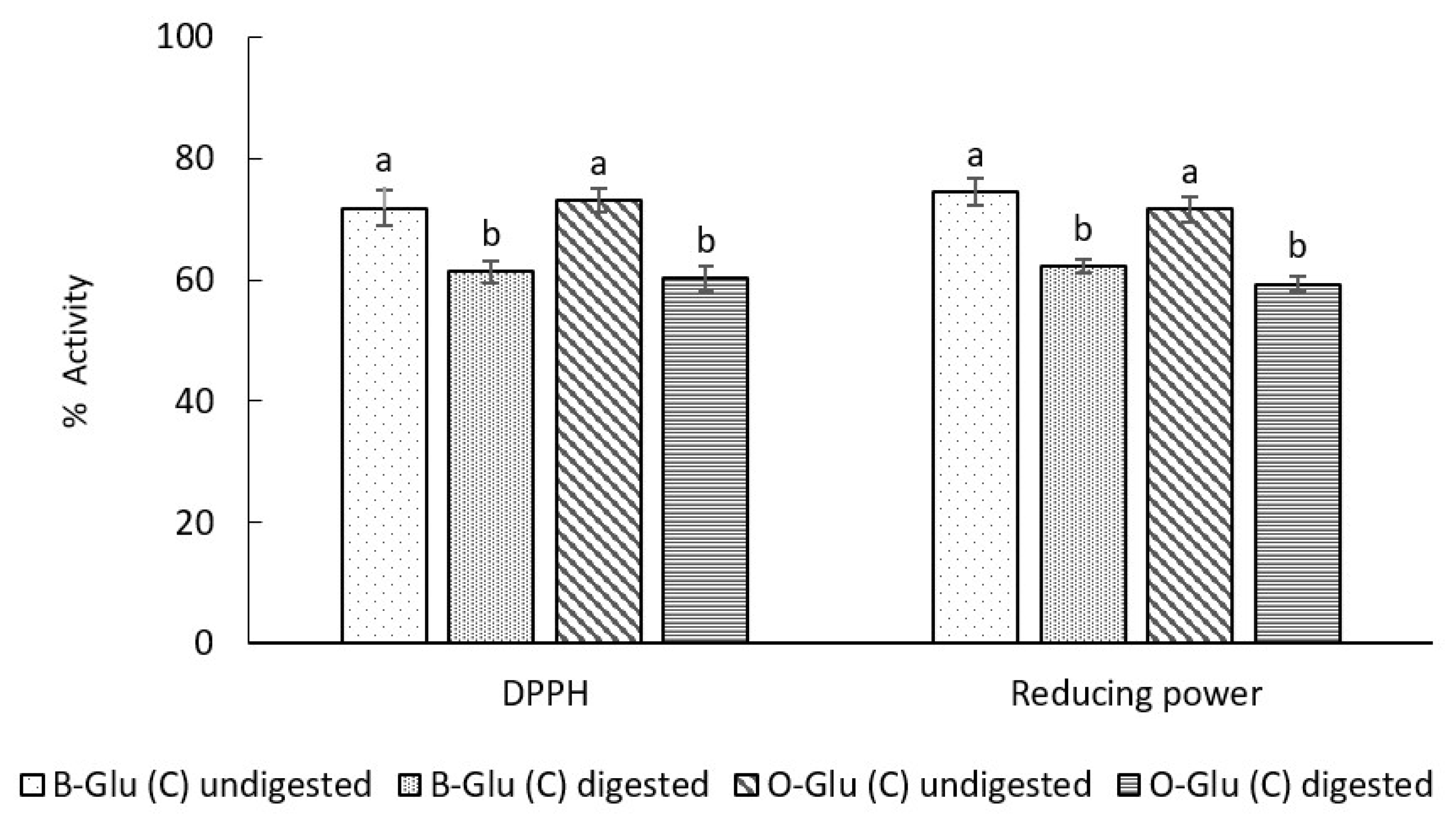
DPPH Activity
Reducing Power
2.10. Statistical Analysis
3. Results and Discussions
3.1. Particle Size, Polydispersity Index, and Zeta Potential
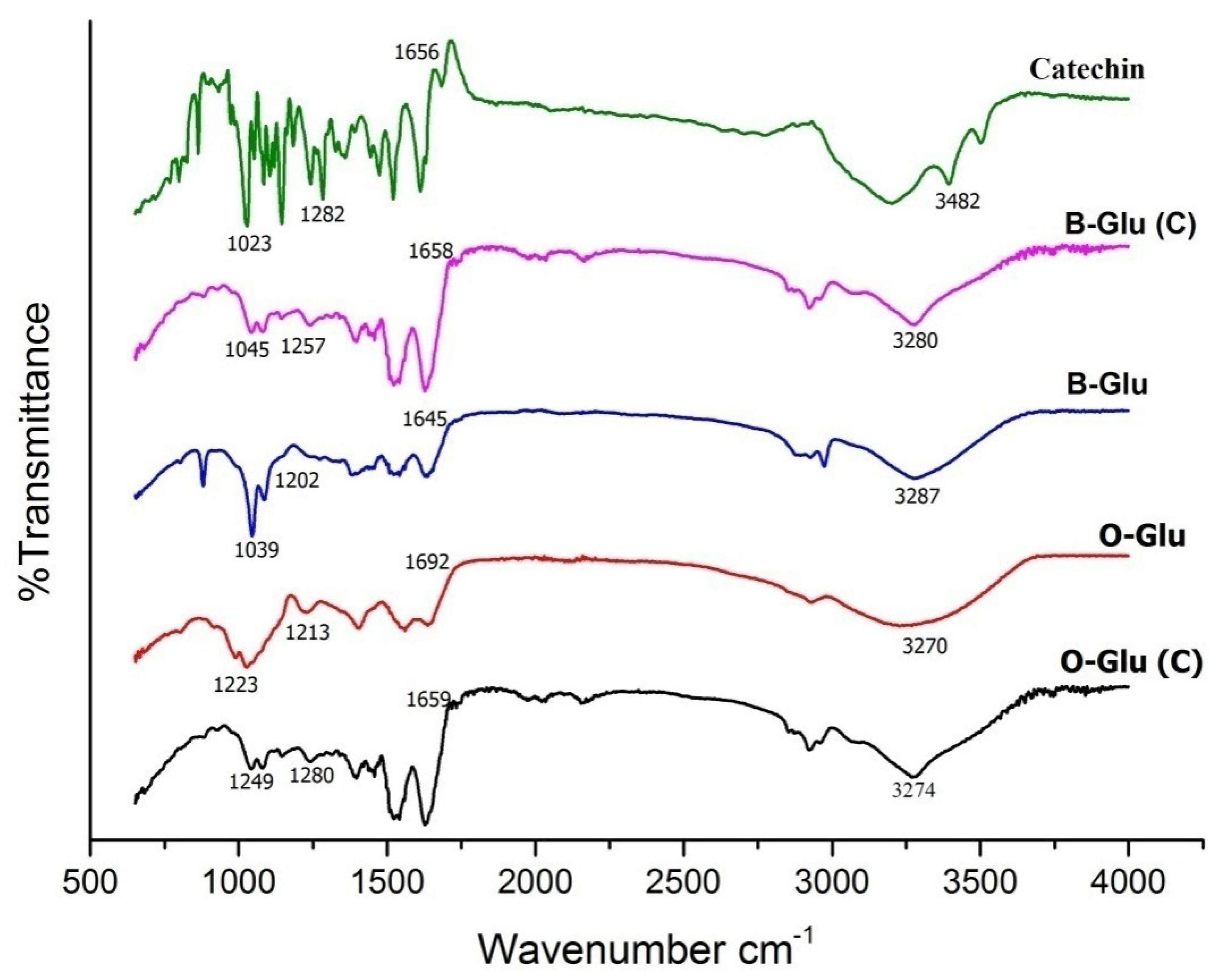
3.2. Structural Elucidation Using FTIR
3.3. Scanning Electron Microscopy
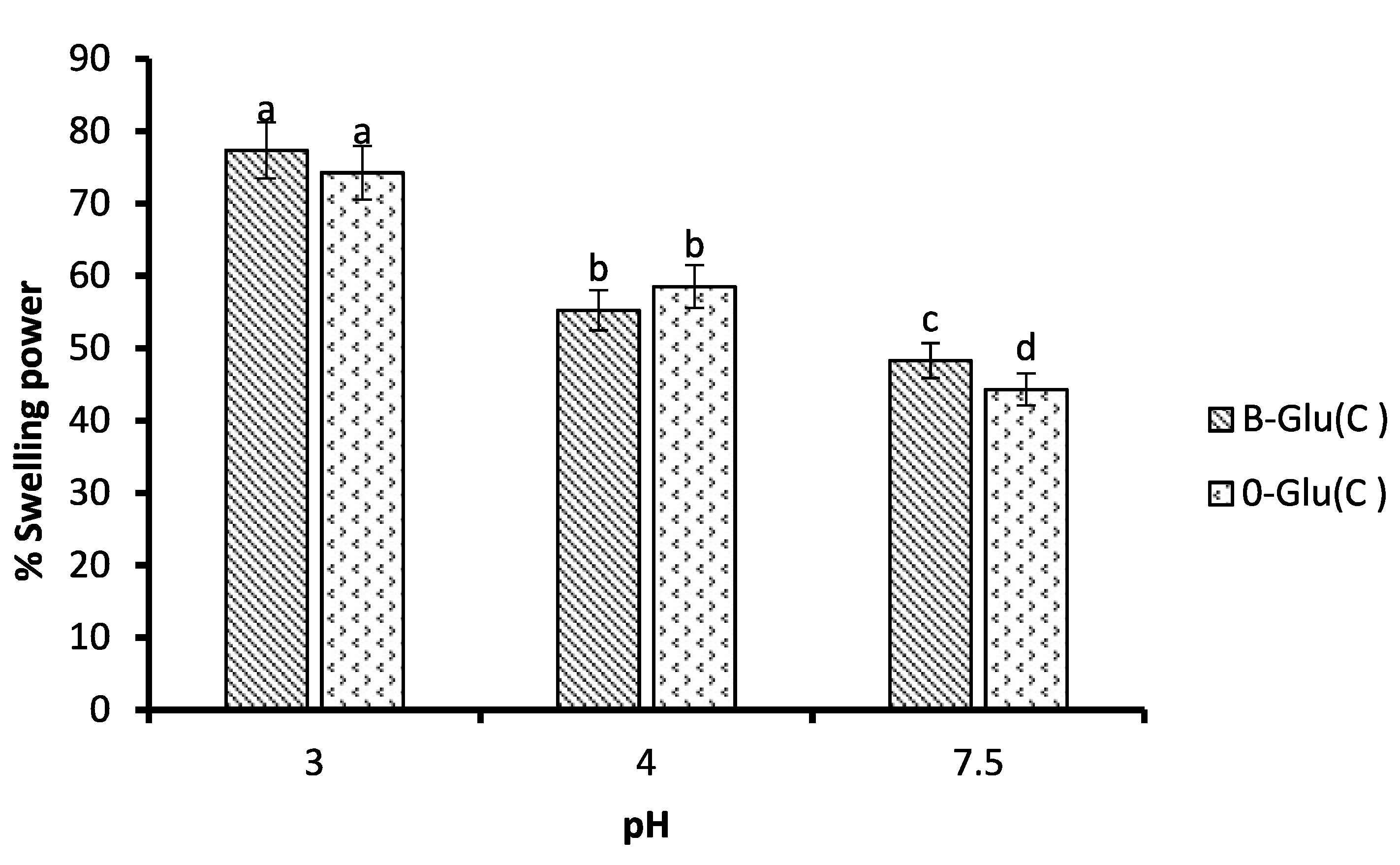
3.4. Swelling Behaviour
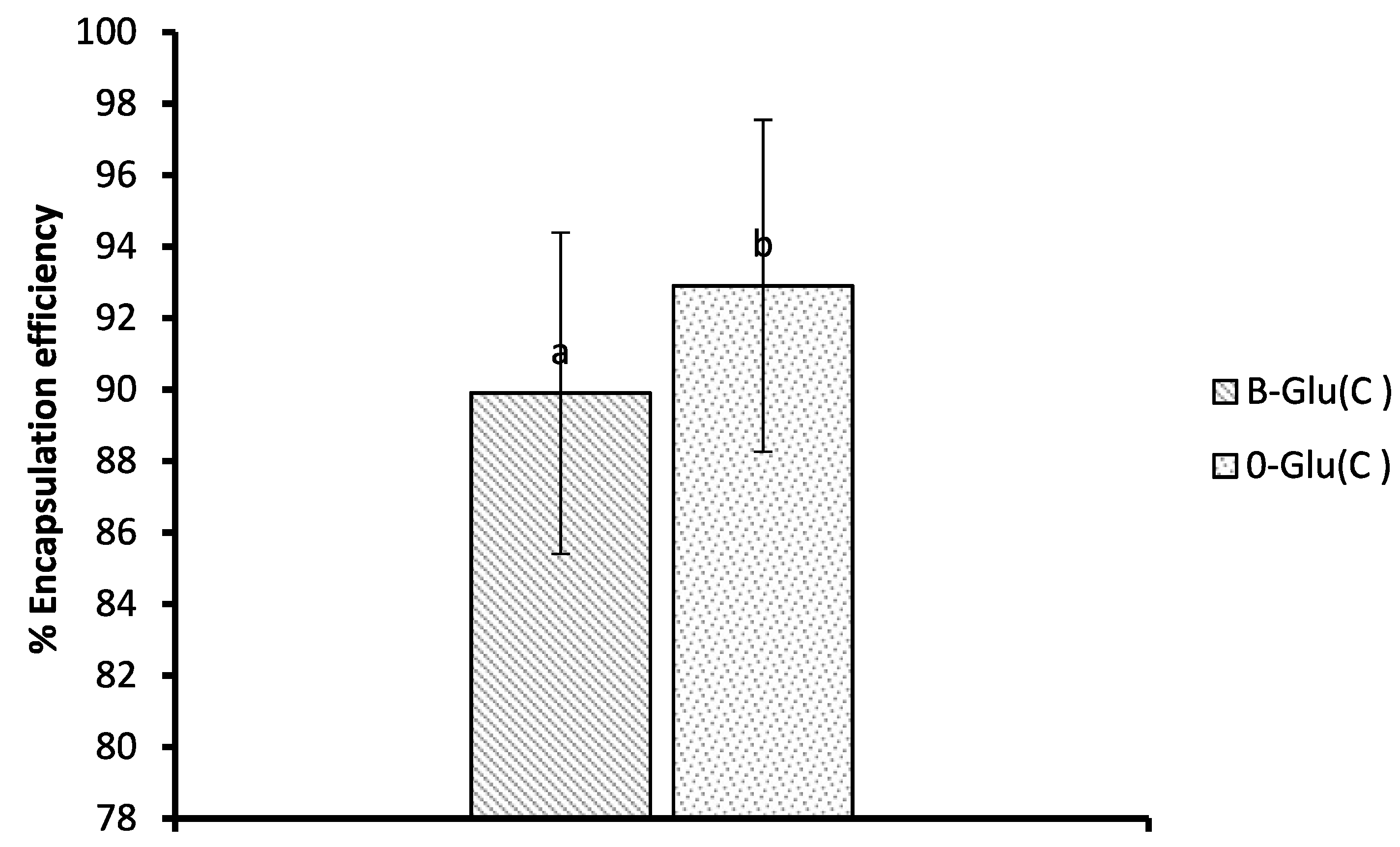
3.5. Encapsulation Efficiency (EE)
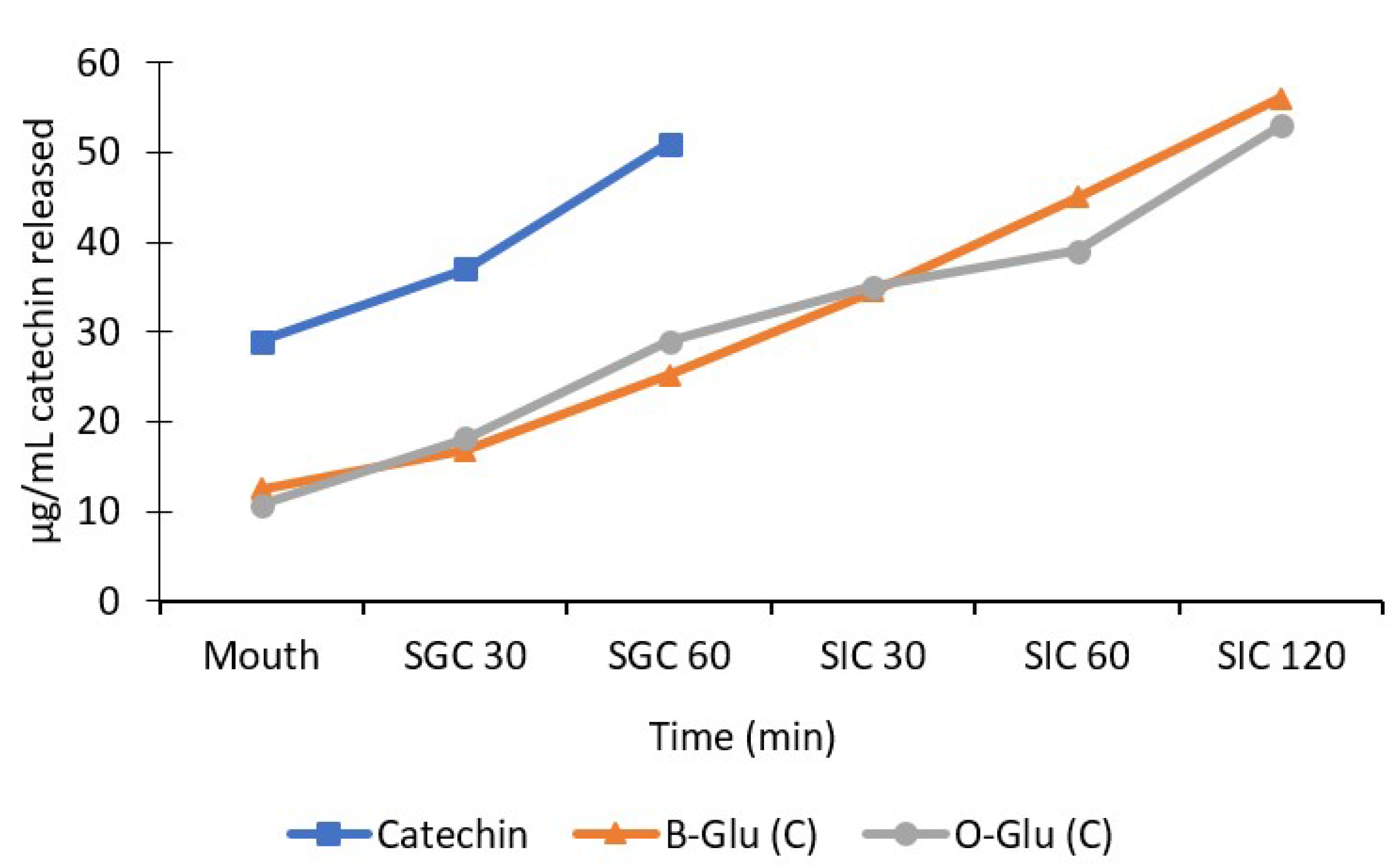
3.6. Release Behaviour of Catechin
3.7. Retention of Biological Activity of Catechin after Digestion
3.7.1. In Vitro Antiobesity Activity
3.7.2. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gadkaria, P.V.; Balaramana, M. Catechins: Sources, extraction and encapsulation: A review. Food Bioprod. Process. 2015, 93, 122–138. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Bajaj, P.R.; Singhal, R.S. Tea Polyphenols as Nutraceuticals. Compr. Rev. Food Sci. 2008, 7, 229–254. [Google Scholar] [CrossRef] [PubMed]
- Rainsa, T.M.; Agarwalb, S.; Makia, K.C. Antiobesity effects of green tea catechins: A mechanistic review. J. Nutr. Biochem. 2011, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.R.; Ghosh, P. A systematic investigation on flavonoids, catechin, β-sitosterol and lignin glycosides from Saracaasoca (ashoka) having anti-cancer & antioxidant properties with no side effect. J. Indian Chem. Soc. 2022, 99, 100293. [Google Scholar]
- Cai, Y.; Anavy, N.D.; Chow, H.H. Contribution of presystemic hepatic extraction to the low oral bioavailability of green tea catechins in rats. Drug Metab. Dispos. 2002, 30, 1246–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krook, M.A.; Hagerman, A.E. Stability of polyphenols epigallocatechin gallate and pentagalloyl glucose in asimulated digestive system. Food Res. Int. 2012, 49, 112–116. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zheng, Y.; Chow, M.S.S.; Zou, Z. Investigation of intestinal absorption and disposition of greentea catechins by Caco-2 monolayer model. Int. J. Pharm. 2004, 287, 1–12. [Google Scholar] [CrossRef]
- Neilson, A.P.; Hop, A.S.; Coope, B.R.; Pereira, M.A.; Bomser, J.A.; Ferruzzi, M.G. Catechin Degradation with Concurrent Formation of Homo- and Heterocatechin Dimers during in Vitro Digestion. J. Agric. Food Chem. 2007, 55, 8941–8949. [Google Scholar] [CrossRef]
- Lam, P.L.; Gambari, R. Advanced progress of microencapsulation technologies: In vivo and in vitro models for studying oral and transdermal drug deliveries. J. Control. Release 2014, 178, 25–45. [Google Scholar] [CrossRef]
- Jhan, F.; Gani, A.; Noor, N.; Ashraf, Z.U.; Gani, A.; Shah, A. Characterisation and utilisation of nano-reduced starch from underutilised cereals for delivery of folic acid through human GI tract. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Noore, S.; Rastogi, N.V.; Donnell, C.; Tiwari, B. Novel Bioactive Extraction and Nano-Encapsulation. Encyclopedia 2021, 1, 632–664. [Google Scholar] [CrossRef]
- Gomez-Guillen, M.C.; Montero, M.P. Enhancement of oral bioavailability of natural compounds and probiotics by mucoadhesive tailored biopolymer-based nanoparticles: A review. Food Hydrocoll. 2021, 118, 106772. [Google Scholar] [CrossRef]
- Gani, A.; Ashraf, Z.U.; Shah, A.; Noor, N.; Gani, A. Encapsulation of Vitamin D3 into β-Glucan Matrix Using the Supercritical Carbon Dioxide. ACS Food Sci. Technol. 2021, 1, 1880–1887. [Google Scholar] [CrossRef]
- Pattawika, H.M.; Seung, L.S.; Baek, J. Nano-encapsulated quercetin by soluble soybean polysaccharide/chitosan enhances anti-cancer, anti-inflammation, and anti-oxidant activities. J. Funct. Foods 2021, 87, 104756. [Google Scholar]
- Wani, T.A.; Masoodi, F.A.; Akhter, R.; Akram, T.; Gani, A.; Shabir, N. Nanoencapsulation of hydroxytyrosol in chitosan crosslinked with sodium bisulfate tandem ultrasonication: Techno-characterization, release and antiproliferative properties. Ultrason. Sonochem. 2021, 82, 105900. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, W.; Wei, Y.; Tong, Z.; Wang, Y.; Gao, Y. Fabrication, characterization and in vitro digestion of food-grade β-carotene high loaded microcapsules: A wet-milling and spray drying coupling approach. LWT 2021, 151, 112176. [Google Scholar] [CrossRef]
- Loh, Z.H.; Samanta, A.K.; Heng, P.W.S. Overview of milling techniques for improving the solubility of poorly water-soluble drugs. Asian J. Pharm. Clin. Res. 2015, 10, 255–274. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.; Ashraf, Z.U.; Gani, A.; Masoodi, F.A.; Gani, A. β-Glucan from mushrooms and dates as a wall material for targeted delivery of model bioactive compound: Nutraceutical profiling and bioavailability. Ultrason. Sonochem. 2022, 82, 105884. [Google Scholar] [CrossRef]
- Noor, N.; Gani, A.; Jhan, F.; Shah, M.A.; Ashraf, Z.U. Ferulic acid loaded pickering emulsions stabilized by resistant starch nanoparticles using ultrasonication: Characterization, in vitro release and nutraceutical potential. Ultrason. Sonochem. 2022, 84, 105967. [Google Scholar] [CrossRef]
- Ashraf, Z.U.; Shah, A.; Gani, A.; Gani, A.; Masoodi, F.A.; Noor, N. Nanoreduction as a technology to exploit β-Glucan from cereal and fungal sources for enhancing its nutraceutical potential. Carbohydr. Polym. 2021, 258, 117664. [Google Scholar] [CrossRef]
- Ashraf, Z.U.; Shah, A.; Gani, A.; Masoodi, F.A.; Noor, N. Effect of nano-reduction on properties of β-glucan and its use as encapsulating agent for release of α-tocopherol. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100230. [Google Scholar] [CrossRef]
- Gani, A.; Jan, R.; Ashwar, B.A.; Ashraf, Z.U.; Shah, A.; Gani, A. Encapsulation of saffron and sea buckthorn bioactives: Its utilization for development of low glycemic baked product for growing diabetic population of the world. LWT Food Sci. Technol. 2021, 142, 111035. [Google Scholar] [CrossRef]
- Gani, A.; Benjakul, S.; Ashraf, Z.U. Nutraceutical profiling of surimi gel containing β-glucan stabilized virgin coconut oil with and without antioxidants after simulated gastro-intestinal digestion. J. Food Sci. Technol. 2019, 57, 3132–3141. [Google Scholar] [CrossRef]
- Johnson, J.L.H.; He, Y.; Yalkowsky, S.H. Prediction of precipitation-induced phlebitis: A statistical validation of an in vitro model. J. Pharmacol. Sci. 2014, 92, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Mudgil, P.; Gani, A.; Hamed, F.; Masoodi, F.A.; Maqsood, S. Nano-encapsulation of catechin in starch nanoparticles: Characterization, release behavior and bioactivity retention during simulated in-vitro digestion. Food Chem. 2019, 270, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.; Gani, A.; Jhan, F.; Jenno, J.L.H.; Dar, M.A. Resistant starch type 2 from lotus stem: Ultrasonic effect on physical and nutraceutical properties. Ultrason. Sonochem. 2022, 76, 105655. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G.J. Lipid Nanoparticles as Carriers for Bioactive Delivery. Front Chem. 2021, 9, 580118. [Google Scholar] [CrossRef]
- Maurya, Y.K.; Aggarwal, M. Phase inversion based nanoemulsion fabrication process to encapsulate vitamin D3 for food applications. J. Steroid Biochem. Mol. Biol. 2019, 190, 88–98. [Google Scholar] [CrossRef]
- Shah, A.; Gani, A.; Masoodi, F.A.; Wani, S.M.; Ashwar, B.A. Structural, Rheological and nutraceutical potential of β-glucan from barley and oat. Bioact. Carbohydr. Diet. Fibre 2017, 10, 10–16. [Google Scholar] [CrossRef]
- Tarun, K.U.; Fatima, N.; Sharma, D.; Saravanakumar, V.; Sharma, R.S. Preparation and characterization of beta-glucan particles containing a payload of nanoembedded rifabutin for enhanced targeted delivery to macrophages. Excli J. 2017, 16, 210–228. [Google Scholar]
- Helena, C.F.C.; Renata, V.T.; Carlos, R.F.G.; Míriam, D.H. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar]
- Zhu, F. Encapsulation and delivery of food ingredients using starch based systems. Food Chem. 2017, 229, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Wongsasulak, S.; Pathumban, S.; Yoovidhya, T. Efect of entrapped α-tocopherol on mucoadhesivity and evaluation of the release, degradation, and swelling characteristics of zein–chitosan composite electrospunfbers. J. Food Eng. 2014, 120, 110–117. [Google Scholar] [CrossRef]
- Chin, S.F.; Yazid, S.N.A.M.; Suh Cem Pang, S.C. Preparation and characterization of starch nanoparticles for controlled release of curcumin. Int. J. Polym. Sci. 2014, 2014, 340121. [Google Scholar] [CrossRef]
- Kumari, A.; Singla, R.; Guliani, A.; Yadav, S.K. Nanoencapsulation for drug delivery. Excli J. 2014, 13, 265–286. [Google Scholar] [PubMed]

| B-Glu | B-Glu (C) | O-Glu | O-Glu (C) | |
|---|---|---|---|---|
| Particle Size (nm) | 130.1 ± 1.2 a | 200 ± 1.5 b | 313.2 ± 2.1 c | 498 ± 1.9 d |
| Polydispersity index (PDI) | 0.1 ± 00 a | 0.2 ± 00 a | 0.23 ± 00 a | 0.4 ± 00 a |
| Zeta potential (mV) | −11 ± 1.1 a | −27 ± 1.3 b | −14 ± 1.1 a | −24 ± 0.2 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, A.; Ashraf, Z.; Gani, A.; Jhan, F.; Gani, A.; Sidiq, M. Encapsulation of Catechin into β-Glucan Matrix Using Wet Milling and Ultrasonication as a Coupled Approach: Characterization and Bioactivity Retention. Foods 2022, 11, 1493. https://doi.org/10.3390/foods11101493
Shah A, Ashraf Z, Gani A, Jhan F, Gani A, Sidiq M. Encapsulation of Catechin into β-Glucan Matrix Using Wet Milling and Ultrasonication as a Coupled Approach: Characterization and Bioactivity Retention. Foods. 2022; 11(10):1493. https://doi.org/10.3390/foods11101493
Chicago/Turabian StyleShah, Asima, ZanoorUl Ashraf, Asir Gani, Faiza Jhan, Adil Gani, and Munazah Sidiq. 2022. "Encapsulation of Catechin into β-Glucan Matrix Using Wet Milling and Ultrasonication as a Coupled Approach: Characterization and Bioactivity Retention" Foods 11, no. 10: 1493. https://doi.org/10.3390/foods11101493
APA StyleShah, A., Ashraf, Z., Gani, A., Jhan, F., Gani, A., & Sidiq, M. (2022). Encapsulation of Catechin into β-Glucan Matrix Using Wet Milling and Ultrasonication as a Coupled Approach: Characterization and Bioactivity Retention. Foods, 11(10), 1493. https://doi.org/10.3390/foods11101493







