More Than Fish—Framing Aquatic Animals within Sustainable Food Systems
Abstract
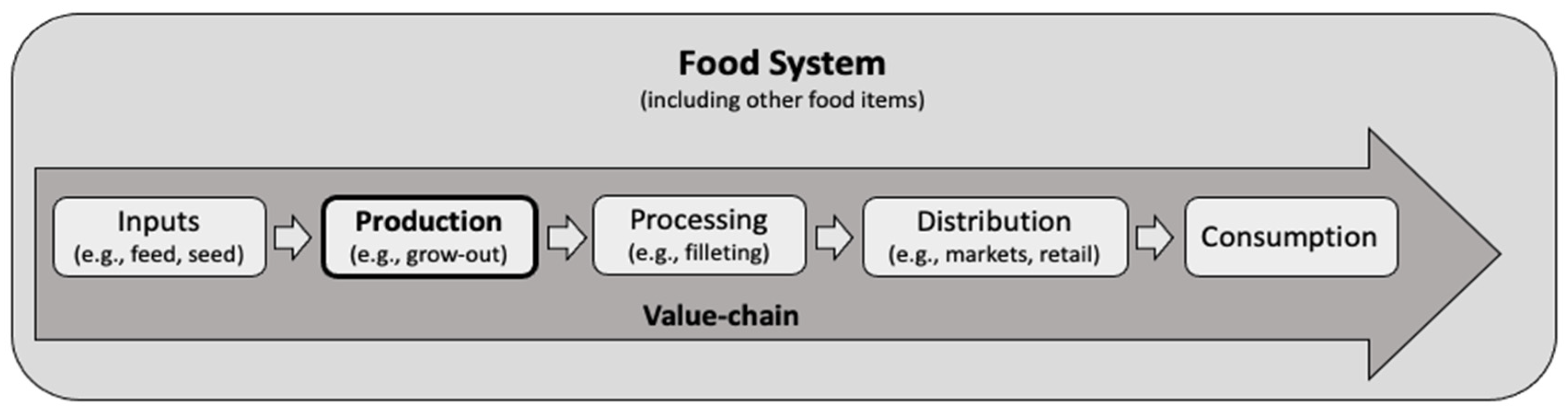
:1. Introduction
2. The Issues
2.1. The Diversity of Aquaculture
2.2. What Is the Edible Yield?
2.3. Broader Benefits from Production Systems
2.4. Emergent Methodologies for Measuring Environmental and Nutritional Outcomes
2.5. Contextual Differences: Delivering Nutritional Benefits from Aquatic Foods
- Food pairings and compound effects: Aquatic animals can supply an array of bioavailable, commonly deficient micronutrients for humans [2,4], a property that is of even greater value when considered as part of the total diet. For example, the co-consumption of fish can increase the bioavailability of certain nutrients in vegetables [75,118]. In addition, traditional recipes may call for certain ingredients to be paired with a fish, that have direct nutritional impacts on the consumer as well as affect the bioavailability of nutrients in the fish (e.g., cod and bacon is a popular food pairing in Norway, and Peruvian ceviche includes lime juice with a white fish such as sea bass or tilapia). As mentioned above, methods by Weidema and Stylianou [18] may be one way of modelling the cumulative effects of these types of food pairings.
- Cooking and preparation methods: The effect of aquatic animal consumption on diet-related health outcomes may vary with how the food is prepared, portioned, and cooked [2]. Available cooking methods alter people’s ability to consume and digest aquatic animals and the nutrition available with possible trade-offs [119,120]. For example, drying small fish softens the bones such that young children can eat the whole fish and benefit from the calcium-rich content [121]; however, drying the fish may also alter its amino acid profile [122]. Baked and canned sardines also contain bones that are edible. Drying, fermenting, or canning may alter the nutritional value of fish. Beyond altering the nutritional value of the product, different production, preparation, or preservation methods can affect health aspects negatively. Preservation chemicals, such as salt and pigments may negatively affect human health, as can contaminants and adulterants post-harvest; yet, preservation methods can prevent spoilage, supporting nutritional needs during lean periods [123]. Furthermore, such processing can increase the affordability of fish while still providing some health benefits, such as the n-3 PUFA content of tinned mackerel, typically much cheaper than fresh alternatives [124]. Regulations that support safe and clean post-harvest practices are critical, particularly given the importance of processed fish in contexts like sub-Saharan Africa, where cold chains are short and cultural tastes lean towards preserved products [125]. The cultural and livelihood implications of processing—an activity often dominated by minority or marginalised groups—as well as the role of processed fish in broader food security are understudied; most literature on processed fish concerns its aesthetic or food safety properties [126].
- Food safety: Nutrition outcomes are influenced by food handling and safety: sanitation practices (i.e., WaSH) and other food safety initiatives have positive effects on disease mitigation [127] and enhanced absorption of nutrients from foods [128]. Aquatic animals and their environments are often central to the transmission of diseases, such as those caused by food-borne trematodes [129], and have been associated with accumulation of contaminants in the food chain [130,131]. Different aquatic species [132] and production systems [133,134] can influence safety and quality aspects of the consumed product; public mis-information that farmed aquatic animals have more contaminants than those from the wild has threatened international trade and food security [135]. Moving from a ‘food safety’ perspective towards a ‘One Health’ lens [136] may be a good basis for integrating environment, human, and animal health. This system framework may also encourage a move away from consumers’ unbalanced focus on negative outcomes associated with aquatic systems such as heavy-metal toxicity, antimicrobial resistance, and plastic contaminants [137].
2.6. Local Versus Global: Perceptions and Realities
2.7. Aquatic Animal Production Systems Span across the Fisheries—Aquaculture Continuum
3. The Way Forward
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Beveridge, M.C.M.; Thilsted, S.H.; Phillips, M.J.; Metian, M.; Troell, M.; Hall, S.J. Meeting the food and nutrition needs of the poor: The role of fish and the opportunities and challenges emerging from the rise of aquaculture. J. Fish Biol. 2013, 83, 1067–1084. [Google Scholar] [CrossRef] [Green Version]
- Cisneros-Montemayor, A.M.; Pauly, D.; Weatherdon, L.V.; Ota, Y. A Global Estimate of Seafood Consumption by Coastal Indigenous Peoples. PLoS ONE 2016, 11, e0166681. [Google Scholar] [CrossRef]
- Golden, C.D.; Allison, E.H.; Cheung, W.W.; Dey, M.M.; Halpern, B.S.; McCauley, D.J.; Smith, M.; Vaitla, B.; Zeller, D.; Myers, S.S. Nutrition: Fall in fish catch threatens human health. Nat. News 2016, 534, 317. [Google Scholar] [CrossRef] [PubMed]
- Belton, B.; Bush, S.R.; Little, D.C. Not just for the wealthy: Rethinking farmed fish consumption in the Global South. Glob. Food Secur. 2018, 16, 85–92. [Google Scholar] [CrossRef]
- Troell, M.; Jonell, M.; Crona, B. The Role of Seafood in Sustainable and Healthy Diets. The EAT-Lancet Commission Report through a Blue Lens; The Beijer Institute: Stockholm, Sweden, 2019. [Google Scholar]
- Blackmore, I.; Lesorogol, C.; Iannotti, L. Small livestock and aquaculture programming impacts on household livelihood security: A systematic narrative review. J. Dev. Eff. 2018, 10, 197–248. [Google Scholar] [CrossRef]
- Little, D.C.; Young, J.A.; Zhang, W.; Newton, R.W.; Al Mamun, A.; Murray, F.J. Sustainable intensification of aquaculture value chains between Asia and Europe: A framework for understanding impacts and challenges. Aquaculture 2018, 493, 338–354. [Google Scholar] [CrossRef]
- Funge-Smith, S.; Bennett, A. A fresh look at inland fisheries and their role in food security and livelihoods. Fish Fish. 2019, 20, 1176–1195. [Google Scholar] [CrossRef] [Green Version]
- Short, R.E.; Gelcich, S.; Little, D.C.; Micheli, F.; Allison, E.H.; Basurto, X.; Belton, B.; Brugere, C.; Bush, S.R.; Cao, L.; et al. Harnessing the diversity of small-scale actors is key to the future of aquatic food systems. Nat. Food 2021, 2, 733–741. [Google Scholar] [CrossRef]
- Filipski, M.; Belton, B. Give a man a fishpond: Modeling the impacts of aquaculture in the rural economy. World Dev. 2018, 110, 205–223. [Google Scholar] [CrossRef]
- Gephart, J.A.; Henriksson, P.J.; Parker, R.W.; Shepon, A.; Gorospe, K.D.; Bergman, K.; Eshel, G.; Golden, C.D.; Halpern, B.S.; Hornborg, S.; et al. Environmental performance of blue foods. Nature 2021, 597, 360–365. [Google Scholar] [CrossRef]
- Golden, C.D.; Koehn, J.Z.; Shepon, A.; Passarelli, S.; Free, C.M.; Viana, D.F.; Matthey, H.; Eurich, J.G.; Gephart, J.A.; Fluet-Chouinard, E.; et al. Aquatic foods to nourish nations. Nature 2021, 45, 1–6. [Google Scholar] [CrossRef]
- Tlusty, M.F.; Tyedmers, P.; Bailey, M.; Ziegler, F.; Henriksson, P.J.; Béné, C.; Bush, S.; Newton, R.; Asche, F.; Little, D.C.; et al. Reframing the sustainable seafood narrative. Glob. Environ. Chang. 2019, 59, 101991. [Google Scholar] [CrossRef]
- HLPE. Nutrition and Food Systems. A Report by the High Level Panel of Experts on Food Security and Nutrition of the Committee on World Food Security; HLPE: Rome, Italy, 2017. [Google Scholar]
- Adesogan, A.T.; Havelaar, A.H.; McKune, S.L.; Eilittä, M.; Dahl, G.E. Animal source foods: Sustainability problem or malnutrition and sustainability solution? Perspective matters. Glob. Food Secur. 2020, 25, 100325. [Google Scholar] [CrossRef]
- Clark, M.A.; Springmann, M.; Hill, J.; Tilman, D. Multiple health and environmental impacts of foods. Proc. Natl. Acad. Sci. USA 2019, 116, 23357–23362. [Google Scholar] [CrossRef] [Green Version]
- Weidema, B.P.; Stylianou, K.S. Nutrition in the life cycle assessment of foods—function or impact? Int. J. Life Cycle Assess. 2020, 74, 1–7. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Poore, J.; Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef] [Green Version]
- Springmann, M.; Clark, M.; Mason-D’Croz, D.; Wiebe, K.; Bodirsky, B.L.; Lassaletta, L.; De Vries, W.; Vermeulen, S.J.; Herrero, M.; Carlson, K.M.; et al. Options for keeping the food system within environmental limits. Nature 2018, 562, 519–525. [Google Scholar] [CrossRef]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S.; Lambin, E.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. Planetary boundaries: Exploring the safe operating space for humanity. Ecol. Soc. 2009, 14, 48–55. [Google Scholar] [CrossRef]
- Roberts, C.A.; Newton, R.; Bostock, J.; Prescott, S.; Honey, D.J.; Telfer, T.; Walmsley, S.F.; Little, D.C.; Hull, S.C. A Risk Benefit Analysis of Mariculture as a means to Reduce the Impacts of Terrestrial Production of Food and Energy. Scottish Aquaculture Research Forum; World Wildlife Fund for Nature (WWF) SARF Project Reports, SARF106. Scott. Aquac. Res. Forum. 2015. Available online: http://www.sarf.org.uk/cms-assets/documents/232492–618987.sarf106.pdf. (accessed on 21 August 2021).
- Roos, E.; Bajželj, B.; Smith, P.; Patel, M.; Little, D.; Garnett, T. Greedy or needy? Land use and climate impacts of food in 2050 under different livestock futures. Glob. Environ. Chang. 2017, 47, 1–12. [Google Scholar] [CrossRef]
- FAO. Working for SDG 14: Healthy Oceans for Food Security. Nutrition. 2017, and Resilient Communities; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
- Stetkiewicz, S.; Norman, R.A.; Allison, E.H.; Andrew, N.L.; Ara, G.; Banner-Stevens, G.; Belton, B.; Beveridge, M.; Bogard, J.R.; Bush, S.; et al. Seafood in Food Security: A call for bridging the terrestrial-aquatic divide. Front. Sustain. Food Syst. 2021, 5, 5–11. [Google Scholar] [CrossRef]
- de Roos, B.; Roos, N.; Mamun, A.A.; Ahmed, T.; Sneddon, A.A.; Murray, F.; Grieve, E.; Little, D.C. Linking agroecosystems producing farmed seafood with food security and health status to better address the nutritional challenges in Bangladesh. Pub. Health Nutr. 2019, 22, 2941–2949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hicks, C.C.; Cohen, P.J.; Graham, N.A.; Nash, K.L.; Allison, E.H.; D’Lima, C.; Mills, D.J.; Roscher, M.; Thilsted, S.H.; Thorne-Lyman, A.L.; et al. Harnessing global fisheries to tackle micronutrient deficiencies. Nature 2019, 574, 95–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallström, E.; Bergman, K.; Mifflin, K.; Parker, R.; Tyedmers, P.; Troell, M.; Ziegler, F. Combined climate and nutritional performance of seafoods. J. Clean. Prod. 2019, 230, 392–411. [Google Scholar] [CrossRef]
- Tezzo, X.; Bush, S.R.; Oosterveer, P.; Belton, B. Food system perspective on fisheries and aquaculture development in Asia. Agric. Hum. Values 2021, 38, 86–90. [Google Scholar] [CrossRef]
- Naylor, R.L.; Kishore, A.; Sumaila, U.R.; Issifu, I.; Hunter, B.P.; Belton, B.; Bush, S.R.; Cao, L.; Gelcich, S.; Gephart, J.A.; et al. Blue food demand across geographic and temporal scales. Nat. Comm. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Belton, B.; Little, D.C.; Zhang, W.; Edwards, P.; Skladany, M.; Thilsted, S.H. Farming fish in the sea will not nourish the world. Nat. Comm. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Zhang, W.; Belton, B.; Edwards, P.; Henriksson, P.J.; Little, D.C.; Newton, R.; Troell, M. Aquaculture will continue to depend more on land than sea. Nature 2022, 603, E2–E4. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, M.; Sadovy de Mitcheson, Y.; Cao, L.; Leadbitter, D.; Newton, R.; Little, D.C.; Li, S.; Yang, Y.; Chen, X.; et al. Fishing for feed in China: Facts, impacts and implications. Fish Fish. 2020, 21, 52–62. [Google Scholar] [CrossRef]
- Tigchelaar, M.; Cheung, W.W.; Mohammed, E.Y.; Phillips, M.J.; Payne, H.J.; Selig, E.R.; Wabnitz, C.C.; Oyinlola, M.A.; Frölicher, T.L.; Gephart, J.A.; et al. Compound climate risks threaten aquatic food system benefits. Nat. Food 2021, 2, 673–682. [Google Scholar] [CrossRef]
- Jennings, S.; Stentiford, G.D.; Leocadio, A.M.; Jeffery, K.R.; Metcalfe, J.D.; Katsiadaki, I.; Auchterlonie, N.A.; Mangi, S.C.; Pinnegar, J.K.; Ellis, T.; et al. Aquatic food security: Insights into challenges and solutions from an analysis of interactions between fisheries, aquaculture, food safety, human health, fish and human welfare, economy and environment. Fish Fish. 2016, 17, 893–938. [Google Scholar] [CrossRef] [Green Version]
- Shepon, A.; Gephart, J.A.; Golden, C.D.; Henriksson, P.J.G.; Jones, R.C.; Koehn, J.Z.; Eshel, G. Exploring sustainable aquaculture development using a nutrition-sensitive approach. Glob. Environ. Chang. 2021, 69, 102285. [Google Scholar] [CrossRef]
- Shepon, A.; Gephart, J.A.; Henriksson, P.J.G.; Jones, R.; Murshed-e-Jahan, K.; Eshel, G.; Golden, C.D. Reorientation of aquaculture production systems can reduce environmental impacts and improve nutrition security in Bangladesh. Nat. Food 2020, 1, 640–647. [Google Scholar] [CrossRef]
- Pullin, A.S.; Knight, T.M.; Watkinson, A.R. Linking reductionist science and holistic policy using systematic reviews: Unpacking environmental policy questions to construct an evidence-based framework. J. Appl. Ecol. 2009, 46, 970–975. [Google Scholar] [CrossRef]
- Belton, B.; Bush, S.R. Beyond net deficits: New priorities for an aquacultural geography. Geogr. J. 2014, 180, 3–14. [Google Scholar] [CrossRef]
- Regueiro, L.; Newton, R.; Soula, M.; Méndez, D.; Kok, B.; Little, D.C.; Pastres, R.; Johansen, J.; Ferreira, M. Opportunities and limitations for the introduction of circular economy principles in EU aquaculture based on the regulatory framework. J. Ind. Ecol. 2020, 18, 16–18. [Google Scholar] [CrossRef]
- Béné, C.; Oosterveer, P.; Lamotte, L.; Brouwer, I.D.; de Haan, S.; Prager, S.D.; Talsma, E.F.; Khoury, C.K. When food systems meet sustainability–Current narratives and implications for actions. World Dev. 2019, 113, 116–130. [Google Scholar] [CrossRef]
- Little, D.C.; Karim, M.; Turongruang, D.; Morales, E.J.; Murray, F.J.; Barman, B.K.; Haque, M.M.; Kundu, N.; Belton, B.; Faruque, G.; et al. Livelihood Impacts of Ponds in Asia-Opportunities and Constraints. Fish Ponds in Farming Systems; Van der Zij, A.J., Verreth, J.A.J., van Mensvoort, M.E.F., Bosma, R.H., Beveridge, M.C.M., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2017; pp. 177–202. [Google Scholar]
- Hernandez, R.; Belton, B.; Reardon, T.; Hu, C.; Zhang, X.; Ahmed, A. The “quiet revolution” in the aquaculture value chain in Bangladesh. Aquaculture 2018, 493, 456–468. [Google Scholar] [CrossRef]
- Brugere, C.; Troell, M.; Eriksson, H. More than fish: Policy coherence and benefit sharing as necessary conditions for equitable aquaculture development. Mar. Policy 2021, 123, 104271. [Google Scholar] [CrossRef]
- Teneva, L.T.; Schemmel, E.; Kittinger, J.N. State of the plate: Assessing present and future contribution of fisheries and aquaculture to Hawai ‘i’s food security. Mar. Policy 2018, 94, 28–38. [Google Scholar] [CrossRef] [Green Version]
- Koehn, J.Z.; Allison, E.H.; Golden, C.D.; Hilborn, R. The role of seafood in sustainable diets. Environ. Res. Lett. 2017, 17, 035003. [Google Scholar] [CrossRef]
- Bohnes, F.A.; Hauschild, M.Z.; Schlundt, J.; Laurent, A. Life cycle assessments of aquaculture systems: A critical review of reported findings with recommendations for policy and system development. Rev. Aquac. 2021, 11, 1061. [Google Scholar] [CrossRef] [Green Version]
- Alsip, P.J.; Zhang, H.; Rowe, M.D.; Mason, D.M.; Rutherford, E.S.; Riseng, C.M.; Su, Z. Lake Michigan’s suitability for bigheaded carp: The importance of diet flexibility and subsurface habitat. Freshw. Biol. 2019, 64, 1921–1939. [Google Scholar] [CrossRef]
- Metian, M.; Troell, M.; Christensen, V.; Steenbeek, J.; Pouil, S. Mapping diversity of species in global aquaculture. Rev. Aquacult. 2020, 12, 1090–1100. [Google Scholar] [CrossRef]
- Little, D.C.; Bunting, S.W. Aquaculture technologies for food security. In Emerging Technologies for Promoting Food Security; Woodhead Publishing: Sawston, UK, 2016; pp. 93–113. [Google Scholar]
- Newton, R.W.; Little, D.C. Mapping the impacts of farmed Scottish salmon from a life cycle perspective. Int. J. Life Cycle Asses. 2018, 23, 1018–1029. [Google Scholar] [CrossRef] [Green Version]
- Pelletier, N.; Klinger, D.H.; Sims, N.A.; Yoshioka, J.R.; Kittinger, J.N. Nutritional attributes, substitutability, scalability, and environmental intensity of an illustrative subset of current and future protein sources for aquaculture feeds: Joint consideration of potential synergies and trade-offs. Environ. Sci. Technol. 2018, 52, 5532–5544. [Google Scholar] [CrossRef]
- Pahlow, M.; van Oel, P.R.; Mekonnen, M.M.; Hoekstra, A.Y. Increasing pressure on freshwater resources due to terrestrial feed ingredients for aquaculture production. Sci. Total Environ. 2015, 536, 847–857. [Google Scholar] [CrossRef] [Green Version]
- Fry, J.P.; Love, D.C.; MacDonald, G.K.; West, P.C.; Engstrom, P.M.; Nachman, K.E.; Lawrence, R.S. Environmental health impacts of feeding crops to farmed fish. Environ. Int. 2016, 91, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Malcorps, W.; Kok, B.; van‘t Land, M.; Fritz, M.; van Doren, D.; Servin, K.; van der Heijden, P.; Palmer, R.; Auchterlonie, N.A.; Rietkerk, M.; et al. The sustainability conundrum of fishmeal substitution by plant ingredients in shrimp feeds. Sustainability 2019, 11, 1212. [Google Scholar] [CrossRef] [Green Version]
- Spiertz, J.H.J.; Ewert, F. Crop production and resource use to meet the growing demand for food, feed and fuel: Opportunities and constraints. NJAS Wagen. J. Life Sci. 2009, 56, 281–300. [Google Scholar] [CrossRef] [Green Version]
- Boissy, J.; Aubin, J.; Drissi, A.; van der Werf, H.M.; Bell, G.J.; Kaushik, S.J. Environmental impacts of plant-based salmonid diets at feed and farm scales. Aquaculture 2011, 321, 61–70. [Google Scholar] [CrossRef]
- Boyd, C.E.; Davis, R.P.; McNevin, A.A. Perspectives on the mangrove conundrum, land use, and benefits of yield intensification in farmed shrimp production: A Review. J. World Aquac. Soc. 2022, 53, 8–46. [Google Scholar] [CrossRef]
- López Cabo, M.; Romalde, L.J.; Simal-Gandara, J.; Gago Martínez, A.; Giráldez Fernández, J.; Bernárdez Costas, M.; Pascual del Hierro, S.; Pousa Ortega, Á.; Manaia, C.M.; Abreu Silva, J.; et al. Identification of emerging hazards in mussels by the Galician emerging food safety risks network (RISEGAL). First Approach Foods 2020, 9, 1641. [Google Scholar] [CrossRef] [PubMed]
- Sprague, M.; Dick, J.R.; Tocher, D.R. Impact of sustainable feeds on omega-3 long-chain fatty acid levels in farmed Atlantic salmon. Sci. Rep. 2006, 6, 21892. [Google Scholar] [CrossRef] [Green Version]
- Sprague, M.; Fawcett, S.; Betancor, M.B.; Struthers, W.; Tocher, D.R. Variation in the nutritional composition of farmed Atlantic salmon (Salmo salar L.) fillets with emphasis on EPA and DHA contents. J. Food Compos. Anal. 2020, 94, 103618. [Google Scholar] [CrossRef]
- Saito, T.; Whatmore, P.; Taylor, J.F.; Fernandes, J.M.; Adam, A.C.; Tocher, D.R.; Espe, M.; Skjærven, K.H. Micronutrient supplementation affects transcriptional and epigenetic regulation of lipid metabolism in a dose-dependent manner. Epigenetics 2021, 48, 1–18. [Google Scholar] [CrossRef]
- Nichols, P.D.; Glencross, B.; Petrie, J.R.; Singh, S.P. Readily available sources of long-chain omega-3 oils: Is farmed Australian seafood a better source of the good oil than wild-caught seafood? Nutrients 2015, 6, 1063. [Google Scholar] [CrossRef]
- Karapanagiotidis, I.T.; Bell, M.V.; Little, D.C.; Yakupitiyage, A.; Rakshit, S.K. Polyunsaturated fatty acid content of wild and farmed tilapias in Thailand: Effect of aquaculture practices and implications for human nutrition. J. Agric. Food Chem. 2006, 54, 4304–4310. [Google Scholar] [CrossRef]
- Mamun, A.A.; Murray, F.J.; Sprague, M.; Mcadam, B.J.; Roos, N.; De Roos, B.; Pounds, A.; and Little, D.C. Export-driven, extensive coastal aquaculture can benefit nutritionally vulnerable people. Front. Sustain. Food Syst. 2021, 713, 140. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Vijayavel, K. Nutritional composition of three estuarine bivalve mussels, Perna viridis, Donax cuneatus and Meretrix meretrix. Int. J. Food Sci. Nutr. 2009, 60, 458–463. [Google Scholar] [CrossRef]
- Küllenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipids Health Dis. 2012, 11, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.N.; Huang, X.H.; Zheng, J.; Sun, Y.H.; Dong, X.P.; Zhou, D.Y.; Zhu, B.W.; Qin, L. Comprehensive metabolomic and lipidomic profiling of the seasonal variation of blue mussels (Mytilus edulis L.): Free amino acids, 5′-nucleotides, and lipids. LWT 2021, 799, 111835. [Google Scholar] [CrossRef]
- Fry, J.P.; Mailloux, N.A.; Love, D.C.; Milli, M.C.; Cao, L. Feed conversion efficiency in aquaculture: Do we measure it correctly? Environ. Res. Lett. 2018, 13, 024017. [Google Scholar] [CrossRef]
- Haque, M.M.; Little, D.C.; Barman, B.K.; Wahab, M.A.; Telfer, T.C. Impacts of decentralized fish fingerling production in irrigated rice fields in Northwest Bangladesh. Aquac. Res. 2014, 45, 655–674. [Google Scholar] [CrossRef]
- Murray, F.J.; Little, D.C. Inland Fisheries Resources and the Current Status of Aquaculture in Sri Lanka; FAO: Rome, Italy, 2000. [Google Scholar]
- Kassam, L.; Dorward, A. Comparative assessment of the poverty impacts of pondand cage aquaculture in Ghana. Aquaculture 2017, 470, 110–122. [Google Scholar] [CrossRef]
- Sproesser, G.; Ruby, M.B.; Arbit, N.; Akotia, C.S.; Alvarenga, M.D.S.; Bhangaokar, R.; Furumitsu, I.; Hu, X.; Imada, S.; Kaptan, G.; et al. Understanding traditional and modern eating: The TEP10 framework. BMC Public Health 2019, 19, 1–14. [Google Scholar] [CrossRef]
- Kawarazuka, N.; Béné, C. The potential role of small fish species in improving micronutrient deficiencies in developing countries: Building evidence. Public Health Nutr. 2011, 14, 1927–1938. [Google Scholar] [CrossRef] [Green Version]
- Roos, N. Fish Consumption and Aquaculture in Rural Bangladesh: Nutritional Contribution and Production Potential of Culturing Small Indigenous Fish Species (SIS) in Pond Polyculture with Commonly Cultured Carps. Ph.D. Thesis, Royal Veterinary and Argicultural University, Copenhagen, Denmark, 2002. [Google Scholar]
- Froese, R.; Pauly, D. (Eds.) FishBase. World Wide Web Electronic Publication. 2022. Available online: www.fishbase.org (accessed on 2 February 2022).
- Batista, I. By-catch, underutilized species and underutilized fish parts as food ingredients. In Maximising the Value of Marine By-Products; Woodhead Publishing: Sawston, UK, 2007; pp. 171–195. [Google Scholar]
- Malcorps, W.; Newton, R.W.; Sprague, M.; Glencross, B.D.; Little, D.C. Nutritional Characterisation of European Aquaculture Processing By-Products to Facilitate Strategic Utilisation. Front. Sustain. Food Syst. 2021, 378, 13–24. [Google Scholar] [CrossRef]
- Woodgate, S.L.; Wilkinson, R.G. The role of rendering in relation to the bovine spongiform encephalopathy epidemic, the development of EU animal by-product legislation and the reintroduction of rendered products into animal feeds. Annu. Appl. Biol. 2021, 178, 430–441. [Google Scholar] [CrossRef]
- Newton, R.W. Assessing Environmental Sustainability and Value Addition Opportunities for by-Products from Aquaculture. Ph.D. Thesis, Institute of Aquaculture, University of Stirling, Stirling, Scotland, 2014. [Google Scholar]
- Stevens, J.R.; Newton, R.W.; Tlusty, M.; Little, D.C. The rise of aquaculture by-products: Increasing food production, value, and sustainability through strategic utilisation. Mar. Policy 2018, 90, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Abbey, L.; Glover-Amengor, M.; Atikpo, M.O.; Atter, A.; Toppe, J. Nutrient content of fish powder from low value fish and fish byproducts. Food Sci. Nutr. 2017, 5, 374–379. [Google Scholar] [CrossRef]
- Malde, M.K.; Bügel, S.; Kristensen, M.; Malde, K.; Graff, I.E.; Pedersen, J.I. Calcium from salmon and cod bone is well absorbed in young healthy men: A double-blinded randomised crossover design. Nutr. Met. 2010, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Franco, C.; Zhang, W. Functions, applications and production of protein hydrolysates from fish processing co-products (FPCP). Food Res. Int. 2013, 50, 289–297. [Google Scholar] [CrossRef]
- Ruddle, K. The impacts of aquaculture development on socioeconomic environments in developing countries: Toward a paradigm for assessment. In Environment and Aquaculture in Developing Countries. Proceedings of the ICLARM Conference; ICLARM: Manila, Philippines, 1993; Volume 31, pp. 20–41. [Google Scholar]
- Edwards, P. Traditional Asian aquaculture. In New Technologies in Aquaculture; Woodhead Publishing: Sawston, UK, 2009; pp. 1029–1063. [Google Scholar]
- Newton, R.; Zhang, W.; Xian, Z.; McAdam, B.; Little, D.C. Intensification, regulation and diversification: The changing face of inland aquaculture in China. Ambio 2021, 65, 1–18. [Google Scholar] [CrossRef]
- Karim, M.; Little, D.C. The impacts of integrated homestead pond-dike systems in relation to production, consumption and seasonality in central north Bangladesh. Aquac. Res. 2018, 49, 313–334. [Google Scholar] [CrossRef] [Green Version]
- Nhan, D.K.; Phong, L.T.; Verdegem, M.J.; Duong, L.T.; Bosma, R.H.; Little, D.C. Integrated freshwater aquaculture, crop and livestock production in the Mekong delta, Vietnam: Determinants and the role of the pond. Agric. Syst. 2007, 94, 445–458. [Google Scholar] [CrossRef]
- Thakur, A.K.; Mohanty, R.K.; Singh, R.; Patil, D.U. Enhancing water and cropping productivity through Integrated System of Rice Intensification (ISRI) with aquaculture and horticulture under rainfed conditions. Agric. Water Manag. 2015, 161, 65–76. [Google Scholar] [CrossRef]
- Bunting, S.W.; Pretty, J.; Edwards, P. Wastewater-fed aquaculture in the East Kolkata Wetlands, India: Anachronism or archetype for resilient ecocultures? Rev. Aquacult. 2010, 2, 138–153. [Google Scholar] [CrossRef]
- Ferreira, J.G.; Bricker, S.B. Goods and services of extensive aquaculture: Shellfish culture and nutrient trading. Aquac. Int. 2021, 24, 803–825. [Google Scholar] [CrossRef]
- Brummett, R.E.; Lazard, J.; Moehl, J. African aquaculture: Realizing the potential. Food Policy 2008, 33, 371–385. [Google Scholar] [CrossRef]
- Islam, F.U.A. Self-Recruiting Species (SRS) in Aquaculture: Their Role in Rural Livelihoods in Two Areas of Bangladesh. Ph.D. Thesis, University of Stirling, Stirling, Scotland, 2007. [Google Scholar]
- Morales, E. Self-Recruiting Species in Farmer Managed Aquatic Systems: Their Importance to the Livelihoods of the Rural Poor in Southeast Asia. Ph.D. Thesis, Institute of Aquaculture, University of Stirling, Stirling, Scotland, 2007. [Google Scholar]
- dos Reis, I.C.; Codeço, C.T.; Degener, C.M.; Keppeler, E.C.; Muniz, M.M.; de Oliveira, F.G.S.; Cortês, J.J.C.; de Freitas Monteiro, A.; de Souza, C.A.A.; Rodrigues, F.C.M.; et al. Contribution of fish farming ponds to the production of immature Anopheles s in a malaria-endemic Amazonian town. Malar. J. 2015, 14, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokolow, S.H.; Jones, I.J.; Jocque, M.; La, D.; Cords, O.; Knight, A.; Lund, A.; Wood, C.L.; Lafferty, K.D.; Hoover, C.M.; et al. Nearly 400 million people are at higher risk of schistosomiasis because dams block the migration of snail-eating river prawns. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, A.F.; Zhou, G.; Omlin, F.X. Malaria mosquito control using edible fish in western Kenya: Preliminary findings of a controlled study. BMC Public Health 2007, 7, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoover, C.M.; Sokolow, S.H.; Kemp, J.; Sanchirico, J.N.; Lund, A.J.; Jones, I.J.; Higginson, T.; Riveau, G.; Savaya, A.; Coyle, S.; et al. Modelled effects of prawn aquaculture on poverty alleviation and schistosomiasis control. Nat. Sustain. 2018, 2, 611–620. [Google Scholar] [CrossRef]
- Edwards, P.; Zhang, W.; Belton, B.; Little, D.C. Misunderstandings, myths and mantras in aquaculture: Its contribution to world food supplies has been systematically over reported. Mar. Policy 2019, 106, 103547. [Google Scholar] [CrossRef]
- Stylianou, K.S.; Heller, M.C.; Fulgoni, V.L.; Ernstoff, A.S.; Keoleian, G.A.; Jolliet, O. A life cycle assessment framework combining nutritional and environmental health impacts of diet: A case study on milk. Int. J. Life Cycle Assess. 2016, 21, 734–746. [Google Scholar] [CrossRef] [Green Version]
- Nijdam, D.; Rood, T.; Westhoek, H. The price of protein: Review of land use and carbon footprints from life cycle assessments of animal food products and their substitutes. Food Policy 2012, 37, 760–770. [Google Scholar] [CrossRef]
- Aakre, I.; Næss, S.; Kjellevold, M.; Markhus, M.W.; Alvheim, A.R.; Dalane, J.Ø.; Kielland, E.; Dahl, L. New datla on nutrient composition in large selection of commercially available seafood products and its impact on micronutrient intake. Food Nutr. Res. 2019, 63, 2035–2058. [Google Scholar] [CrossRef] [Green Version]
- Smetana, S.M.; Bornkessel, S.; Heinz, V. A path from sustainable nutrition to nutritional sustainability of complex food systems. Front. Nutr. 2019, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Hallström, E.; Davis, J.; Woodhouse, A.; Sonesson, U. Using dietary quality scores to assess sustainability of food products and human diets: A systematic review. Ecol. Ind. 2018, 93, 219–230. [Google Scholar] [CrossRef]
- Cooper, K.A.; Quested, T.E.; Lanctuit, H.; Zimmermann, D.; Espinoza-Orias, N.; Roulin, A. Nutrition in the bin: A nutritional and environmental assessment of food wasted in the UK. Front. Nutr. 2018, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Lucas, E.; Galán-Martín, Á.; Pozo, C.; Guo, M.; Guillén-Gosálbez, G. Global environmental and nutritional assessment of national food supply patterns: Insights from a data envelopment analysis approach. Sci. Total Environ. 2021, 755, 142826. [Google Scholar] [CrossRef]
- Boulay, A.M.; Bare, J.; Benini, L.; Berger, M.; Lathuillière, M.J.; Manzardo, A.; Margni, M.; Motoshita, M.; Núñez, M.; Pastor, A.V.; et al. The WULCA consensus characterization model for water scarcity footprints: Assessing impacts of water consumption based on available water remaining (AWARE). Int. J. Life Cycle Assess. 2018, 23, 368–378. [Google Scholar] [CrossRef] [Green Version]
- Dekker, E.; Zijp, M.C.; van de Kamp, M.E.; Temme, E.H.; van Zelm, R. A taste of the new ReCiPe for life cycle assessment: Consequences of the updated impact assessment method on food product LCAs. Int. J. Life Cycle Assess. 2020, 25, 2315–2324. [Google Scholar] [CrossRef] [Green Version]
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Gephart, J.A.; Golden, C.D.; Asche, F.; Belton, B.; Brugere, C.; Froehlich, H.E.; Fry, J.P.; Halpern, B.S.; Hicks, C.C.; Jones, R.C. Scenarios for global aquaculture and its role in human nutrition. Rev. Fish. Sci. Aquac. 2021, 75, 1–17. [Google Scholar] [CrossRef]
- Ruiz-Salmón, I.; Laso, J.; Margallo, M.; Villanueva-Rey, P.; Rodríguez, E.; Quinteiro, P.; Dias, A.C.; Almeida, C.; Nunes, M.L.; Marques, A. Life cycle assessment of fish and seafood processed products–a review of methodologies and new challenges. Sci. Total Environ. 2020, 63, 144094. [Google Scholar] [CrossRef]
- FAO. FAO/INFOODS Global Food Composition Database for Fish and Shellfish Version 1.0-uFiSh1.0; FAO: Rome, Italy, 2016. [Google Scholar]
- Moxness Reksten, A.; Bøkevoll, A.; Frantzen, S.; Lundebye, A.K.; Kögel, T.; Kolås, K.; Aakre, I.; Kjellevold, M. Sampling protocol for the determination of nutrients and contaminants in fish and other seafood–The EAF-Nansen Programme. Methods 2020, 7, 1063. [Google Scholar] [CrossRef]
- Bogard, J.R.; Farook, S.; Marks, G.C.; Waid, J.; Belton, B.; Ali, M.; Toufique, K.; Mamun, A.; Thilsted, S.H. Higher fish but lower micronutrient intakes: Temporal changes in fish consumption from capture fisheries and aquaculture in Bangladesh. PLoS ONE 2017, 12, e0175098. [Google Scholar] [CrossRef] [Green Version]
- Kent, G. Fisheries, food security, and the poor. Food Policy 1997, 22, 393–404. [Google Scholar] [CrossRef]
- Toure, F.; Lucas, E.; Stoecker, B. Fish and shrimp added bioavailable iodine to cassava and millet-based diets. Ecol. Food Nutr. 2003, 42, 223–239. [Google Scholar] [CrossRef]
- Ersoy, B.; Özeren, A. The effect of cooking methods on mineral and vitamin contents of African catfish. Food Chem. 2009, 115, 419–422. [Google Scholar] [CrossRef]
- Uran, H.; Gokoglu, N. Effects of cooking methods and temperatures on nutritional and quality characteristics of anchovy (Engraulis encrasicholus). J. Food Sci. Technol. 2014, 51, 722–728. [Google Scholar] [CrossRef] [Green Version]
- Gibson, R.S.; Yeudall, F.; Drost, N.; Mtitimuni, B.M.; Cullinan, T.R. Experiences of a community-based dietary intervention to enhance micronutrient adequacy of diets low in animal source foods and high in phytate: A case study in rural Malawian children. J. Nutr. 2003, 133, 3992S–3999S. [Google Scholar] [CrossRef]
- Teixeira, B.; Mendes, R. The Nutritional Quality of Dried Salted Cod: The Effect of Processing and Polyphosphates Addition. J. Food Nutr. Res. 2020, 8, 304–312. [Google Scholar] [CrossRef]
- Gelli, A.; Nguyen, P.H.; Santacroce, M.; Twalibu, A.; Margolies, A.; Katundu, M. A Community-Based Early Childhood Development Center Platform Promoting Diversified Diets and Food Production Increases the Mean Probability of Adequacy of Intake of Preschoolers in Malawi: A Cluster Randomized Trial. J. Nutr. 2020, 150, 350–355. [Google Scholar] [CrossRef] [Green Version]
- Singer, P.; Wirth, M.; Singer, K. Canned seawater fish with declared content of omega-3 fatty acids: A novel benefit for dietary practice and research. Eur. J. Clin. Nutr. 2016, 70, 1093–1094. [Google Scholar] [CrossRef]
- Kaminski, A.M.; Cole, S.M.; Al Haddad, R.E.; Kefi, A.S.; Chilala, A.D.; Chisule, G.; Mukuka, K.N.; Longley, C.; Teoh, S.J.; Ward, A.R. Fish Losses for Whom? A Gendered Assessment of Post-Harvest Losses in the Barotse Floodplain Fishery, Zambia. Sustainability 2020, 12, 91. [Google Scholar] [CrossRef]
- Belton, B.; Johnson, D.S.; Thrift, E.; Olsen, J.; Hossain, M.A.R.; Thilsted, S.H. Dried fish at the intersection of food science, economy, and culture: A global survey. Fish Fish. 2020, 86, 89–96. [Google Scholar] [CrossRef]
- Gizaw, Z.; Addisu, A. Evidence of households’ water, sanitation, and hygiene (WASH) performance improvement following a WASH education program in rural Dembiya, Northwest Ethiopia. Environ. Health Insights 2022, 14, 1178630220903100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mbuya, M.N.; Humphrey, J.H. Preventing environmental enteric dysfunction through improved water, sanitation and hygiene: An opportunity for stunting reduction in developing countries. Matern. Child Nutr. 2016, 12, 106–120. [Google Scholar] [CrossRef] [Green Version]
- Keiser, J.; Utzinger, J. Food-borne trematodiases. Clin. Microbiol. Rev. 2009, 22, 466–483. [Google Scholar] [CrossRef] [Green Version]
- Bosch, A.C.; O’Neill, B.; Sigge, G.O.; Kerwath, S.E.; Hoffman, L.C. Heavy metals in marine fish meat and consumer health: A review. J. Sci. Food Agric. 2016, 96, 32–48. [Google Scholar] [CrossRef]
- Waring, R.H.; Harris, R.M.; Mitchell, S.C. Plastic contamination of the food chain: A threat to human health? Maturitas 2018, 115, 64–68. [Google Scholar] [CrossRef]
- Barone, G.; Storelli, A.; Meleleo, D.; Dambrosio, A.; Garofalo, R.; Busco, A.; Storelli, M.M. Levels of mercury, methylmercury and selenium in fish: Insights into children food safety. Toxics 2021, 9, 39. [Google Scholar] [CrossRef]
- Lundebye, A.K.; Lock, E.J.; Rasinger, J.D.; Nøstbakken, O.J.; Hannisdal, R.; Karlsbakk, E.; Wennevik, V.; Madhun, A.S.; Madsen, L.; Graff, I.E. Lower levels of persistent organic pollutants, metals and the marine omega 3-fatty acid DHA in farmed compared to wild Atlantic salmon (Salmo salar). Environ. Res. 2017, 155, 49–59. [Google Scholar] [CrossRef]
- Annibaldi, A.; Truzzi, C.; Carnevali, O.; Pignalosa, P.; Api, M.; Scarponi, G.; Illuminati, S. Determination of Hg in farmed and wild atlantic bluefin tuna (Thunnus thynnus L.) muscle. Molecules 2019, 24, 1273. [Google Scholar] [CrossRef] [Green Version]
- Little, D.C.; Bush, S.R.; Belton, B.; Phuong, N.T.; Young, J.A.; Murray, F.J. Whitefish wars: Pangasius, politics and consumer confusion in Europe. Mar. Policy 2012, 36, 738–745. [Google Scholar] [CrossRef] [Green Version]
- Stentiford, G.D.; Bateman, I.J.; Hinchliffe, S.J.; Bass, D.; Hartnell, R.; Santos, E.M.; Devlin, M.J.; Feist, S.W.; Taylor, N.G.H.; Verner-Jeffreys, D.W. Sustainable aquaculture through the One Health lens. Nat. Food 2020, 1, 468–474. [Google Scholar] [CrossRef]
- Newton, R.; Zhang, W.; Leaver, M.; Murray, F.; Little, D.C. Assessment and communication of the toxicological risk of consuming shrimp in the EU. Aquaculture 2019, 500, 148–159. [Google Scholar] [CrossRef]
- Chambers, S.; Lobb, A.; Butler, L.T.; Traill, W.B. The influence of age and gender on food choice: A focus group exploration. Int. J. Consum. Stud. 2008, 32, 356–365. [Google Scholar] [CrossRef]
- Cantillo, J.; Martín, J.C.; Román, C. Discrete choice experiments in the analysis of consumers’ preferences for finfish products: A systematic literature review. Food Qual. Prefer. 2020, 84, 103952. [Google Scholar] [CrossRef]
- Trondsen, T.; Scholderer, J.; Lund, E.; Eggen, A.E. Perceived barriers to consumption of fish among Norwegian women. Appetite 2003, 41, 301–314. [Google Scholar] [CrossRef]
- Myrland, Ø.; Trondsen, T.; Johnston, R.S.; Lund, E. Determinants of seafood consumption in Norway: Lifestyle, revealed preferences, and barriers to consumption. Food Qual. Prefer. 2000, 11, 169–188. [Google Scholar] [CrossRef]
- Másílko, J.; Zajíc, T.; Hlaváč, D. The Culture System Affects Organoleptic Properties and Lipid Composition of Common Carp (Cyprinus Carpio, L.) Meat. J. Text. Stud. 2015, 46, 345–352. [Google Scholar] [CrossRef]
- Bronnmann, J.; Asche, F. Sustainable seafood from aquaculture and wild fisheries: Insights from a discrete choice experiment in Germany. Ecol. Econom. 2017, 142, 113–119. [Google Scholar] [CrossRef]
- Grunert, K.G.; Hieke, S.; Wills, J. Sustainability labels on food products: Consumer motivation, understanding and use. Food Policy 2014, 44, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Murray, F.; Little, D. Rural consumer preferences for inland fish and their substitutes in the Dry-Zone of Sri Lanka and implications for aquaculture development. Frontiers 2022, 2022, 11–56. [Google Scholar] [CrossRef]
- Jaffry, S.; Pickering, H.; Ghulam, Y.; Whitmarsh, D.; Wattage, P. Consumer choices for quality and sustainability labelled seafood products in the UK. Food Policy 2004, 29, 215–228. [Google Scholar] [CrossRef]
- Cawthorn, D.M.; Steinman, H.A.; Witthuhn, R.C. Evaluating the availability of fish species on the South African market and the factors undermining sustainability and consumer choice. Food Control 2011, 22, 1748–1759. [Google Scholar] [CrossRef]
- McClenachan, L.; Dissanayake, S.T.; Chen, X. Fair trade fish: Consumer support for broader seafood sustainability. Fish Fish. 2016, 17, 825–838. [Google Scholar] [CrossRef]
- Malcorps, W.; Newton, R.W.; Maiolo, S.; Eltholth, M.; Zhu, C.; Zhang, W.; Li, S.; Tlusty, M.; Little, D.C. Global Seafood Trade: Insights in Sustainability Messaging and Claims of the Major Producing and Consuming Regions. Sustainability 2021, 13, 1720. [Google Scholar] [CrossRef]
- Bogard, J.R.; Marks, G.C.; Mamun, A.; Thilsted, S.H. Non-farmed fish contribute to greater micronutrient intakes than farmed fish: Results from an intra-household survey in rural Bangladesh. Public Health Nutr. 2017, 20, 702–711. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.; Wang, H.H.; Shogren, J.F. Fishing or Aquaculture? Chinese Consumers’ Stated Preference for the Growing Environment of Salmon through a Choice Experiment and the Consequentiality Effect. Mar. Resour. Econ. 2021, 36, 23–42. [Google Scholar] [CrossRef]
- Tien, N.H.; Hung, N.T.; Vu, N.T.; Bien, B.X. Risks of Vietnamese Enterprises in Trade Relations with China. Int. J. Res. Financ. Manag. 2019, 3, 1–6. [Google Scholar]
- Verbeke, W.; Vackier, I. Individual determinants of fish consumption: Application of the theory of planned behaviour. Appetite 2005, 44, 67–82. [Google Scholar] [CrossRef]
- Mohan Dey, M.; Rab, M.A.; Paraguas, F.J.; Piumsombun, S.; Bhatta, R.; Ferdous Alam, M.; Ahmed, M. Fish consumption and food security: A disaggregated analysis by types of fish and classes of consumers in selected Asian countries. Aquac. Econ. Manag. 2005, 9, 89–111. [Google Scholar] [CrossRef]
- Sonowal, C.J. Sustainability of Fishing as a Caste-Based Traditional Occupation: An Analysis of Studies on the Kaibartas of Assam. India J. Hum. Ecol. 2020, 72, 60–76. [Google Scholar] [CrossRef]
- Fabinyi, M.; Foale, S.; Macintyre, M. Managing inequality or managing stocks? An ethnographic perspective on the governance of small-scale fisheries. Fish Fish. 2015, 16, 471–485. [Google Scholar] [CrossRef]
- Andersen, A.B.; Schmidt, L.K.; Faurholt-Jepsen, D.; Roos, N.; Friis, H.; Kongsbak, K.; Wahed, M.A.; Thilsted, S.H. The effect of daily consumption of the small fish Amblypharyngodon mola or added vitamin A on iron status: A randomised controlled trial among Bangladeshi children with marginal vitamin A status. Asia Pac. J. Clin. Nutr. 2016, 25, 464–471. [Google Scholar]
- Nurhasan, M.; Roos, N.; Skau, J.K.; Wieringa, F.T.; Friis, H.; Michaelsen, K.F.; Dijkhuizen, M.A.; Stark, K.D.; Ritz, C.; Chhoun, C. Effect of complementary food with small amounts of freshwater fish on whole blood n-3 fatty acids in Cambodian infants age 6–15 months. Prostaglandins Leukot. Essent. Fat. Acids 2016, 135, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Skau, J.K.; Touch, B.; Chhoun, C.; Chea, M.; Unni, U.S.; Makurat, J.; Filteau, S.; Wieringa, F.T.; Dijkhuizen, M.A.; Ritz, C. Effects of animal source food and micronutrient fortification in complementary food products on body composition, iron status, and linear growth: A randomized trial in Cambodia. Am. J. Clin. Nutr. 2015, 101, 742–751. [Google Scholar] [CrossRef]
- Marinda, P.A.; Genschick, S.; Khayeka-Wandabwa, C.; Kiwanuka-Lubinda, R.; Thilsted, S.H. Dietary diversity determinants and contribution of fish to maternal and under-five nutritional status in Zambia. PLoS ONE 2018, 13, e0204009. [Google Scholar] [CrossRef]
- O’Meara, L.; Cohen, P.J.; Simmance, F.; Marinda, P.; Nagoli, J.; Teoh, S.J.; Funge-Smith, S.; Mills, D.J.; Thilsted, S.H.; Byrd, K.A. Inland fisheries critical for the diet quality of young children in sub-Saharan Africa. Glob. Food Secur. 2021, 28, 100483. [Google Scholar] [CrossRef]
- Kwasek, K.; Thorne-Lyman, A.L.; Phillips, M. Can human nutrition be improved through better fish feeding practices? A review paper. Crit. Rev. Food Sci. Nutr. 2020, 60, 3822–3835. [Google Scholar] [CrossRef]
- Roos, N.; Chamnan, C.; Loeung, D.; Jakobsen, J.; Thilsted, S.H. Freshwater fish as a dietary source of vitamin A in Cambodia. Food Chem. 2007, 103, 1104–1111. [Google Scholar] [CrossRef]
- Roos, N.; Leth, T.; Jakobsen, J.; Thilsted, S.H. High vitamin A content in some small indigenous fish species in Bangladesh: Perspectives for food-based strategies to reduce vitamin A deficiency. Int. J. Food Sci. Nutr. 2002, 53, 425–437. [Google Scholar] [CrossRef]
- Roos, N.; Mazharul Islam, M.; Thilsted, S.H. Small fish is an important dietary source of vitamin A and calcium in rural Bangladesh. Int. J. Food Sci. Nutr. 2003, 54, 329–339. [Google Scholar] [CrossRef]
- Roos, N.; Thorseng, H.; Chamnan, C.; Larsen, T.; Gondolf, U.H.; Bukhave, K.; Thilsted, S.H. Iron content in common Cambodian fish species: Perspectives for dietary iron intake in poor, rural households. Food Chem. 2007, 104, 1226–1235. [Google Scholar] [CrossRef]
- Sigh, S.; Roos, N.; Sok, D.; Borg, B.; Chamnan, C.; Laillou, A.; Dijkhuizen, M.A.; Wieringa, F.T. Development and acceptability of locally made fish-based, ready-to-use products for the prevention and treatment of malnutrition in Cambodia. Food Nutr. Bull. 2018, 39, 420–434. [Google Scholar] [CrossRef] [Green Version]
- Risius, A.; Hamm, U.; Janssen, M. Target groups for fish from aquaculture: Consumer segmentation based on sustainability attributes and country of origin. Aquaculture 2019, 499, 341–347. [Google Scholar] [CrossRef]
- Maesano, G.; Di Vita, G.; Chinnici, G.; Pappalardo, G.; D’Amico, M. The role of credence attributes in consumer choices of sustainable fish products: A review. Sustainability 2020, 12, 8. [Google Scholar] [CrossRef]
- Paciarotti, C.; Torregiani, F. The logistics of the short food supply chain: A literature review. Sustain. Prod. Consum. 2021, 26, 428–442. [Google Scholar] [CrossRef]
- Stein, A.J.; Santini, F. The sustainability of “local” food: A review for policy-makers. Rev. Agric. Food Environ. Stud. 2021, 96, 1–13. [Google Scholar] [CrossRef]
- Ritchie, H. You want to reduce the carbon footprint of your food? Focus on what you eat, not whether your food is local. Our World Data 2020, 24, 4–65. [Google Scholar]
- Farmery, A.K.; Gardner, C.; Green, B.S.; Jennings, S.; Watson, R.A. Domestic or imported? An assessment of carbon footprints and sustainability of seafood consumed in Australia. Environ. Sci. Policy 2015, 54, 35–43. [Google Scholar] [CrossRef]
- Henriksson, P.J.; Rico, A.; Zhang, W.; Ahmad-Al-Nahid, S.; Newton, R.; Phan, L.T.; Zhang, Z.; Jaithiang, J.; Dao, H.M.; Phu, T.M. Comparison of Asian aquaculture products by use of statistically supported life cycle assessment. Environ. Sci. Technol. 2015, 49, 14176–14183. [Google Scholar] [CrossRef]
- Bush, S.R.; Belton, B.; Little, D.C.; Islam, M.S. Emerging trends in aquaculture value chain research. Aquaculture 2019, 498, 428–434. [Google Scholar] [CrossRef] [Green Version]
- Bustos-Gallardo, B. The post 2008 Chilean Salmon industry: An example of an enclave economy. Geogr. J. 2017, 183, 152–163. [Google Scholar] [CrossRef]
- Belton, B.; Little, D. The development of aquaculture in central Thailand: Domestic demand versus export-led production. J. Agrar. Chang. 2008, 8, 123–143. [Google Scholar] [CrossRef]
- Phuong, N.T.; Oanh, D.T.H. Striped catfish aquaculture in Vietnam: A decade of unprecedented development. In Success Stories in Asian Aquaculture; Springer: Dordrecht, Germany, 2010; pp. 131–147. [Google Scholar]
- Joffre, O.; Prein, M.; Tung, P.B.V.; Saha, S.B.; Hao, N.V.; Alam, M.J. Evolution of shrimp aquaculture systems in the coastal zones of Bangladesh and Vietnam: A comparison. In Tropical Deltas and Coastal Zones: Food Production, Communities and Environment at the Land–Water Interface; Hoanh, C.T., Szuster, B.W., Ismail, S.-P.K.A.M., Noble, A.E., Eds.; CABI: Oxforshire, UK, 2010; pp. 48–63. [Google Scholar]
- Shamshak, G.L.; Anderson, J.L.; Asche, F.; Garlock, T.; Love, D.C. US seafood consumption. J. World Aquac. Soc. 2019, 50, 715–727. [Google Scholar] [CrossRef]
- Kaminski, A.M.; Genschick, S.; Kefi, A.S.; Kruijssen, F. Commercialization and upgrading in the aquaculture value chain in Zambia. Aquaculture 2018, 493, 355–364. [Google Scholar] [CrossRef]
- Claret, A.; Guerrero, L.; Ginés, R.; Grau, A.; Hernández, M.D.; Aguirre, E.; Peleteiro, J.B.; Fernández-Pato, C.; Rodríguez-Rodríguez, C. Consumer beliefs regarding farmed versus wild fish. Appetite 2014, 79, 25–31. [Google Scholar] [CrossRef]
- Budhathoki, M.; Zølner, A.; Nielsen, T.; Reinbach, H.C. The role of production method information on sensory perception of smoked salmon—A mixed-method study from Denmark. Food Qual. Prefer. 2021, 94, 104325. [Google Scholar] [CrossRef]
- Verbeke, W.; Sioen, I.; Brunsø, K.; De Henauw, S.; Van Camp, J. Consumer perception versus scientific evidence of farmed and wild fish: Exploratory insights from Belgium. Aquac. Int. 2007, 15, 121–136. [Google Scholar] [CrossRef]
- Froehlich, H.E.; Gentry, R.R.; Rust, M.B.; Grimm, D.; Halpern, B.S. Public perceptions of aquaculture: Evaluating spatiotemporal patterns of sentiment around the world. PLoS ONE 2017, 12, e0169281. [Google Scholar] [CrossRef] [Green Version]
- Osmundsen, T.C.; Olsen, M.S. The imperishable controversy over aquaculture. Mar. Policy 2017, 76, 136–142. [Google Scholar] [CrossRef]
- Wang, D.; Hsieh, Y.H.P. The use of imported pangasius fish in local restaurants. Food Control 2016, 65, 136–142. [Google Scholar] [CrossRef]
- Williams, M.; Hernandez-Jover, M.; Shamsi, S. Fish substitutions which may increase human health risks from zoonotic seafood borne parasites: A review. Food Control 2020, 118, 107429. [Google Scholar] [CrossRef]
- López-Mas, L.; Claret, A.; Reinders, M.J.; Banovic, M.; Krystallis, A.; Guerrero, L. Farmed or wild fish? Segmenting European consumers based on their beliefs. Aquaculture 2021, 532, 735992. [Google Scholar] [CrossRef]
- Claret, A.; Guerrero, L.; Gartzia, I.; Garcia-Quiroga, M.; Ginés, R. Does information affect consumer liking of farmed and wild fish? Aquaculture 2016, 454, 157–162. [Google Scholar] [CrossRef]
- Farmery, A.K.; Alexander, K.; Anderson, K.; Blanchard, J.L.; Carter, C.G.; Evans, K.; Fischer, M.; Fleming, A.; Frusher, S.; Fulton, E.A. Food for all: Designing sustainable and secure future seafood systems. Rev. Fish Biol. Fish. 2021, 32, 1–21. [Google Scholar] [CrossRef]
- Bevitt, K. Illuminating Hidden Harvests: The Conversation around a Global and Collaborative Small-Scale Fisheries Study Highlights the Under-Recognition and Under-Reporting of Women’s Work; ICSF: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Fluet-Chouinard, E.; Funge-Smith, S.; McIntyre, P.B. Global hidden harvest of freshwater fish revealed by household surveys. Proc. Natl. Acad. Sci. USA 2018, 115, 7623–7628. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Belton, B.; Edwards, P.; Henriksson, P.; Little, D.C.; Newton, R.; Troell, M. Freshwater Aquaculture: The Silent Majority. Submitted. Available online: https://www.susaquastirling.net/blog/2022/3/8/the-silent-majority (accessed on 5 May 2022).
- Naylor, R.L.; Goldburg, R.J.; Primavera, J.H.; Kautsky, N.; Beveridge, M.C.; Clay, J.; Folke, C.; Lubchenco, J.; Mooney, H.; Troell, M. Effect of aquaculture on world fish supplies. Nature 2000, 405, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Kok, B.; Malcorps, W.; Tlusty, M.F.; Eltholth, M.M.; Auchterlonie, N.A.; Little, D.C.; Harmsen, R.; Newton, R.W.; Davies, S.J. Fish as feed: Using economic allocation to quantify the Fish In: Fish Out ratio of major fed aquaculture species. Aquaculture 2020, 528, 735474. [Google Scholar] [CrossRef]
- Amilhat, E.; Lorenzen, E.J.; Morales, A.; Yakupitiyage, D.C. Fisheries production in Southeast Asian farmer managed aquatic systems (FMAS) I. Characterization of systems. Aquaculture 2021, 296, 219–226. [Google Scholar] [CrossRef]
- Phomsouvanh, A.; Saphakdy, B.; De Silva, S.S. Production trends, monetary returns and benefit sharing protocols from the extensive aquaculture practice of culture-based fisheries in rural communities in Lao PDR. Aquaculture 2015, 439, 29–38. [Google Scholar] [CrossRef]
- De Silva, S.S. Culture based fisheries in Asia are a strategy to augment food security. Food Secur. 2016, 8, 585–596. [Google Scholar] [CrossRef]
- Sarkar, U.K.; Lianthuamluaia, L.; Panda, D.; Kumari, S.; Parida, P.K.; Karnatak, G.; Mishal, P. Evaluation and impact assessment of culture-based fisheries to enhance fish yield in small reservoirs in Odisha State, India. Fish. Manag. Ecol. 2020, 27, 481–489. [Google Scholar] [CrossRef]
- Lovatelli, A.; Holthus, P.F. Capture-Based Aquaculture; FAO: Rome, Italy, 2008. [Google Scholar]
- Halpern, B.S.; Cottrell, R.S.; Blanchard, J.L.; Bouwman, L.; Froehlich, H.E.; Gephart, J.A.; Jacobsen, N.S.; Kuempel, C.D.; McIntyre, P.B.; Metian, M. Opinion: Putting all foods on the same table: Achieving sustainable food systems requires full accounting. Proc. Natl. Acad. Sci. USA 2019, 116, 18152–18156. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pounds, A.; Kaminski, A.M.; Budhathoki, M.; Gudbrandsen, O.; Kok, B.; Horn, S.; Malcorps, W.; Mamun, A.-A.; McGoohan, A.; Newton, R.; et al. More Than Fish—Framing Aquatic Animals within Sustainable Food Systems. Foods 2022, 11, 1413. https://doi.org/10.3390/foods11101413
Pounds A, Kaminski AM, Budhathoki M, Gudbrandsen O, Kok B, Horn S, Malcorps W, Mamun A-A, McGoohan A, Newton R, et al. More Than Fish—Framing Aquatic Animals within Sustainable Food Systems. Foods. 2022; 11(10):1413. https://doi.org/10.3390/foods11101413
Chicago/Turabian StylePounds, Alexandra, Alexander M. Kaminski, Mausam Budhathoki, Oddrun Gudbrandsen, Björn Kok, Stephanie Horn, Wesley Malcorps, Abdullah-Al Mamun, Amy McGoohan, Richard Newton, and et al. 2022. "More Than Fish—Framing Aquatic Animals within Sustainable Food Systems" Foods 11, no. 10: 1413. https://doi.org/10.3390/foods11101413
APA StylePounds, A., Kaminski, A. M., Budhathoki, M., Gudbrandsen, O., Kok, B., Horn, S., Malcorps, W., Mamun, A.-A., McGoohan, A., Newton, R., Ozretich, R., & Little, D. C. (2022). More Than Fish—Framing Aquatic Animals within Sustainable Food Systems. Foods, 11(10), 1413. https://doi.org/10.3390/foods11101413






