Structure-Activity Relationship of Pine Nut-Derived Peptides and Their Protective Effect on Nerve-Cell Mitochondria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Molecular Docking
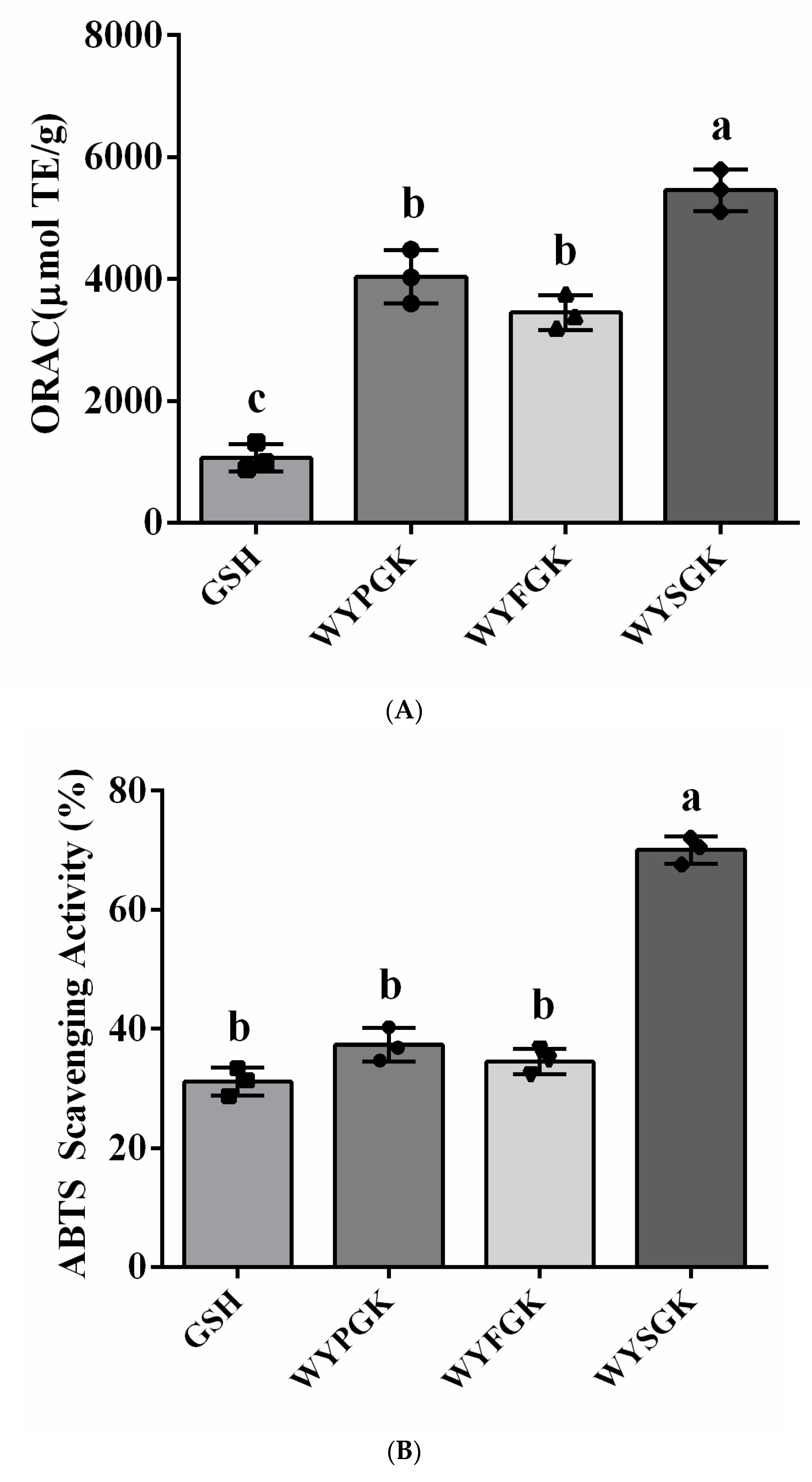
2.3. Antioxidant Activity Assay
2.3.1. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.3.2. ABTS Assay
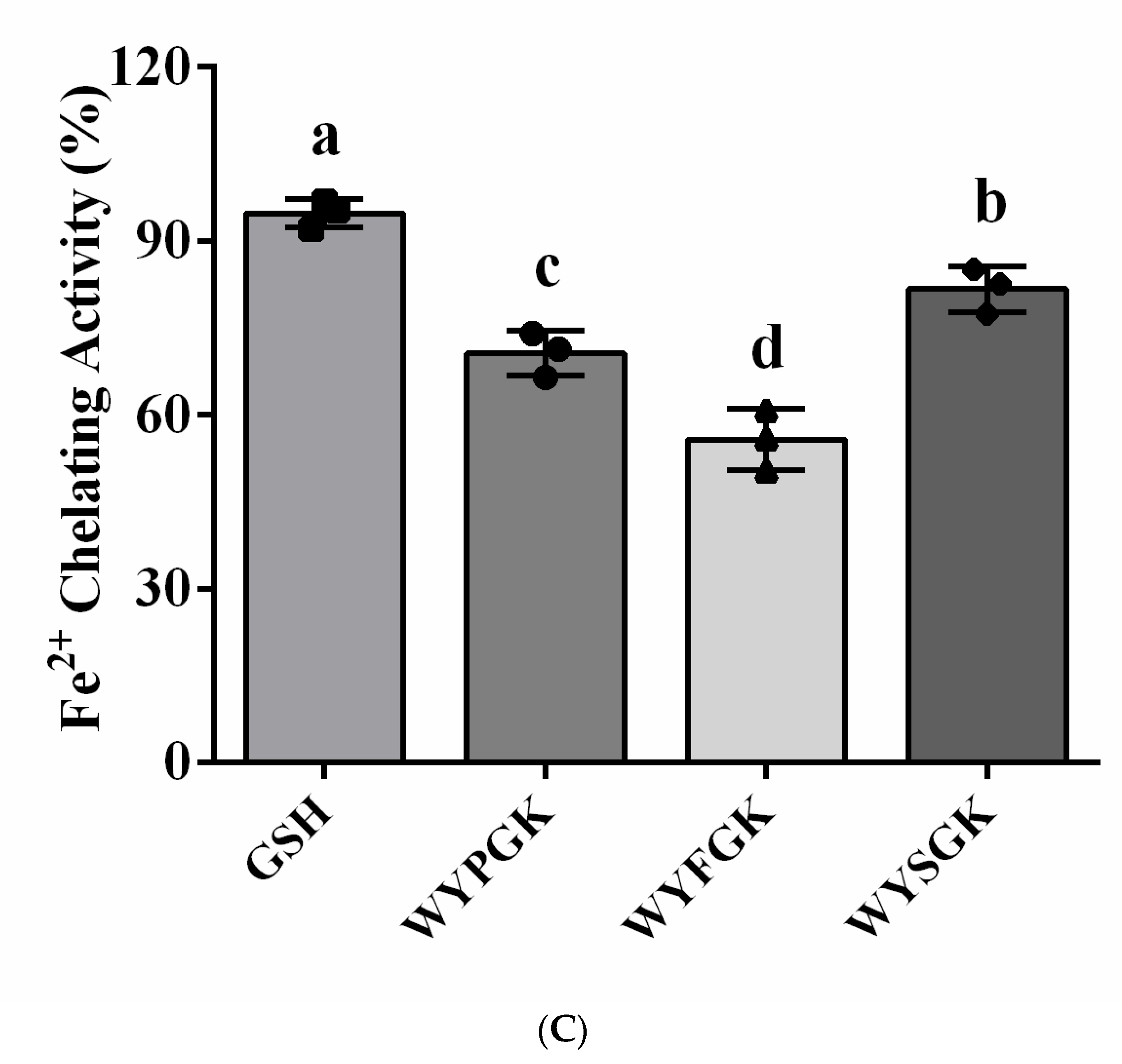
2.3.3. Fe2+-Chelating Activity Assay
2.4. CD Measurements
2.5. NMR Measurements
2.6. Cell Culture
2.7. Determination of ROS, MDA and ATP Levels
2.8. Transmission Electron Microscopy (TEM) Measurements
2.9. Mitochondrial Respiration Measurements
2.10. Statistical Analysis
3. Results and Discussion
3.1. Construction and Screening of Pine Nut-Derived Peptides
3.2. Antioxidant Activity of the Pine Nut Peptide WYPGK and Its Derived Peptides WYFGK and WYSGK
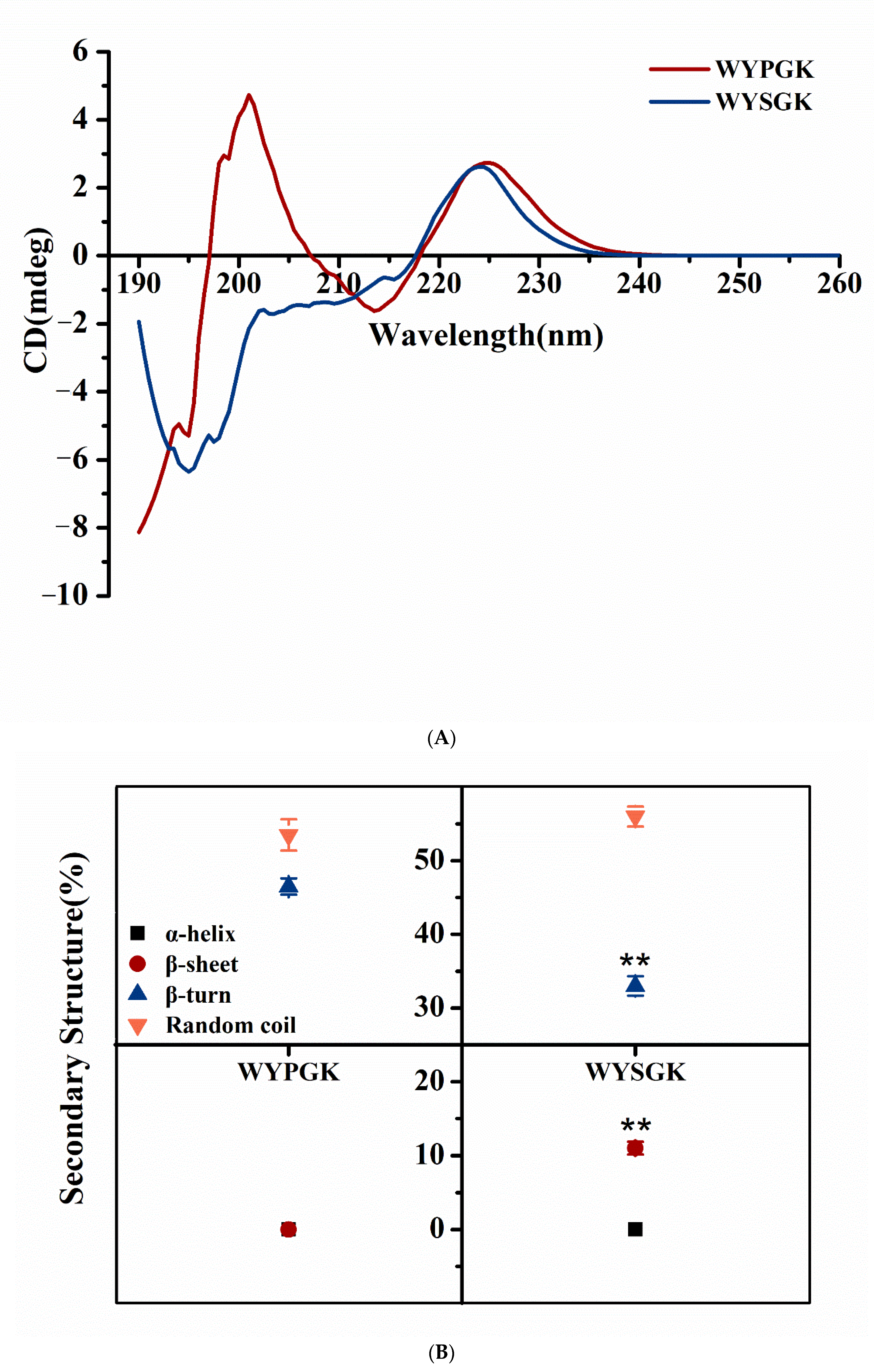
3.3. Analysis of WYPGK and WYSGK by CD Spectroscopy
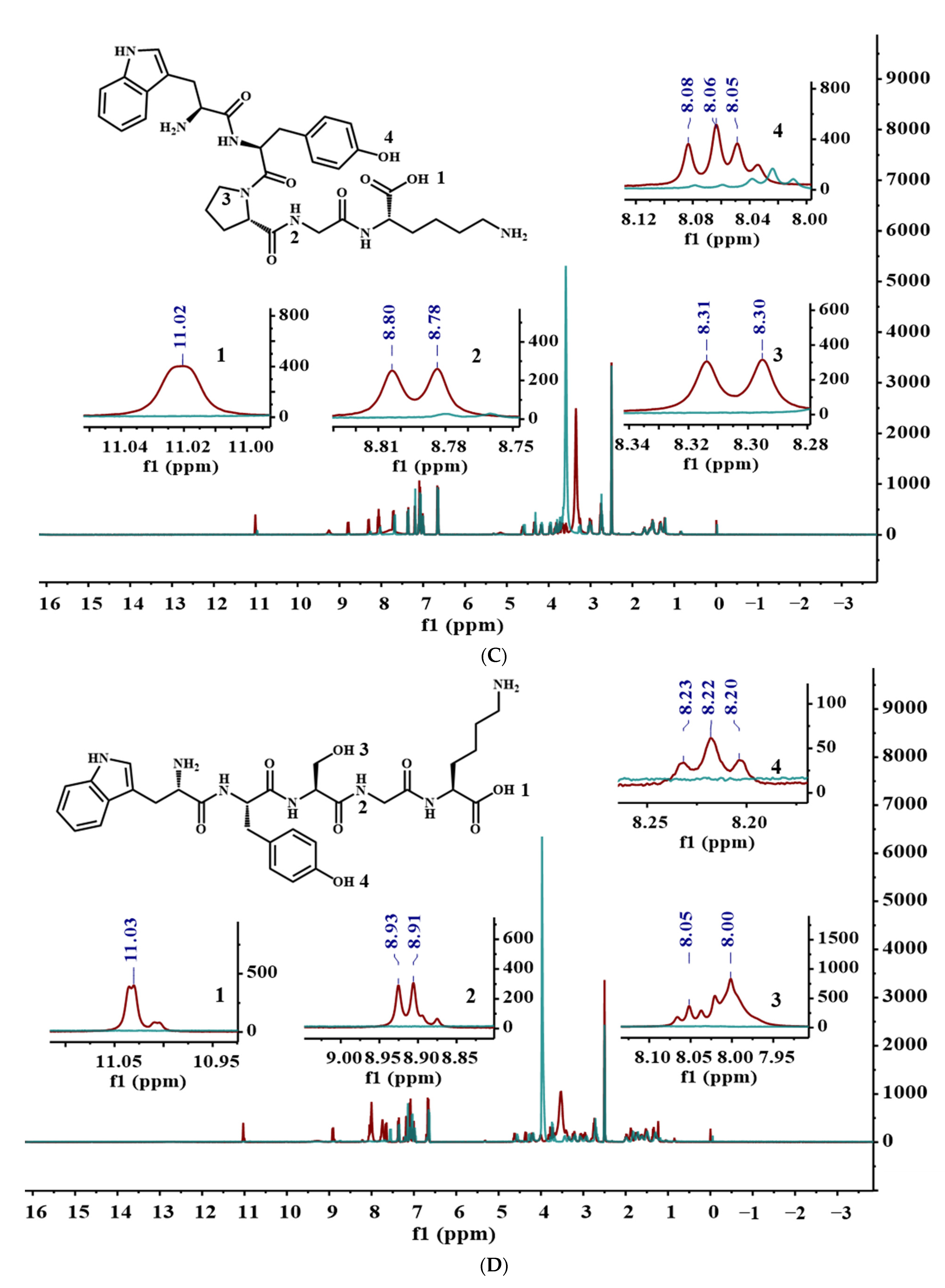
3.4. Analysis of WYPGK and WYSGK by NMR Spectroscopy
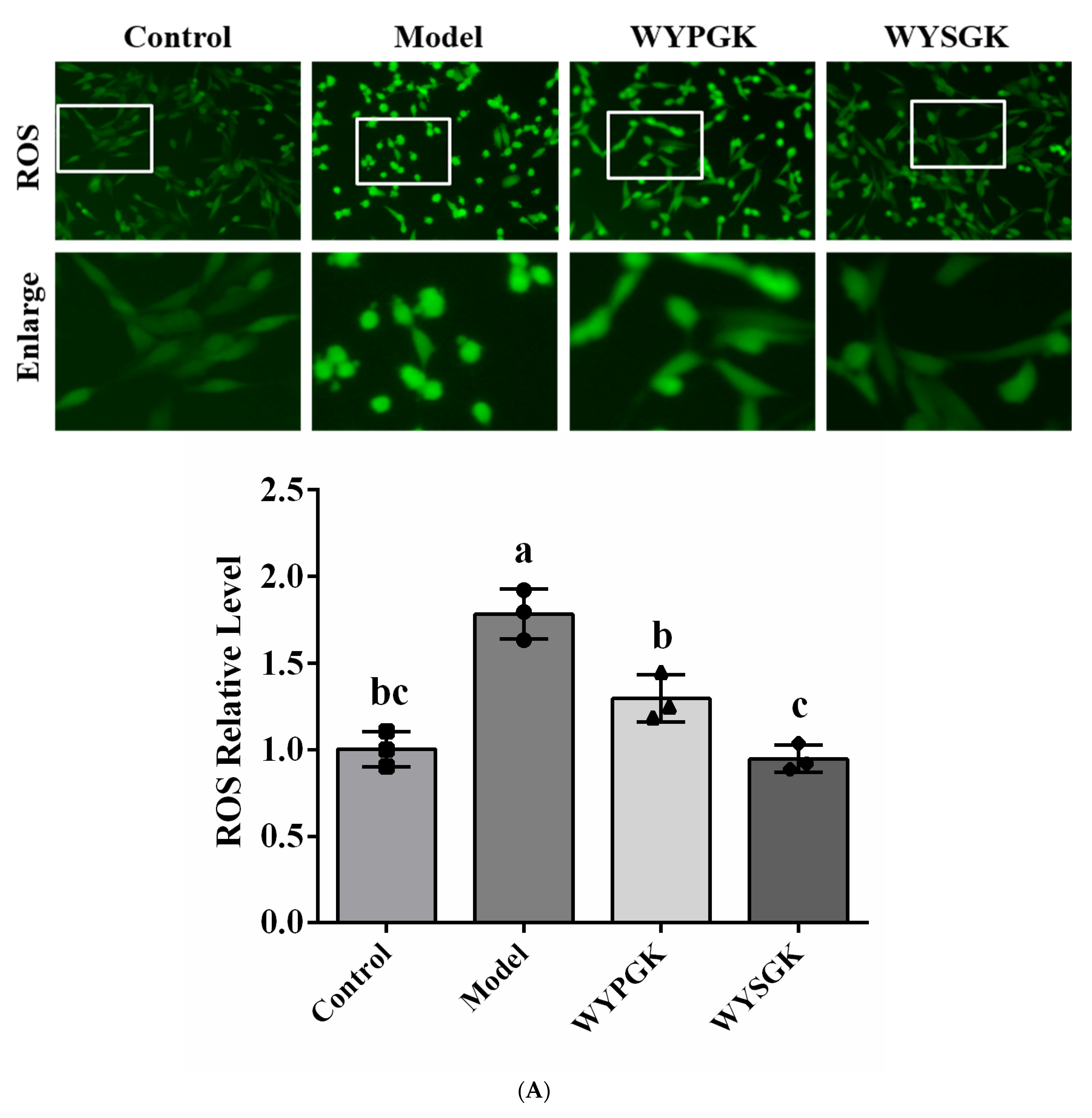
3.5. Effects of WYPGK and WYSGK on ROS, MDA, and ATP Levels in H2O2-Treated PC12 Cells
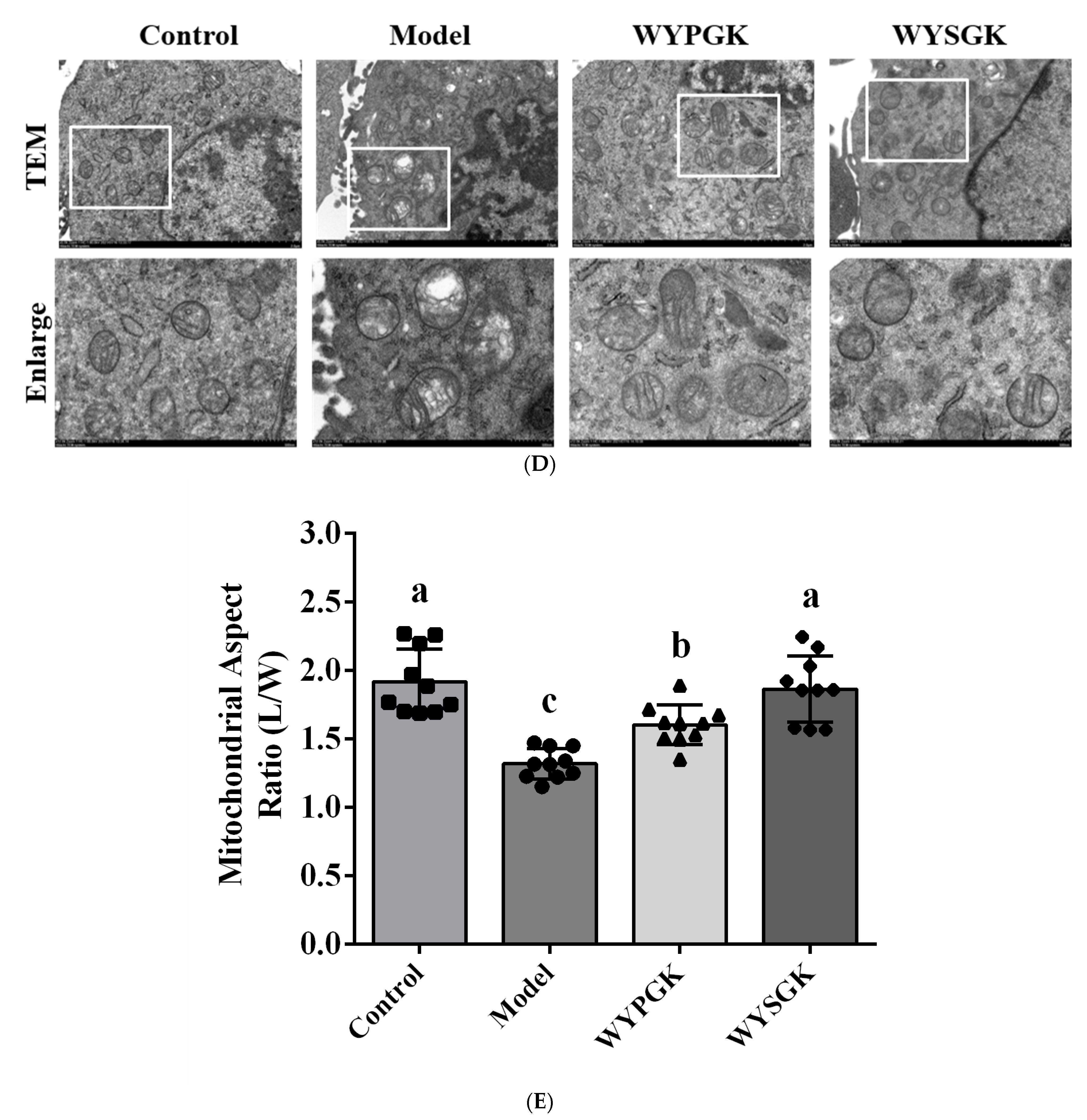
3.6. Effects of WYPGK and WYSGK on Mitochondrial Damage in H2O2-Treated PC12 Cells
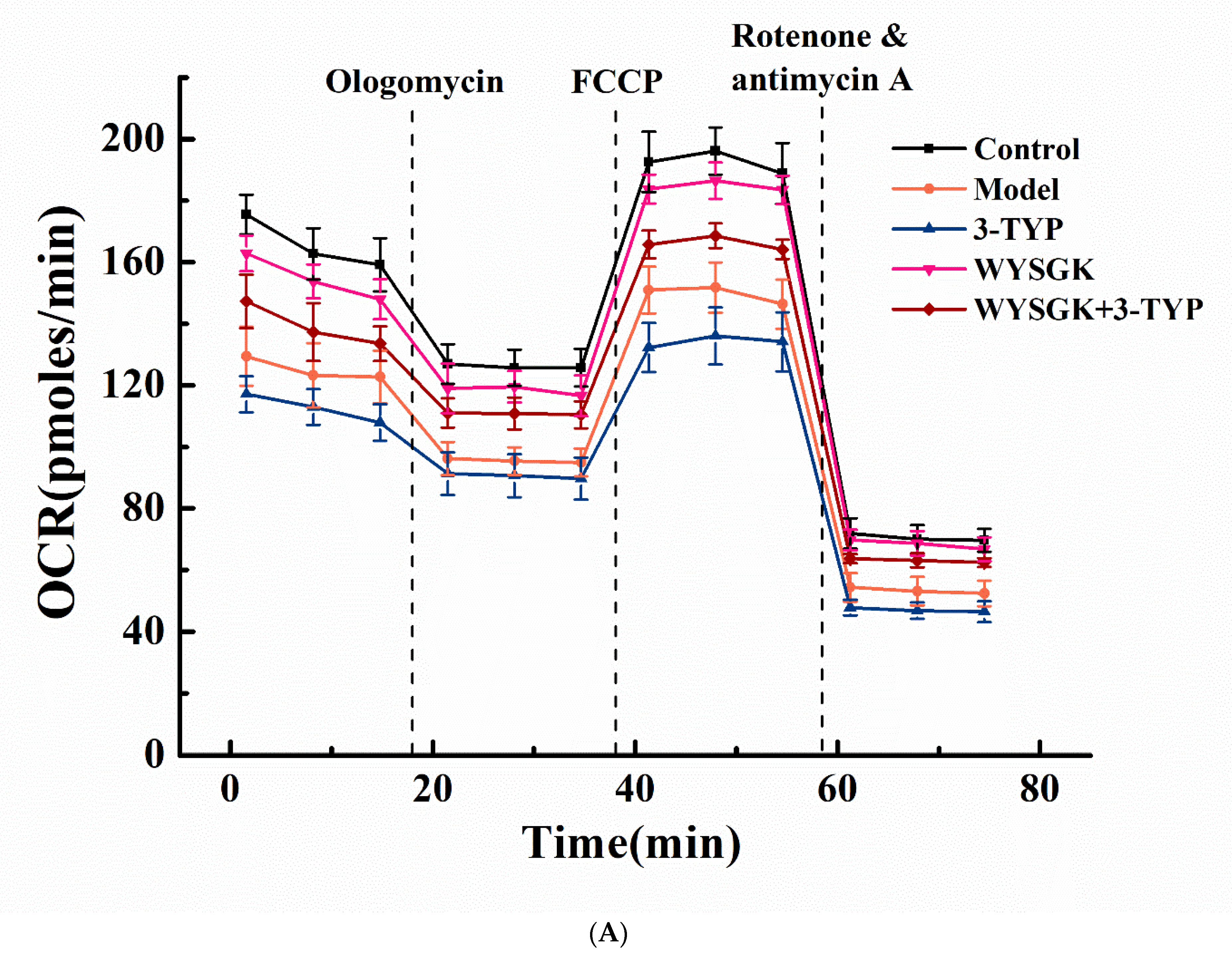
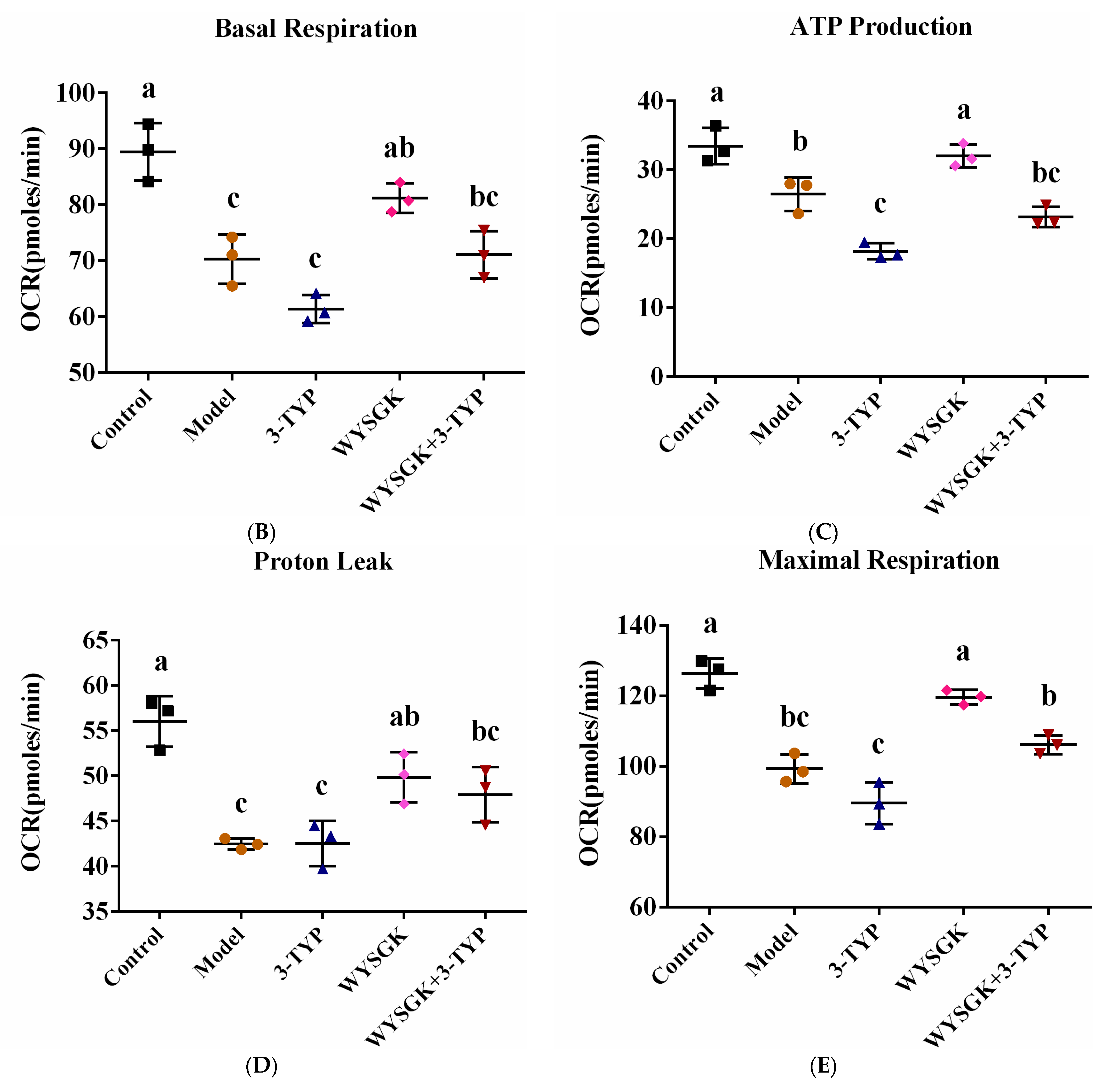
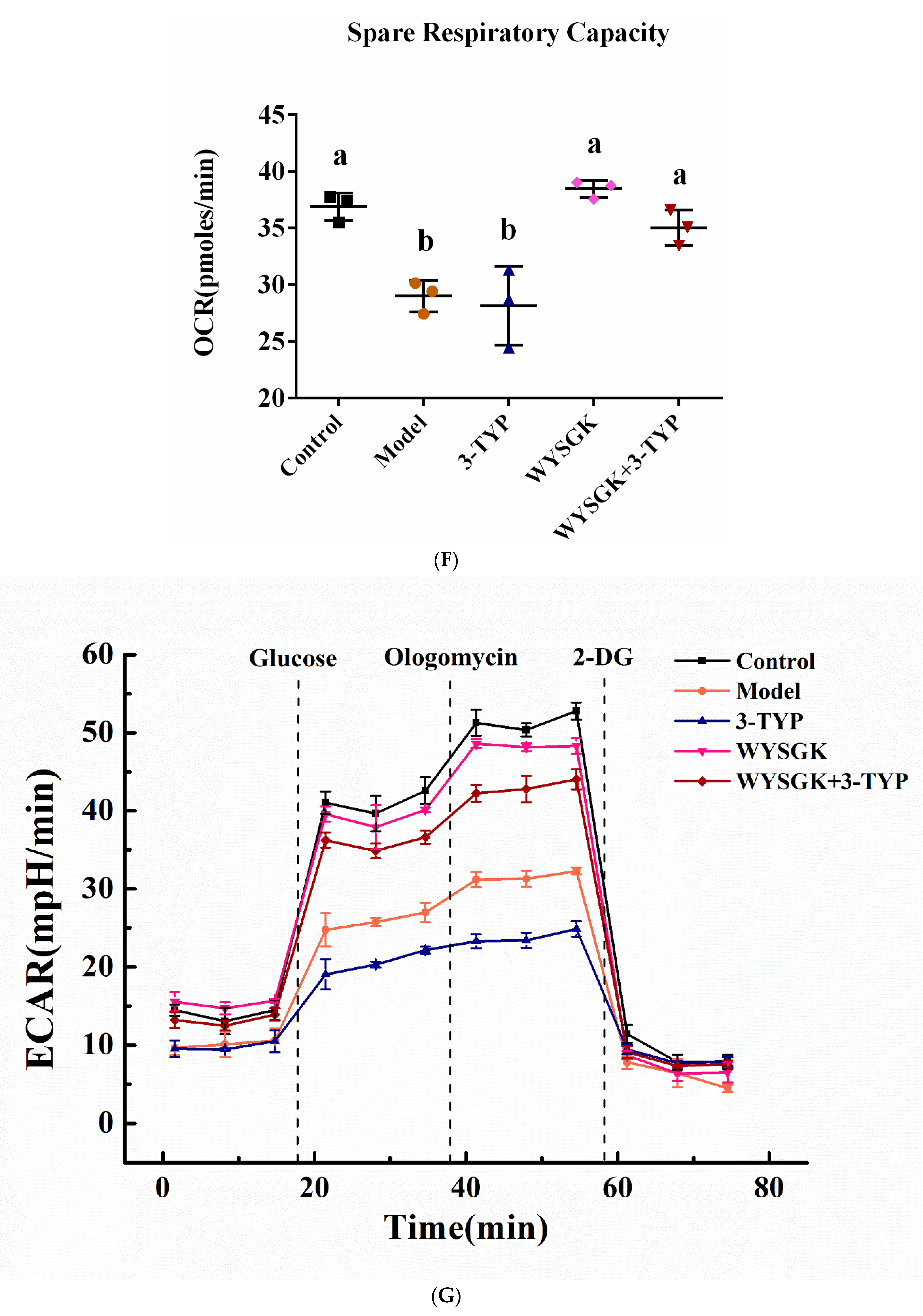
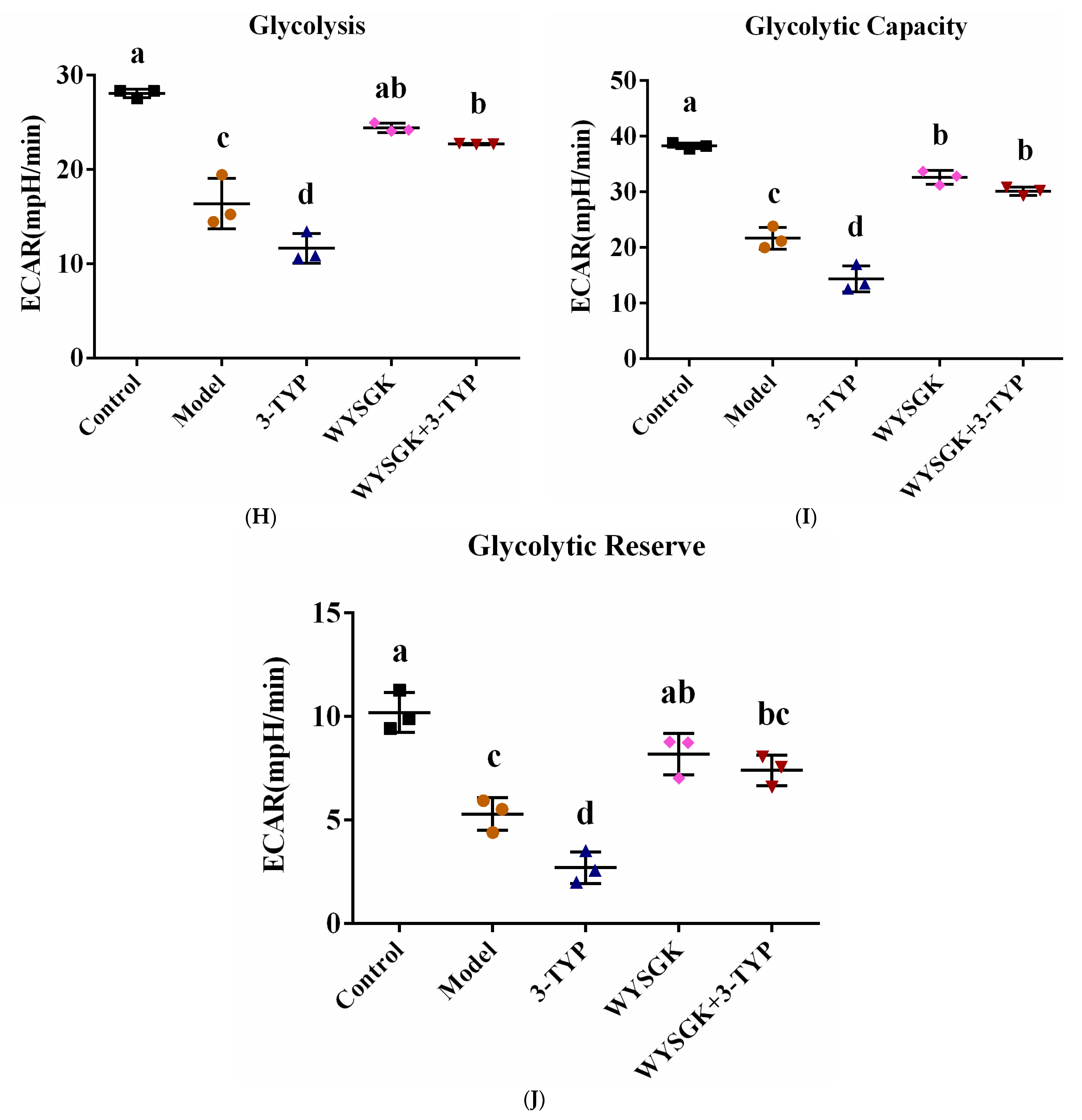
3.7. Effects of WYSGK on Mitochondrial Respiration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lei, L.; Zou, Z.; Liu, J.; Xu, Z.; Fu, Y.; Tian, Y.; Zhang, W. Multifunctional peptide-assembled micelles for simultaneously reducing amyloid-beta and reactive oxygen species. Chem. Sci. 2021, 12, 6449–6457. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Liu, X.; Wu, C.; Wu, Y.; Li, P.; Guo, X.; Tang, B. A new endoplasmic reticulum-targeted two-photon fluorescent probe for imaging of superoxide anion in diabetic mice. Biosens. Bioelectron. 2017, 91, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Moradi-Marjaneh, R.; Hassanian, S.M.; Mehramiz, M.; Rezayi, M.; Ferns, G.A.; Khazaei, M.; Avan, A. Reactive oxygen species in colorectal cancer: The therapeutic impact and its potential roles in tumor progression via perturbation of cellular and physiological dysregulated pathways. J. Cell. Physiol. 2018, 234, 10072–10079. [Google Scholar] [CrossRef] [PubMed]
- Hamacher-Brady, A. CMLS forum reviews: Mitochondrial damage control. Cell. Mol. Life Sci. 2021, 78, 3763–3765. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.E.; Arias-Durán, C.; Ávalos-Guajardo, Y.; Aedo, G.; Verdejo, H.E.; Parra, V.; Lavandero, S. Emerging role of mitophagy in cardiovascular physiology and pathology. Mol. Asp. Med. 2020, 71, 100822. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.-B.; He, T.-P.; Li, H.-B.; Tang, H.-W.; Xia, E.-Q. The Structure-Activity Relationship of the Antioxidant Peptides from Natural Proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef]
- Yang, R.; Wang, J.; Lin, S.; Ye, H.; Chen, F. In vitro antioxidant activities of the novel pentapeptides Ser-His-Glu-Cys-Asn and Leu-Pro-Phe-Ala-Met and the relationship between activity and peptide secondary structure. J. Sci. Food Agric. 2017, 97, 1945–1952. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, Y.; Li, L. Relationship between primary structure or spatial conformation and functional activity of antioxidant peptides from Pinctada fucata. Food Chem. 2018, 264, 108–117. [Google Scholar] [CrossRef]
- Abdelhameed, S.A.M.; Vandebroek, L.; De Azambuja, F.; Parac-Vogt, T.N. Redox Activity of Ce(IV)-Substituted Polyoxometalates toward Amino Acids and Peptides. Inorg. Chem. 2020, 59, 10569–10577. [Google Scholar] [CrossRef]
- Ajibola, C.F.; Fashakin, J.B.; Fagbemi, T.N.; Aluko, R.E. Effect of Peptide Size on Antioxidant Properties of African Yam Bean Seed (Sphenostylis stenocarpa) Protein Hydrolysate Fractions. Int. J. Mol. Sci. 2011, 12, 6685–6702. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Su, G.; Zhou, F.; Zhang, J.; Zheng, L.; Zhao, M. Protective Effect of Bovine Elastin Peptides against Photoaging in Mice and Identification of Novel Antiphotoaging Peptides. J. Agric. Food Chem. 2018, 66, 10760–10768. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, I.D.; Aluko, R.E. Structural and functional properties of food protein-derived antioxidant peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, G.T.; Gertz, M.; Steegborn, C. Crystal structures of Sirt3 complexes with 4’-bromo-resveratrol reveal binding sites and inhi-bition mechanism. Chem. Biol. 2013, 20, 1375–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Fang, L.; Wang, J.; Zhao, F.; Liu, C.; Gao, Y.; Liu, J.; Min, W. Pine nut antioxidant peptides ameliorate the memory impairment in a scopolamine-induced mouse model via SIRT3-induced synaptic plasticity. Food Funct. 2021, 12, 8026–8036. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, A.C.; Bartolomé, B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC−Fluorescein) Assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Zhang, M.; Mu, T.H. Optimisation of antioxidant hydrolysate production from sweet potato protein and effect of invitro gastrointestinal digestion. Int. J. Food Sci. Technol. 2016, 51, 1844–1850. [Google Scholar] [CrossRef]
- Delaglio, F.; Grzesiek, S.; Vuister, G.W.; Zhu, G.; Pfeifer, J.; Bax, A. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 1995, 6, 277–293. [Google Scholar] [CrossRef]
- Jin, L.; Wei, W.; Jiang, Y.; Peng, H.; Cai, J.; Mao, C.; Dai, H.; Choy, W.; Bemis, J.E.; Jirousek, M.R.; et al. Crystal Structures of Human SIRT3 Displaying Substrate-induced Conformational Changes. J. Biol. Chem. 2009, 284, 24394–24405. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Li, J.; Gu, H.; Wei, D.; Xu, Y.C.; Fu, W.; Yu, Z. Conformational Preferences of π-π Stacking Between Ligand and Protein, Analysis Derived from Crystal Structure Data Geometric Preference of π-π Interaction. Interdiscip. Sci. 2015, 7, 211–220. [Google Scholar] [CrossRef]
- Xu, F.; Wang, L.; Ju, X.; Zhang, J.; Yin, S.; Shi, J.; He, R.; Yuan, Q. Transepithelial Transport of YWDHNNPQIR and Its Metabolic Fate with Cytoprotection against Oxidative Stress in Human Intestinal Caco-2 Cells. J. Agric. Food Chem. 2017, 65, 2056–2065. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, W.; Li, Q.; Chen, Y.; Zheng, D.; Zou, Y.; Zhang, M.; Zhao, T.; Mao, G.; Feng, W.; et al. Physicochemical, functional properties and antioxidant activities of porcine cerebral hydrolysate peptides produced by ultrasound processing. Process Biochem. 2016, 51, 431–443. [Google Scholar] [CrossRef]
- Habinshuti, I.; Mu, T.-H.; Zhang, M. Ultrasound microwave-assisted enzymatic production and characterisation of antioxidant peptides from sweet potato protein. Ultrason. Sonochem. 2020, 69, 105262. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Toldrá, F. Characterisation of the antioxidant peptide AEEEYPDL and its quantification in Spanish dry-cured ham. Food Chem. 2018, 258, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Fric, I.; Flegel, M.; Zaoral, M.; Kodicek, M. Circular-Dichroic Spectra of Vasopressin Analogues and Their Cyclic Fragments. JBIC J. Biol. Inorg. Chem. 1975, 56, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Cheng, S.; Wang, X. Secondary structure changes induced by pulsed electric field affect antioxidant activity of pentapeptides from pine nut (Pinus koraiensis) protein. Food Chem. 2018, 254, 170–184. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhou, J.; Duan, Y.; Zhang, H.; Ma, H. Effects of slit divergent ultrasound and enzymatic treatment on the structure and antioxidant activity of arrowhead protein. Ultrason. Sonochem. 2018, 49, 294–302. [Google Scholar] [CrossRef]
- Liu, C.; Ren, D.; Li, J.; Fang, L.; Wang, J.; Liu, J.; Min, W. Cytoprotective effect and purification of novel antioxidant peptides from hazelnut (C. heterophylla Fisch) protein hydrolysates. J. Funct. Foods 2018, 42, 203–215. [Google Scholar] [CrossRef]
- Fossel, E.T.; Easwaran, K.R.K.; Blout, E.R. A13C spin-lattice relaxation study of dipeptides containing glycine and proline: Mobility of the cyclic proline side chain. Biopolymers 1975, 14, 927–935. [Google Scholar] [CrossRef]
- Anglister, J.; Srivastava, G.; Naider, F. Detection of intermolecular NOE interactions in large protein complexes. Prog. Nucl. Magn. Reson. Spectrosc. 2016, 97, 40–56. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, S.; Sun, N.; Zhu, B.; Lin, S. Neuroprotective Function of a Novel Hexapeptide QMDDQ from Shrimp via Activation of the PKA/CREB/BNDF Signaling Pathway and Its Structure–Activity Relationship. J. Agric. Food Chem. 2020, 68, 6759–6769. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, M.; Tonan, K.; Ikawa, S.-I. Effect of solvent on the cis–trans conformational equilibrium of a proline imide bond of short model peptides in solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 1305–1316. [Google Scholar] [CrossRef]
- Lai, Y.; Lin, P.; Chen, M.; Zhang, Y.; Chen, J.; Zheng, M.; Liu, J.; Du, H.; Chen, R.; Pan, X.; et al. Restoration of L-OPA1 alleviates acute ischemic stroke injury in rats via inhibiting neuronal apoptosis and preserving mitochondrial function. Redox Biol. 2020, 34, 101503. [Google Scholar] [CrossRef] [PubMed]
- Sisalli, M.J.; Ianniello, G.; Savoia, C.; Cuomo, O.; Annunziato, L.; Scorziello, A. Knocking-out the Siah2 E3 ubiquitin ligase prevents mitochondrial NCX3 degradation, regulates mitochondrial fission and fusion, and restores mitochondrial function in hypoxic neurons. Cell Commun. Signal. 2020, 18, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.; Liu, C.; Fang, L.; Lu, H.; Wang, J.; Gao, Y.; Gabbianelli, R.; Min, W. Walnut-derived peptide activates PINK1 via the NRF2/KEAP1/HO-1 pathway, promotes mitophagy, and alleviates learning and memory impairments in a mice model. J. Agric. Food Chem. 2021, 69, 2758–2772. [Google Scholar] [CrossRef]
- Wang, N.N.; Xu, H.H.; Zhou, W.; Yang, H.X.; Wang, J.; Ma, Z.C.; Gao, Y. Aconitine attenuates mitochondrial dysfunction of cardio-myocytes via promoting deacetylation of cyclophilin-D mediated by sirtuin-3. J. Ethnopharmacol. 2021, 270, 113765. [Google Scholar] [CrossRef]
- Lin, X.; Wang, L.; Zhao, L.; Zhu, Z.; Chen, T.; Chen, S.; Tao, Y.; Zeng, T.; Zhong, Y.; Sun, H.; et al. Curcumin micelles suppress gastric tumor cell growth by upregulating ROS generation, disrupting redox equilibrium and affecting mitochondrial bioenergetics. Food Funct. 2020, 11, 4146–4159. [Google Scholar] [CrossRef]
- Camacho-Pereira, J.; Tarragó, M.G.; Chini, C.C.; Nin, V.; Escande, C.; Warner, G.M.; Puranik, A.; Schoon, R.A.; Reid, J.M.; Galina, A.; et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab. 2016, 23, 1127–1139. [Google Scholar] [CrossRef] [Green Version]
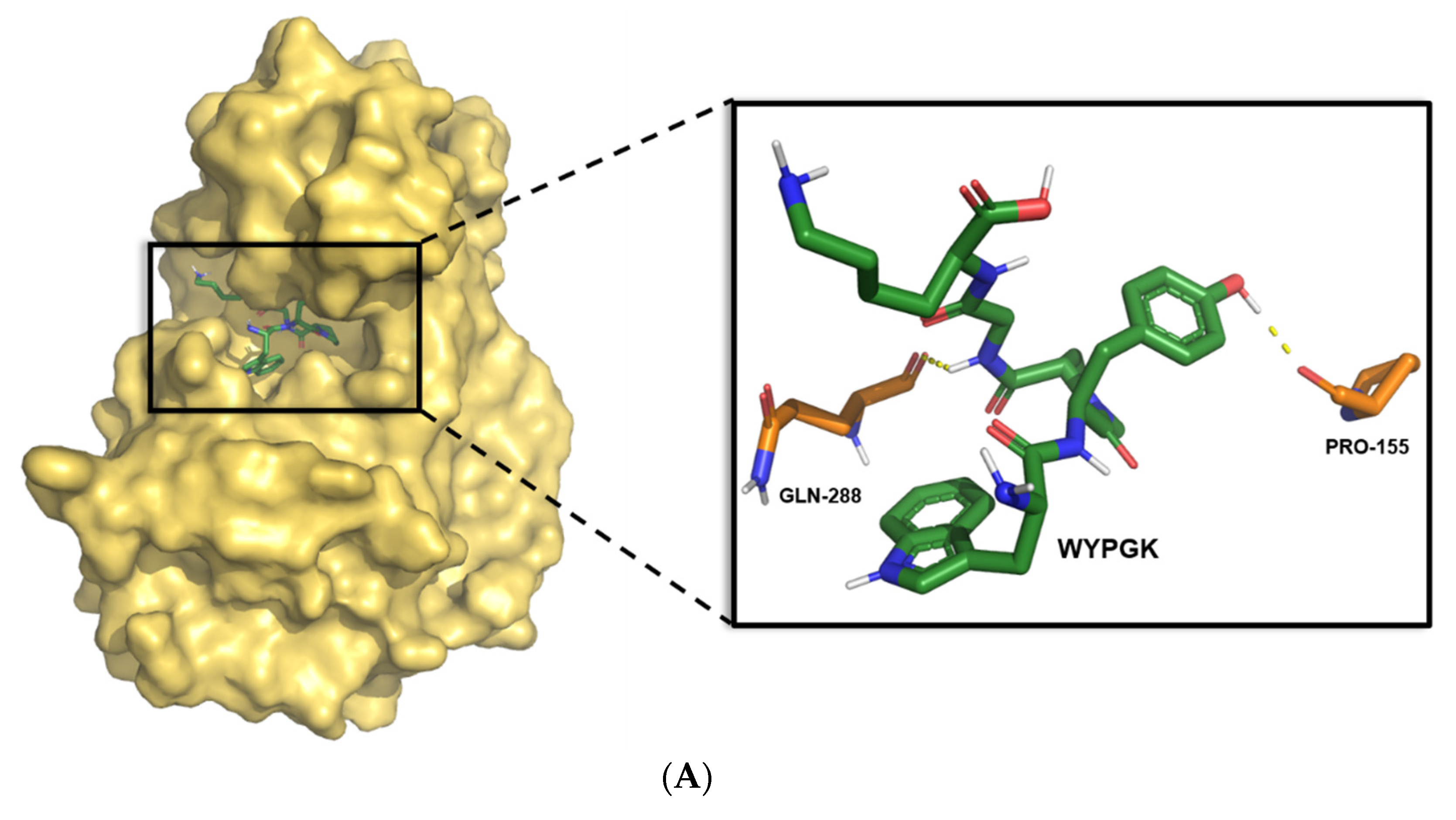

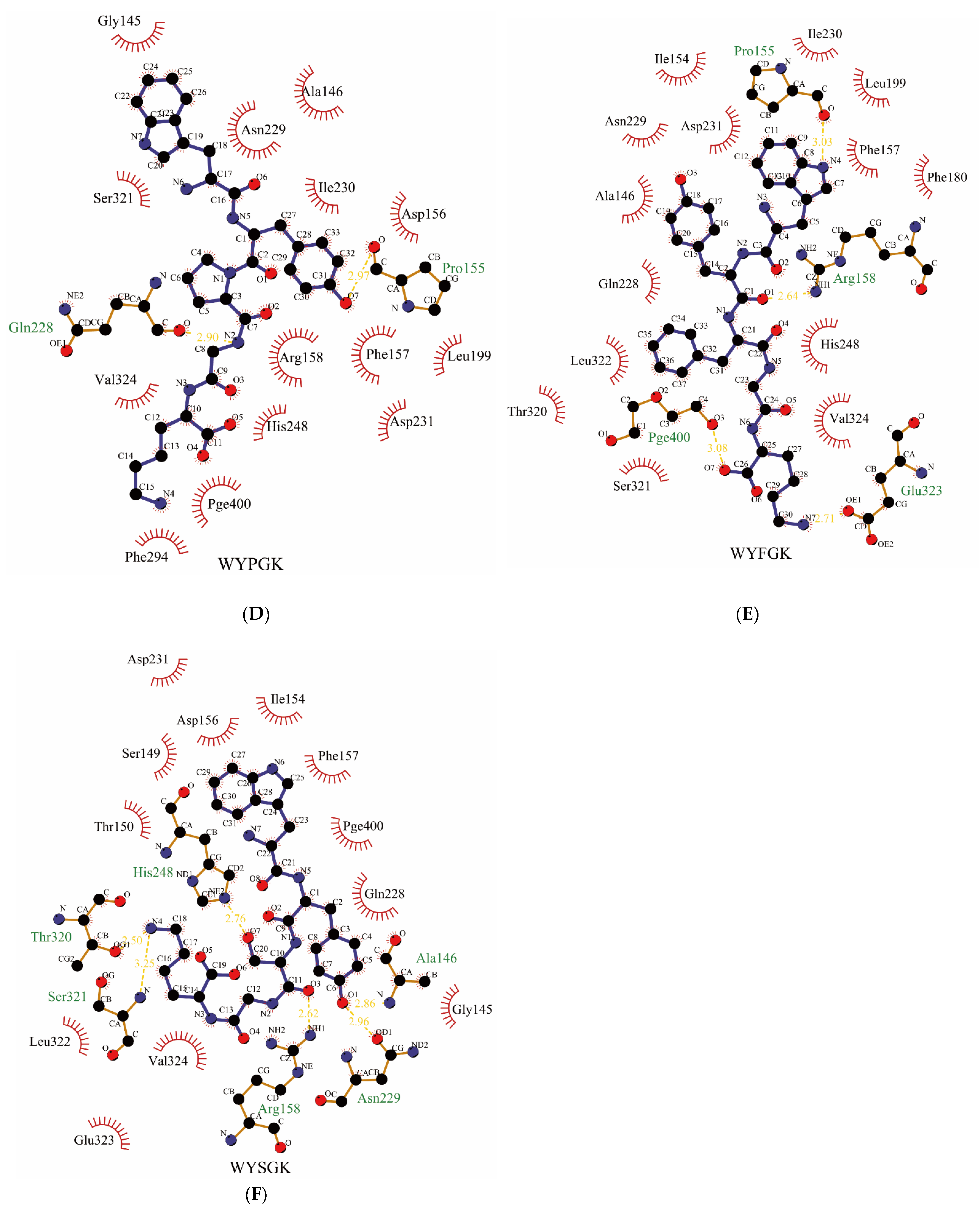


| Number | Sequence | Binding Energy (kcal/mol) | Hydrogen Bonds Number | π-π Interactions Number | Intermolecular Energy (kcal/mol) | Internal Energy (kcal/mol) | Torsional Energy (kcal/mol) |
|---|---|---|---|---|---|---|---|
| 1 | WYDGK | −3.59 | 8 | 1 | −10.75 | −3.66 | 7.16 |
| 2 | WYEGK | −1.44 | 4 | 1 | −8.90 | −3.99 | 7.46 |
| 3 | WYKGK | −1.94 | 6 | 1 | −9.69 | −4.41 | 7.76 |
| 4 | WYRGK | −3.95 | 8 | - | −11.41 | −1.76 | 7.46 |
| 5 | WYHGK | −3.64 | 3 | - | −10.50 | −6.53 | 6.86 |
| 6 | WYGGK | −4.34 | 6 | - | −10.60 | −3.63 | 6.26 |
| 7 | WYAGK | −4.29 | 6 | - | −10.56 | −6.42 | 6.26 |
| 8 | WYLGK | −4.32 | 4 | - | −11.18 | −2.36 | 6.86 |
| 9 | WYIGK | −5.37 | 5 | - | −12.23 | −4.31 | 6.86 |
| 10 | WYVGK | −5.40 | 2 | - | −11.96 | −6.08 | 6.56 |
| 11 | WYFGK | −6.08 | 4 | - | −12.94 | −5.29 | 6.86 |
| 12 | WYPGK | −4.66 | 2 | - | −10.63 | −6.75 | 5.97 |
| 13 | WYMGK | −4.84 | 5 | 1 | −12.00 | −4.12 | 7.16 |
| 14 | WYWGK | −5.16 | 5 | - | −12.03 | −5.93 | 6.86 |
| 15 | WYSGK | −5.87 | 6 | 1 | −12.73 | −6.07 | 6.86 |
| 16 | WYQGK | −4.97 | 4 | - | −12.13 | −5.75 | 7.16 |
| 17 | WYTGK | −4.72 | 6 | - | −11.58 | −4.66 | 6.86 |
| 18 | WYCGK | −5.18 | 2 | - | −12.04 | −4.36 | 6.86 |
| 19 | WYNGK | −4.73 | 8 | - | −11.59 | −5.11 | 6.86 |
| 20 | WYYGK | −3.43 | 2 | - | −10.59 | −4.18 | 7.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Fang, L.; Wang, X.; Wu, D.; Liu, C.; Liu, X.; Wang, J.; Gao, Y.; Min, W. Structure-Activity Relationship of Pine Nut-Derived Peptides and Their Protective Effect on Nerve-Cell Mitochondria. Foods 2022, 11, 1428. https://doi.org/10.3390/foods11101428
Lu H, Fang L, Wang X, Wu D, Liu C, Liu X, Wang J, Gao Y, Min W. Structure-Activity Relationship of Pine Nut-Derived Peptides and Their Protective Effect on Nerve-Cell Mitochondria. Foods. 2022; 11(10):1428. https://doi.org/10.3390/foods11101428
Chicago/Turabian StyleLu, Hongyan, Li Fang, Xiyan Wang, Dan Wu, Chunlei Liu, Xiaoting Liu, Ji Wang, Yawen Gao, and Weihong Min. 2022. "Structure-Activity Relationship of Pine Nut-Derived Peptides and Their Protective Effect on Nerve-Cell Mitochondria" Foods 11, no. 10: 1428. https://doi.org/10.3390/foods11101428
APA StyleLu, H., Fang, L., Wang, X., Wu, D., Liu, C., Liu, X., Wang, J., Gao, Y., & Min, W. (2022). Structure-Activity Relationship of Pine Nut-Derived Peptides and Their Protective Effect on Nerve-Cell Mitochondria. Foods, 11(10), 1428. https://doi.org/10.3390/foods11101428






