Consumption of Phenolic-Rich Food and Dietary Supplements as a Key Tool in SARS-CoV-19 Infection
Abstract
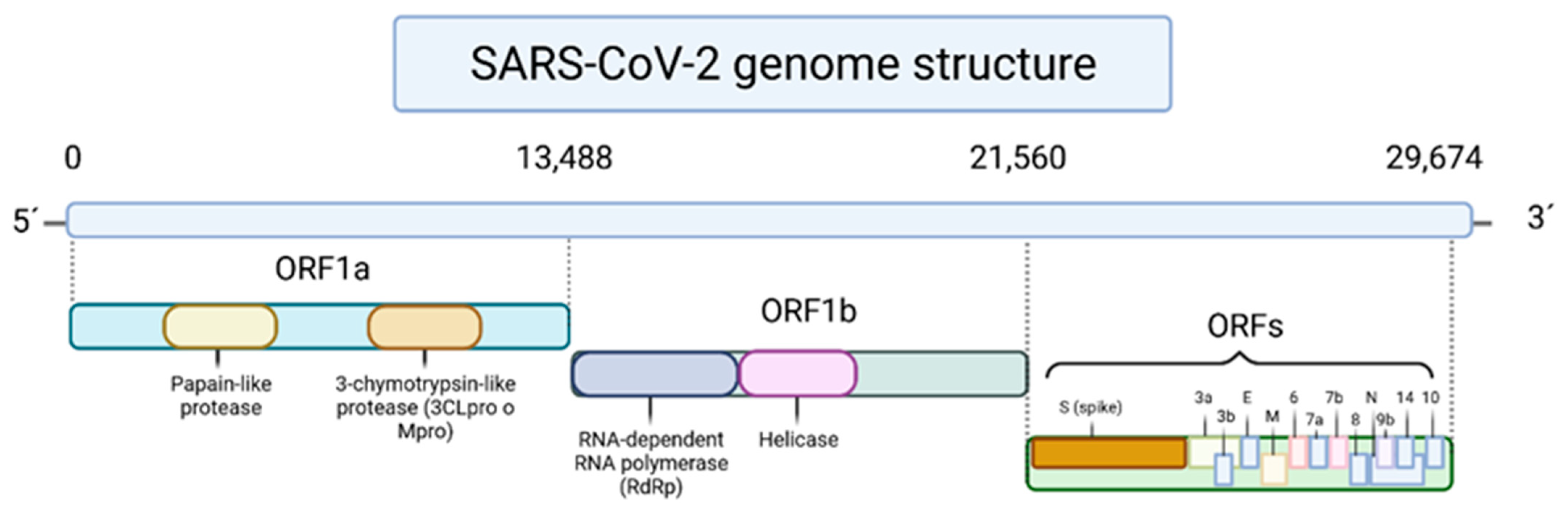
:1. Introduction
2. Phenolic Compounds in Human Health
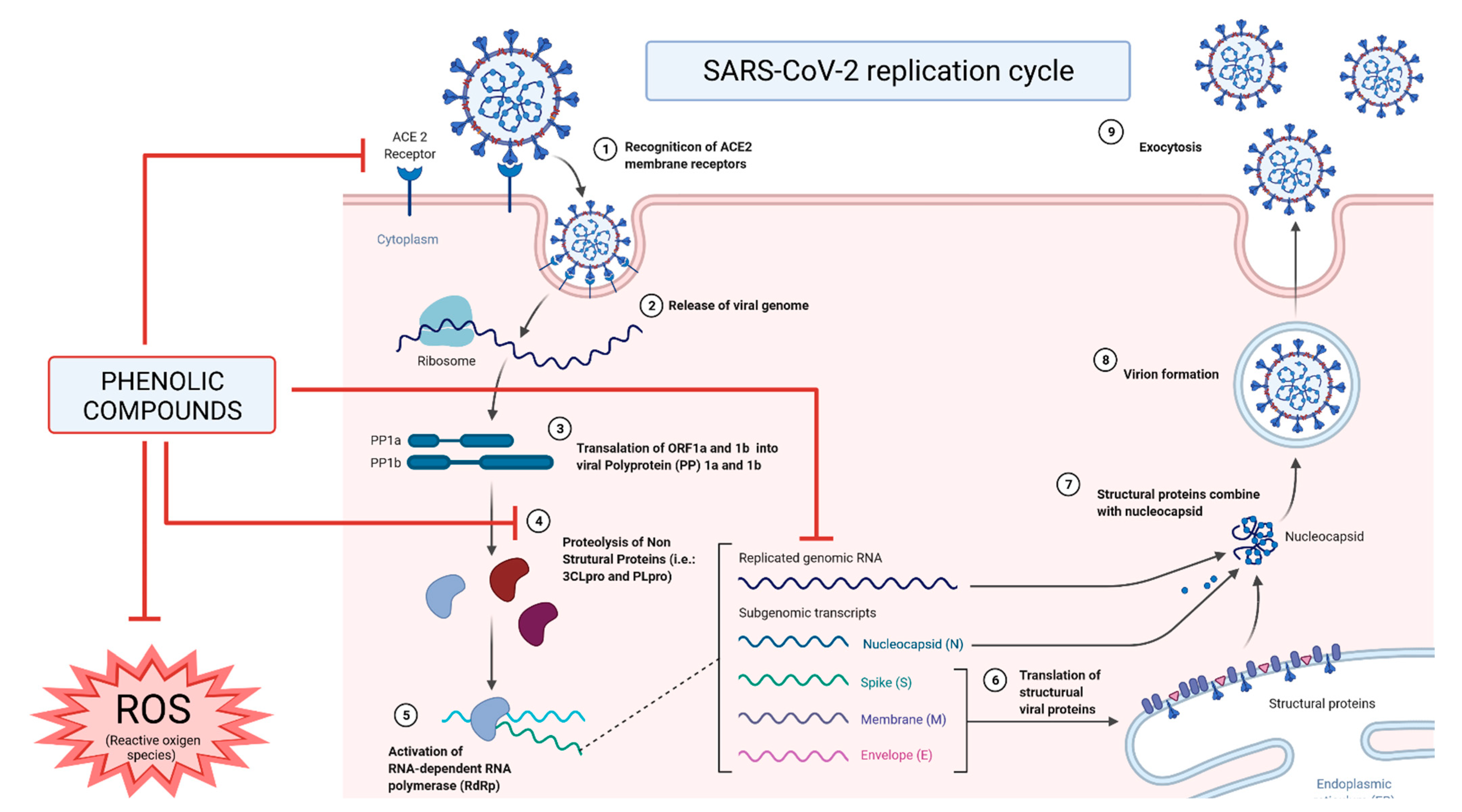
Phenolic Compounds in COVID-19
| Phenolic Compounds | Main Outcome | Reference |
|---|---|---|
| Cyanidin | Mpro inhibitor | [64,65,66,67] |
| Daidzein | ||
| Dieckol | ||
| Genistein | ||
| Mearnsitrin | ||
| Myricitrin | ||
| Psoralidin | ||
| Quercetin 3-O-β-D-glucoside | ||
| Rutin | ||
| Xanthoangelol E | ||
| Benzoic acid | RdRp inhibitor | [68,69] |
| Cyanidin | ||
| Daidzein | ||
| Ellagic acid | ||
| Gallic acid | ||
| Genistein | ||
| Kaempferol 3-O-rutinoside | ||
| Naringenin | ||
| Oleuropein | ||
| Quercetin | ||
| Quercetin 3-O-rutinoside | ||
| Resveratrol | ||
| Myrcetin | Non-structural SARS-CoV-2 helicases inhibitor | [70] |
| Scutellarein | ||
| Cyanidin 3-O-glucoside | PLpro inhibitor | [57,71,72] |
| Epigallocatechin | ||
| Epigallocatechin gallate | ||
| Hypericin | ||
| Kaempferol | ||
| Quercetin | ||
| Cryptotanshinone | TMPRSS2 inhibitor | [10,70,73] |
| Ellagic acid | ||
| Gallic acid | ||
| Kaempferol | ||
| Luteolin | ||
| Quercetin | ||
| Afzelin | ACE2 inhibitor | [44,70,73,74,75] |
| Apigenin | ||
| Baicalin | ||
| Biorobin | ||
| Caffeic acid | ||
| Catechin | ||
| Chlorogenic acid | ||
| Chrysin | ||
| Ellagic acid | ||
| Curcumin | ||
| Cyanidin | ||
| Delphinidin | ||
| Epigallocatechin | ||
| Epigallocatechin gallate | ||
| Ferulic acid | ||
| Galangin | ||
| Gallic acid | ||
| Hesperetin | ||
| Isoferulic acid | ||
| Kaempferol | ||
| Luteolin | ||
| Myricitrin | ||
| Naringenin | ||
| Nobiletin | ||
| Nympholide A | ||
| Pinocembrin | ||
| Quercetin | ||
| Rhoifolin | ||
| Rutin | ||
| Scutellarein | ||
| Taiwanhomoflavone A | ||
| Tangeretin | ||
| ε-Viniferin | ||
| Chrysin | Interact with Spike protein | [44,70,73,74,75] |
| Ellagic acid | ||
| Gallic acid | ||
| Hesperetin | ||
| Pinocembrin | ||
| Artepillin C | Inhibit p21-activated kinase 1 | [76] |
| Ellagic acid | Inhibit furin | [77] |
| Gallic acid |
3. Uptake of Phenolic Compounds in Dietary Supplements in COVID-19
| Origin | Compound | Type of Study | Drug Target | References |
|---|---|---|---|---|
| Theobroma cacao (Cocoa) | Flavonoids, hydroxybenzoic acids, hydroxycinnamic acids and N-phenylpropenoyl-L-amido acids | Molecular docking | Mpro | [86] |
| Camellia sinensis (green tea) | Epigallocatechin-3-gallate, theaflavin | in vitro | Mpro | [81,82] |
| Camellia sinensis (green tea) | Epigallocatechin-3-gallate, theaflavin | Molecular docking | 3CLpro, Spike protein, PLpro, RdRp and ACE2 | [84] |
| Camellia sinensis (green tea) | 16 phenolic compounds | Molecular docking | Nsp 6 | [85] |
| Different sources | 100 phenolic compounds (epigallocatechin, hesperidin, myricetin, quercetagetin and theaflavins | Molecular docking | RdRp | [60] |
| Berries | 18 anthocyanins | Molecular docking | Spike protein and Mpro | [97] |
| Allium sativum (black garlic) | Total extract, 49 polyphenols and 1 combination treatment of tannic acid with myricetin, puerarin, and/or daidzein | in vitro | Mpro | [88] |
| Euphorbia cuneata | Naringenin | Molecular docking and in vitro | Mpro | [89] |
| Red wine, Chinese hawthorn and blackberry | Quercetin, luteolin, and isorhamnetin | Molecular docking and metadata analysis | Several mechanisms | [68] |
| Citrus | Naringenin Neohesperidin Nobiletin | Molecular docking | Spike protein and ACE-inhibitor | [101] |
| Citrus aurantium Citri Reticulate Pericarpium | Hesperitin | Molecular docking | Spike protein and ACE-inhibitor | [75,102] |
| Coffee | 28 caffeic acid derivatives | Molecular docking | Mpro, Spike protein, Nsp15 and fusion protein subunit S2 | [94] |
| Different sources | Cyanidin 3-O-glucoside and resveratrol | Molecular docking and in vitro | Mpro | [57] |
| Different sources | Gallic acid, quercetin, caffeine, ribavirin, resveratrol, naringenin, benzoic acid, oleuropein, ellagic acid | Molecular docking | RdRp | [92] |
| Erigenon breviscapus (Vant.) | Scutellarin | Molecular docking | Spike protein and ACE-inhibitor | [75] |
| Propolis from Apis mellifera | Propolis (caffeic acid, p-coumaric acid, ferulic acid, t-cinnamic acid, hesperetin, chrysin, pinocembrin, CAPE) | Molecular docking and in vitro | ACE2 and Spike protein | [44] |
| Propolis from Apis mellifera | Propolis (Caffeic acid, p-coumaric acid, t-cinnamic acid, hesperetin, chrysin, pinocembrin, CAPE, rutin, myricetin, luteolin, resveratrol | Molecular docking | ACE2 | [103] |
| Propolis form Apis mellifera | Propolis extract | Clinical trial | n.a. | [104] |
| Rheum officinale (rhubarb) Reymoutria multiflora tuber | Emodin | in vitro and in vivo | Spike protein and ACE-inhibitor | [105] |
| Curcuma longa | Nano-curcumin (micromicelar curcumin) | Clinical trials | Inmune response (Interleucine modulation) | [99] |
| Moringa oliefera | Apigenin, chlorogenic acid, chrysin, ellagic acid, myricetin, and quercetin | Molecular docking | Nsp9 and Nsp10 | [106] |
| Ginkgo biloba | Amentoflavone, ginkgetin, bilobetin, isoginkgetin, sciadopitysin, kaempferol, quercetin, apigenin, isorhamnetin, genkwanin, luteolin, quercetin) | Molecular docking | Mpro | [107] |
| Glycine max (Soya bean) | Nicotinamine | in vitro and in vivo | Spike protein and ACE-inhibitor | [108] |
| Glycyrrhiza radix (Licorice root) | Glycyrrhizin | in vitro | Spike protein and ACE-inhibitor | [75] |
| Scutellaria baicalensis | Baicalin | in vitro | Spike protein and ACE-inhibitor | [75] |
| Traditional Indian medicine plants (Azadirachta indica, Syzygium aromaticum, Ocimum sanctum, Zingiber officinale, Curcuma longa, Camellia sinensis, Nigella sativa, Luffa cylindrica, Allium sativum and Allium sativum) | Nimbaflavone, epigallocatechin, catechins and curcumin | Molecular docking | Mpro | [109] |
| Several plants from traditional Indian medicine | 23 bioactive compounds, including quercetin, apigenin, curcumin, carvacrol and gingerol | Molecular docking | ACE2, Mpro, Spike protein, RdRp | [66] |
| Traditional Chinese medicine formulations | Kaempferol, luteolin, isorhamnetin, epigallocatechin-3-gallate, naringenin and wogonin | Molecular docking, in vitro and clinical trials | Mpro | [110] |
| Traditional Chinese medicine formulations | Isorhamnetin | Molecular docking, in vitro and clinical trials | ACE2 and Mpro | [111] |
4. Uptake of Phenolic Compounds in Dietary Supplements in COVID-19
5. Population Experiences of Phenolic Compounds, Diet and COVID-19
6. Conclusions and Future Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jiang, S.; Du, L.; Shi, Z. An emerging coronavirus causing pneumonia outbreak in Wuhan, China: Calling for developing therapeutic and prophylactic strategies. Emerg. Microbes Infect. 2020, 9, 275–277. [Google Scholar] [CrossRef]
- Lytras, S.; Hughes, J.; Xia, W.; Jiang, X.; Robertson, D.L. Exploring the natural origins of SARS-CoV-2. bioRxiv. 2021. Available online: https://www.biorxiv.org/content/10.1101/2021.01.22.427830v3.abstract (accessed on 27 June 2021).
- Sallard, E.; Halloy, J.; Casane, D.; Decroly, E.; van Helden, J. Tracing the origins of SARS-COV-2 in coronavirus phylogenies: A review. Environ. Chem. Lett. 2021, 19, 769–785. [Google Scholar] [CrossRef]
- Wacharapluesadee, S.; Tan, C.W.; Maneeorn, P.; Duengkae, P.; Zhu, F.; Joyjinda, Y.; Kaewpom, T.; Chia, W.N.; Ampoot, W.; Lim, B.L.; et al. Evidence for SARS-CoV-2 related coronaviruses circulating in bats and pangolins in Southeast Asia. Nat. Commun. 2021, 12, 1–9. [Google Scholar]
- Makarenkov, V.; Mazoure, B.; Rabusseau, G.; Legendre, P. Horizontal gene transfer and recombination analysis of SARS-CoV-2 genes helps discover its close relatives and shed light on its origin. BMC Ecol. Evol. 2021, 21, 1–18. [Google Scholar]
- Boopathi, S.; Poma, A.B.; Kolandaivel, P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J. Biomol. Struct. Dyn. 2020, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 1–10. [Google Scholar] [CrossRef]
- Sriram, K.; Insel, P. A hypothesis for pathobiology and treatment of COVID-19: The centrality of ACE1/ACE2 imbalance. Authorea 2020, 177, 4825–4844. [Google Scholar] [CrossRef]
- Singh, A.; Gautam, A.; Chandel, S.; Ghosh, A.; Dey, D.; Roy, S.; Ravichandiran, V.; Gosh, D. Protease inhibitory effect of natural polyphenolic compounds on SARS-CoV-2: An in silico study. Molecules 2020, 25, 4604. [Google Scholar] [CrossRef]
- Spelta, A.; Flori, A.; Pierri, F.; Bonaccorsi, G.; Pammolli, F. After the lockdown: Simulating mobility, public health and economic recovery scenarios. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Atalan, A. Is the lockdown important to prevent the COVID-19 pandemic? Effects on psychology, environment and economy-perspective. Ann. Med. Surg. 2020, 56, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1–42. [Google Scholar] [CrossRef] [Green Version]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Bartolomé, B.; Gómez-Cordovés, C. Updated Knowledge About the Presence of Phenolic Compounds in Wine. Crit. Rev. Food Sci. Nutr. 2005, 45, 85–118. [Google Scholar] [CrossRef]
- Jesus, F.; Gonçalves, A.C.A.; Alves, G.; Silva, L.R. Health Benefits of Prunus avium Plant Parts: An Unexplored Source Rich in Phenolic Compounds. Food Rev. Int. 2020, 1–29. [Google Scholar] [CrossRef]
- Calumpang, C.L.; Saigo, T.; Watanabe, M.; Tohge, T. Cross-Species Comparison of Fruit-Metabolomics to Elucidate Metabolic Regulation of Fruit Polyphenolics Among Solanaceous Crops. Metabolites 2020, 10, 209. [Google Scholar] [CrossRef]
- Quiles, J.L.; Rivas-García, L.; Varela-López, A.; Llopis, J.; Battino, M.; Sánchez-González, C. Do nutrients and other bioactive molecules from foods have anything to say in the treatment against COVID-19? Environ. Res. 2020, 191, 110053. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Kay, C.D.; Pereira-Caro, G.; Ludwig, I.A.; Clifford, M.N.; Crozier, A. Anthocyanins and flavanones are more bioavailable than previously perceived: A review of recent rvidence. Annu. Rev. Food Sci. Technol. 2017, 8, 155–180. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, I.A.; Mena, P.; Calani, L.; Borges, G.; Pereira-Caro, G.; Bresciani, L.; Del Rio, D.; Lean, M.E.; Crozier, A. New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free. Radic. Biol. Med. 2015, 89, 758–769. [Google Scholar] [CrossRef] [Green Version]
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Richling, E. Human intervention study to investigate the intestinal accessibility and bioavailability of anthocyanins from bilberries. Food Chem. 2017, 231, 275–286. [Google Scholar] [CrossRef]
- Halliwell, B. Dietary polyphenols: Good, bad, or indifferent for your health? Cardiovasc. Res. 2007, 73, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Thrimawithana, T.; Shukla, R.; Adhikari, B. Managing obesity through natural polyphenols: A review. Futur. Foods 2020, 1–2, 100002. [Google Scholar] [CrossRef]
- Anhê, F.F.; Desjardins, Y.; Pilon, G.; Dudonné, S.; Genovese, M.I.; Lajolo, F.M.; Marette, A. Polyphenols and type 2 diabetes: A prospective review. Pharma Nutr. 2013, 1, 105–114. [Google Scholar] [CrossRef]
- Chait, Y.A.; Gunenc, A.; Bendali, F.; Hosseinian, F. Simulated gastrointestinal digestion and in vitro colonic fermentation of carob polyphenols: Bioaccessibility and bioactivity. LWT 2020, 117, 108623. [Google Scholar] [CrossRef]
- Del Mondo, A.; Smerilli, A.; Ambrosino, L.; Albini, A.; Noonan, D.M.; Sansone, C.; Brunet, C. Insights into phenolic compounds from microalgae: Structural variety and complex beneficial activities from health to nutraceutics. Crit. Rev. Biotechnol. 2021, 41, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Pap, N.; Fidelis, M.; Azevedo, L.; Do, C.M.A.V.; Wang, D.; Mocan, A.; Pereira, E.P.R.; Xavier-Santos, D.; Sant’Ana, A.S.; Yang, B.; et al. Berry polyphenols and human health: Evidence of antioxidant, anti-inflammatory, microbiota modulation, and cell-protecting effects. Curr. Opin. Food Sci. 2021, 42, 167–186. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Visioli, F. Polyphenol-based nutraceuticals for the prevention and treatment of cardiovascular disease: Review of human evidence. Phytomedicine 2016, 23, 1145–1174. [Google Scholar] [CrossRef]
- Lorenz, M.; Lehmann, S.; Djordjevic, I.; Düsterhöft, T.; Zimmermann, B.F.; Stangl, K.; Stangl, V. Vasodilation of Tea Polyphenols Ex Vivo Is Mediated by Hydrogen Peroxide under Rapid Compound Decay. Antioxidants 2020, 9, 390. [Google Scholar] [CrossRef]
- Markovics, A.; Biró, A.; Kun-Nemes, A.; Fazekas, M.É.; Rácz, A.A.; Paholcsek, M.; Lukács, J.; Stündl, L.; Remenyik, J. Effect of Anthocyanin-Rich Extract of Sour Cherry for Hyperglycemia-Induced Inflammatory Response and Impaired Endothelium-Dependent Vasodilation. Nutrients 2020, 12, 3373. [Google Scholar] [CrossRef]
- Sanches-Silva, A.; Testai, L.; Nabavi, S.F.; Battino, M.; Devi, K.P.; Tejada, S.; Sureda, A.; Xu, S.; Yousefi, B.; Majidinia, M.; et al. Therapeutic potential of polyphenols in cardiovascular diseases: Regulation of mTOR signaling pathway. Pharmacol. Res. 2020, 152, 104626. [Google Scholar] [CrossRef]
- Ávila-Román, J.; Soliz-Rueda, J.R.; Bravo, F.I.; Aragonès, G.; Suárez, M.; Arola-Arnal, A.; Mulero, M.; Salvadó, M.J.; Arola, L.; Torres-Fuentes, C.; et al. Phenolic compounds and biological rhythms: Who takes the lead? Trends Food Sci. Technol. 2021, 113, 77–85. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Gędas, A.; Simões, M. Antimicrobial polyphenol-rich extracts: Applications and limitations in the food industry. Food Res. Int. 2020, 134, 109214. [Google Scholar] [CrossRef] [PubMed]
- Loaiza-Cano, V.; Monsalve-Escudero, L.M.; Filho, C.D.S.M.B.; Martinez-Gutierrez, M.; de Sousa, D.P. Antiviral Role of Phenolic Compounds against Dengue Virus: A Review. Biomolecules 2020, 11, 11. [Google Scholar] [CrossRef]
- El-Aziz, N.; Shehata, M.; Awad, O.; El-Sohaimy, S. Inhibition of COVID-19 RNA-dependent RNA polymerase by natural bioactive compounds: Molecular docking analysis. Egypt. J. Chem. 2021, 64, 1989–2001. [Google Scholar]
- Ogawa, M.; Shimojima, M.; Saijo, M.; Fukasawa, M. Several catechins and flavonols from green tea inhibit severe fever with thrombocytopenia syndrome virus infection in vitro. J. Infect. Chemother. 2021, 27, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.-T.; Karimi, A.; Lorigooini, Z.; Pourgheysari, B.; Alidadi, S. In vitro anti influenza virus activity, antioxidant potential and total phenolic content of twelve Iranian medicinal plants. Marmara Pharm. J. 2017, 21, 843–851. [Google Scholar] [CrossRef] [Green Version]
- Moradi, M.-T.; Karimi, A.; Shahrani, M.; Hashemi, L.; Ghaffari-Goosheh, M.-S. Anti-Influenza Virus Activity and Phenolic Content of Pomegranate (Punica granatum L.) Peel Extract and Fractions. Avicenna J. Med. Biotech. 2020, 11, 285–291. [Google Scholar]
- Xie, Y.; Huang, B.; Yu, K.; Shi, F.; Liu, T.; Xu, W. Caffeic acid derivatives: A new type of influenza neuraminidase inhibitors. Bioorganic Med. Chem. Lett. 2013, 23, 3556–3560. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; López-Sabater, M.C.; Covas, M.I.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; et al. Predimed study investigators. Polyphenol intake and mortality risk: A re-analysis of the Predimed trial. BMC Med. 2014, 12, 77. [Google Scholar] [CrossRef] [Green Version]
- Guler, H.I.; Sal, F.A.; Can, Z.; Kara, Y.; Yildiz, O.; Belduz, O.; Çanakci, S.; Kolayli, S. Targeting CoV-2 Spike RBD and ACE-2 Interaction with flavonoids of Anatolian propolis by in silico and in vitro studies in terms of possible COVID-19 therapeutics. Turkish J. Biol. 2021, 45, 530–548. [Google Scholar]
- Hamed, A.N.; Samy, M.N.; Mahmoud, B.K.; Shihata, E.Z.A.; Ali, T.F.S.; Afifi, A.; Kamel, M.S. Flavonoidal glycosides and in vitro antioxidant activity of Bignonia binata Thunb. leaves Family Bignoniaceae and in silico evidence of their potential anti-COVID-19 activity. J. Adv. Biomed. Pharm. Sci. 2021, 4, 98–106. [Google Scholar]
- Gowrishankar, S.; Muthumanickam, S.; Kamaladevi, A.; Karthika, C.; Jothi, R.; Boomi, P.; Maniazhagu, D.; Pandian, S.K. Promising phytochemicals of traditional Indian herbal steam inhalation therapy to combat COVID-19—An in silico study. Food Chem. Toxicol. 2021, 148, 111966. [Google Scholar] [CrossRef]
- Singh, R.; Singh, P.K.; Kumar, R.; Kabir, T.; Kamal, M.A.; Rauf, A.; Albadrani, G.M.; Sayed, A.A.; Mousa, S.A.; Abdel-Daim, M.M.; et al. Multi-Omics Approach in the Identification of Potential Therapeutic Biomolecule for COVID-19. Front. Pharmacol. 2021, 12, 652335. [Google Scholar] [CrossRef]
- Limanaqi, F.; Busceti, C.L.; Biagioni, F.; Lazzeri, G.; Forte, M.; Schiavon, S.; Sciarretta, S.; Frati, G.; Fornai, F. Cell Clearing Systems as Targets of Polyphenols in Viral Infections: Potential Implications for COVID-19 Pathogenesis. Antioxidants 2020, 9, 1105. [Google Scholar] [CrossRef]
- Lin, C.-W.; Tsai, F.-J.; Tsai, C.-H.; Lai, C.-C.; Wan, L.; Ho, T.-Y.; Hsieh, C.-C.; Chao, P.-D.L. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antivir. Res. 2005, 68, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Junior, J.A.C.N.; Santos, A.M.; Quintans-Júnior, L.J.; Walker, C.I.B.; Borges, L.P.; Serafini, M.R. SARS, MERS and SARS-CoV-2 (COVID-19) treatment: A patent review. Expert Opin. Ther. Pat. 2020, 30, 567–579. [Google Scholar] [CrossRef]
- Zabetakis, I.; Lordan, R.; Norton, C.; Tsoupras, A. COVID-19: The Inflammation Link and the Role of Nutrition in Potential Mitigation. Nutrients 2020, 12, 1466. [Google Scholar] [CrossRef] [PubMed]
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020, 40, 1–7. [Google Scholar] [CrossRef]
- Giovinazzo, G.; Gerardi, C.; Uberti-Foppa, C.; Lopalco, L. Can Natural Polyphenols Help in Reducing Cytokine Storm in COVID-19 Patients? Molecules 2020, 25, 5888. [Google Scholar] [CrossRef]
- Margină, D.; Ungurianu, A.; Purdel, C.; Nițulescu, G.M.; Tsoukalas, D.; Sarandi, E.; Thanasoula, M.; Burykina, T.I.; Tekos, F.; Buha, A.; et al. Analysis of the intricate effects of polyunsaturated fatty acids and polyphenols on inflammatory pathways in health and disease. Food Chem. Toxicol. 2020, 143, 111558. [Google Scholar] [CrossRef]
- Tallei, T.E.; Tumilaar, S.G.; Niode, N.J.; Kepel, B.J.; Idroes, R.; Effendi, Y.; Sakib, S.A.; Emran, T. Bin potential of plant bioactive compounds as SARS-CoV-2 main protease (Mpro) and Spike (S) glycoprotein inhibitors: A molecular docking study. Scientifica 2020, 2020, 6307457. [Google Scholar] [CrossRef] [PubMed]
- Mishra, C.B.; Pandey, P.; Sharma, R.D.; Malik, M.Z.; Mongre, R.K.; Lynn, A.M.; Prasad, R.; Jeon, R.; Prakash, A. Identifying the natural polyphenol catechin as a multi-targeted agent against SARS-CoV-2 for the plausible therapy of COVID-19: An integrated computational approach. Brief. Bioinform. 2021, 22, 1346–1360. [Google Scholar] [CrossRef]
- Pitsillou, E.; Liang, J.; Karagiannis, C.; Ververis, K.; Darmawan, K.K.; Ng, K.; Hung, A.; Karagiannis, T.C. Interaction of small molecules with the SARS-CoV-2 main protease in silico and in vitro validation of potential lead compounds using an enzyme-linked immunosorbent assay. Comput. Biol. Chem. 2020, 89, 107408. [Google Scholar] [CrossRef]
- Elfiky, A.A. SARS-CoV-2 RNA dependent RNA polymerase (RdRp) targeting: An in silico perspective. J. Biomol. Struct. Dyn. 2020, 39, 3204–3212. [Google Scholar] [PubMed]
- Kokic, G.; Hillen, H.S.; Tegunov, D.; Dienemann, C.; Seitz, F.; Schmitzova, J.; Farnung, L.; Siewert, A.; Höbartner, C.; Cramer, P. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat. Commun. 2021, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sk, F.; Sonawane, A.; Kar, P.; Sadhukhan, S.; Sk, M.F.; Sonawane, A.; Kar, P.; Sadhukhan, S. Plant-derived natural polyphenols as potential antiviral drugs against SARS-CoV-2 via RNA—Dependent RNA polymerase (RdRp) inhibition: An in-silico analysis. J. Biomol. Struct. Dyn. 2020, 28, 1–16. [Google Scholar] [CrossRef]
- Karkhanei, B.; Ghane, E.T.; Mehri, F. Evaluation of oxidative stress level: Total antioxidant capacity, total oxidant status and glutathione activity in patients with COVID-19. New Microbes New Infect. 2021, 42, 100897. [Google Scholar] [CrossRef] [PubMed]
- Mrityunjaya, M.; Pavithra, V.; Neelam, R.; Janhavi, P.; Halami, P.M.; Ravindra, P.V. Immune-Boosting, Antioxidant and Anti-inflammatory Food Supplements Targeting Pathogenesis of COVID-19. Front. Immunol. 2020, 11, 570122. [Google Scholar] [CrossRef]
- Iddir, M.; Brito, A.; Dingeo, G.; Del Campo, S.S.F.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef]
- Abd El-Mordy, F.M.; El-Hamouly, M.M.; Ibrahim, M.T.; El-Rheem, G.A.; Aly, O.M.; Abd El-Kader, A.M.; Youssif, K.A.; Abdelmohsen, U.R. Inhibition of SARS-CoV-2 main protease by phenolic compounds from Manilkara hexandra (Roxb.) Dubard assisted by metabolite profiling and in silico virtual screening. RSC Adv. 2020, 10, 32148–32155. [Google Scholar] [CrossRef]
- Pendyala, B.; Patras, A. In silico screening of food bioactive compounds to predict potential inhibitors of COVID-19 main protease (Mpro) and RNA-dependent RNA polymerase (RdRp). ChemRxiv. 2020. Available online: https://chemrxiv.org/engage/chemrxiv/article-details/60c749a3567dfef273ec4c31 (accessed on 15 June 2021).
- Kumar Verma, A.; Kumar, V.; Singh, S.; Goswami, B.C.; Camps, I.; Sekar, A.; Yoon, S.; Lee, K.W. Repurposing potential of Ayurvedic medicinal plants derived active principles against SARS-CoV-2 associated target proteins revealed by molecular docking, molecular dynamics and MM-PBSA studies. Biomed. Pharmacother. 2021, 137, 111356. [Google Scholar] [CrossRef]
- Cherrak, S.A.; Merzouk, H.; Mokhtari-Soulimane, N. Potential bioactive glycosylated flavonoids as SARS-CoV-2 main protease inhibitors: A molecular docking and simulation studies. PLoS ONE 2020, 15, e0240653. [Google Scholar] [CrossRef]
- Xu, J.; Gao, L.; Liang, H.; Chen, S.-D. In silico screening of potential anti–COVID-19 bioactive natural constituents from food sources by molecular docking. Nutrition 2021, 82, 111049. [Google Scholar] [CrossRef]
- Silva, F.M.A.; Silva, K.P.A.; Oliveira, L.P.M.; Costa, E.V.; Koolen, H.H.F.; Pinheiro, M.L.B.; Souza, A.Q.L.; Souza, A.D.L. Flavonoid glycosides and their putative human metabolites as potential inhibitors of the SARS-CoV-2 main protease (Mpro) and RNA-dependent RNA polymerase (RDRP). Mem. Inst. Oswaldo Cruz 2020, 115, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Benarba, B.; Pandiella, A. Medicinal Plants as Sources of Active Molecules Against COVID-19. Front. Pharmacol. 2020, 11, 1189. [Google Scholar] [CrossRef]
- Swargiary, A.; Mahmud, S.; Saleh, M.A. Screening of phytochemicals as potent inhibitor of 3-chymotrypsin and papain-like proteases of SARS-CoV2: An in silico approach to combat COVID-19. J. Biomol. Struct. Dyn. 2020. Available online: https://www.tandfonline.com/doi/ref/10.1080/07391102.2020.1835729?scroll=top (accessed on 10 June 2021).
- Piccolella, S.; Crescente, G.; Faramarzi, S.; Formato, M.; Pecoraro, M.T.; Pacifico, S. Polyphenols vs. Coronaviruses: How Far Has Research Moved Forward? Molecules 2020, 25, 4103. [Google Scholar] [CrossRef]
- Suručić, R.; Tubić, B.; Stojiljković, M.P.; Djuric, D.M.; Travar, M.; Grabež, M.; Šavikin, K.; Škrbić, R. Computational study of pomegranate peel extract polyphenols as potential inhibitors of SARS-CoV-2 virus internalization. Mol. Cell. Biochem. 2021, 476, 1179–1193. [Google Scholar] [CrossRef] [PubMed]
- Muchtaridi, M.; Fauzi, M.; Ikram, N.K.K.; Gazzali, A.M.; Wahab, H.A. Natural flavonoids as potential angiotensin-converting enzyme 2 inhibitors for Anti-SARS-CoV-2. Molecules 2020, 25, 3980. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Du, Q. Potential natural compounds for preventing 2019-nCoV infection. Preprints 2020, 2020, 358. [Google Scholar]
- Shahinozzaman, M.; Basak, B.; Emran, R.; Rozario, P.; Obanda, D.N. Artepillin C: A comprehensive review of its chemistry, bioavailability, and pharmacological properties. Fitoterapia 2020, 147, 104775. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zheng, M.; Yang, Y.; Gu, X.; Yang, K.; Li, M.; Liu, Y.; Zhang, Q.; Zhang, P.; Wang, Y.; et al. Furin: A Potential Therapeutic Target for COVID-19. Iscience 2020, 23, 101642. [Google Scholar] [CrossRef]
- Finicelli, M.; Squillaro, T.; Di Cristo, F.; Di Salle, A.; Melone, M.A.B.; Galderisi, U.; Peluso, G. Metabolic syndrome, Mediterranean diet, and polyphenols: Evidence and perspectives. J. Cell. Physiol. 2019, 234, 5807–5826. [Google Scholar] [CrossRef]
- Yang, M.; Wei, J.; Huang, T.; Lei, L.; Shen, C.; Lai, J.; Yang, M.; Liu, L.; Yang, Y.; Liu, G.; et al. Resveratrol inhibits the replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in cultured Vero cells. Phyther. Res. 2021, 35, 1127–1129. [Google Scholar] [CrossRef]
- Mittra, I.; Souza, R.; Bhadade, R.; Madke, T.; Shankpal, P.D.; Joshi, M.; Qayyumi, B.; Bhattacharya, A.; Gota, V.; Gupta, S.; et al. Resveratrol and copper for treatment of severe COVID-19: An observational study (RESCU 002) (preprint). medRxiv 2020. Available online: https://www.medrxiv.org/content/10.1101/2020.07.21.20151423v1 (accessed on 12 June 2021).
- Jang, M.; Park, R.; Park, Y.-I.; Cha, Y.-E.; Yamamoto, A.; Lee, J.I.; Park, J. EGCG, a green tea polyphenol, inhibits human coronavirus replication in vitro. Biochem. Biophys. Res. Commun. 2021, 547, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Park, Y.I.; Cha, Y.E.; Park, R.; Namkoong, S.; Lee, J.I.; Park, J. Tea polyphenols EGCG and theaflavin inhibit the activity of SARS-CoV-2 3CL-protease in vitro. Evid. Based Complement. Alternat. Med. 2020, 2020, 5630838. [Google Scholar]
- Mhatre, S.; Srivastava, T.; Naik, S.; Patravale, V. Antiviral activity of green tea and black tea polyphenols in prophylaxis and treatment of COVID-19: A review. Phytomedicine 2021, 85, 153286. [Google Scholar] [CrossRef]
- Mhatre, S.; Naik, S.; Patravale, V. A molecular docking study of EGCG and theaflavin digallate with the druggable targets of SARS-CoV-2. Comput. Biol. Med. 2021, 129, 104137. [Google Scholar] [CrossRef]
- Singh, R.; Bhardwaj, V.K.; Sharma, J.; Purohit, R.; Kumar, S. In-Silico evaluation of bioactive compounds from tea as potential SARS-CoV-2 nonstructural protein 16 inhibitors. J. Tradit. Complement. Med. 2021. Available online: https://www.sciencedirect.com/science/article/pii/S2225411021000596 (accessed on 13 June 2021).
- Yañez, O.; Osorio, M.I.; Areche, C.; Vasquez-Espinal, A.; Bravo, J.; Sandoval-Aldana, A.; Pérez-Donoso, J.M.; González-Nilo, F.; Matos, M.J.; Osorio, E.; et al. Theobroma cacao L. compounds: Theoretical study and molecular modeling as inhibitors of main SARS-CoV-2 protease. Biomed. Pharmacother. 2021, 140, 111764. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, N.; Qiao, X.; Qiu, Z.; Liu, P. Composition analysis and antioxidant properties of black garlic extract. J. Food Drug Anal. 2017, 25, 340–349. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Jung, J.-H.; Kim, M.-K.; Lim, S.; Choi, J.-M.; Chung, B.; Kim, D.-W.; Kim, D. The Inhibitory Effects of Plant Derivate Polyphenols on the Main Protease of SARS Coronavirus 2 and Their Structure–Activity Relationship. Molecules 2021, 26, 1924. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.; El-Halawany, A.; Sirwi, A.; El-Araby, A.; Mohamed, G.; Ibrahim, S.; Koshak, A.; Asfour, H.; Awan, Z.; Elfaky, M.A. Repurposing of Some Natural Product Isolates as SARS-COV-2 Main Protease Inhibitors via In Vitro Cell Free and Cell-Based Antiviral Assessments and Molecular Modeling Approaches. Pharmaceuticals 2021, 14, 213. [Google Scholar] [CrossRef]
- Alam, S.; Bhuiyan, F.R.; Emon, T.H.; Hasan, M. Prospects of nutritional interventions in the care of COVID-19 patients. Heliyon 2021, 7, e06285. [Google Scholar] [CrossRef]
- Kow, C.S.; Hadi, M.A.; Hasan, S.S. Vitamin D supplementation in influenza and COVID-19 infections comment on: “Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths”. Nutrients 2020, 12, 1626. [Google Scholar] [CrossRef]
- Abd El-Aziz, N.M.; Eldin Awad, O.M.; Shehata, M.G.; El-Sohaimy, S.A. Inhibition of the SARS-CoV-2 RNA-dependent RNA polymerase by natural bioactive compounds: Molecular docking analysis. Egypt. J. Chem. 2021, 64, 1989–2001. [Google Scholar]
- Carneiro, S.M.; Oliveira, M.B.P.; Alves, R.C. Neuroprotective properties of coffee: An update. Trends Food Sci. Technol. 2021, 113, 167–179. [Google Scholar] [CrossRef]
- Adem, Ş.; Eyupoglu, V.; Sarfraz, I.; Rasul, A.; Zahoor, A.F.; Ali, M.; Abdalla, M.; Ibrahim, I.M.; Elfiky, A.A. Caffeic acid derivatives (CAFDs) as inhibitors of SARS-CoV-2: CAFDs-based functional foods as a potential alternative approach to combat COVID-19. Phytomedicine 2021, 85, 153310. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, D.; Nandi, R.; Jagadeesan, R.; Kumar, N.; Prakash, A.; Kumar, D. Identification of potential inhibitors against SARS-CoV-2 by targeting proteins responsible for envelope formation and virion assembly using docking based virtual screening, and pharmacokinetics approaches. Infect. Genet. Evol. 2020, 84, 104451. [Google Scholar] [CrossRef]
- Luís, Â.; Duarte, A.P.; Pereira, L.; Domingues, F. Interactions between the major bioactive polyphenols of berries: Effects on antioxidant properties. Eur. Food Res. Technol. 2018, 244, 175–185. [Google Scholar] [CrossRef]
- Messaoudi, O.; Gouzi, H.; El-Hoshoudy, A.N.; Benaceur, F.; Patel, C.; Goswami, D.; Boukerouis, D.; Bendahou, M. Berries anthocyanins as potential SARS-CoV–2 inhibitors targeting the viral attachment and replication; molecular docking simulation. Egypt. J. Pet. 2021, 30, 33–43. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valizadeh, H.; Abdolmohammadi-Vahid, S.; Danshina, S.; Gencer, M.Z.; Ammari, A.; Sadeghi, A.; Roshangar, L.; Aslani, S.; Esmaeilzadeh, A.; Ghaebi, M.; et al. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int. Immunopharmacol. 2020, 89, 107088. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, S.; Saeed, B.Q.; Temirgalieva, E.; Yumashev, A.V.; El-Esawi, M.A.; Navashenaq, J.G.; Valizadeh, H.; Sadeghi, A.; Aslani, S.; Yousefi, M.; et al. Nanocurcumin improves Treg cell responses in patients with mild and severe SARS-CoV2. Life Sci. 2021, 276, 119437. [Google Scholar] [CrossRef] [PubMed]
- Yudi Utomo, R.; Meiyanto, E. Revealing the potency of citrus and galangal constituents to halt SARS-CoV-2 infection. Preprints. 2020. Available online: https://www.preprints.org/manuscript/202003.0214/v1 (accessed on 12 June 2021).
- Cheng, L.; Zheng, W.; Li, M.; Huang, J.; Bao, S.; Xu, Q.; Ma, Z. Citrus fruits are rich in flavonoids for immunoregulation and potential targeting ACE2. Preprints 2020, 2020, 313. [Google Scholar]
- Guler, H.I.; Tatar, G.; Yildiz, O.; Belduz, A.O.; Kolayli, S. Investigation of potential inhibitor properties of ethanolic propolis extracts against ACE-II receptors for COVID-19 treatment by molecular docking study. Arch. Microbiol. 2021, 203, 3557–3564. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.A.D.; Jong, D.; Galvão, E.B.S.; Ribeiro, J.C.; Silva, T.C.; Berretta, A.A.; Amorim, T.C.; Martin, R.L.A.S.; Conceiçao, L.F.M.R.; Gomes, M.M.D.; et al. Efficacy of propolis as an adjunct treatment for hospitalized COVID-19 patients: A randomized, controlled clinical trial. Biomed. Pharmacother. 2021. Available online: https://www.medrxiv.org/content/10.1101/2021.01.08.20248932v1.full (accessed on 13 June 2021).
- Dai, W.; Bi, J.; Li, F.; Wang, S.; Huang, X.; Meng, X.; Sun, B.; Wang, D.; Kong, W.; Jiang, C.; et al. Antiviral Efficacy of Flavonoids against Enterovirus 71 Infection in Vitro and in Newborn Mice. Viruses 2019, 11, 625. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, S.; Hassan, S.H.; Al-Sehemi, A.G.; Shakir, H.A.; Khan, M.; Irfan, M.; Iqbal, J. Exploring the new potential antiviral constituents of Moringa oliefera for SARS-COV-2 pathogenesis: An in silico molecular docking and dynamic studies. Chem. Phys. Lett. 2021, 767, 138379. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhu, G.H.; Wang, H.N.; Hu, Q.; Chen, L.L.; Guan, X.Q.; Li, H.L.; Chen, H.Z.; Tang, H.; Ge, G.B. Discovery of naturally occurring inhibitors against SARS-CoV-2 3CLpro from Ginkgo biloba leaves via large-scale screening. Fitoterapia 2021, 152, 104909. [Google Scholar] [CrossRef]
- Takahashi, S.; Yoshiya, T.; Yoshizawa-Kumagaye, K.; Sugiyama, T. Nicotianamine is a novel angiotensin-converting enzyme 2 inhibitor in soybean. Biomed. Res. 2015, 36, 219–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halder, P.; Pal, U.; Paladhi, P.; Dutta, S.; Paul, P.; Pal, S.; Das, D.; Ganguly, A.; Dutta, I.; Mandal, S.; et al. Evaluation of potency of the selected bioactive molecules from Indian medicinal plants with MPro of SARS-CoV-2 through in silico analysis. J. Ayurveda Integr. Med. 2021. Available online: https://www.sciencedirect.com/science/article/pii/S0975947621000759 (accessed on 15 June 2021).
- Du, A.; Zheng, R.; Disoma, C.; Li, S.; Chen, Z.; Li, S.; Liu, P.; Zhou, Y.; Shen, Y.; Liu, S.; et al. Epigallocatechin-3-gallate, an active ingredient of Traditional Chinese Medicines, inhibits the 3CLpro activity of SARS-CoV-2. Int. J. Biol. Macromol. 2021, 176, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Shao, X.-X.; Li, X.-X.; Jia, X.-H.; Song, T.; Zhou, W.-Y.; Wang, P.; Li, Y.; Wang, X.-L.; Cui, Q.-H.; et al. Identifying potential treatments of COVID-19 from Traditional Chinese Medicine (TCM) by using a data-driven approach. J. Ethnopharmacol. 2020, 258, 112932. [Google Scholar] [CrossRef]
- Lordan, R.; Rando, H.M.; Greene, C.S. Dietary Supplements and Nutraceuticals Under Investigation for COVID-19 Prevention and Treatment. Msystems 2021, 6, e00122-21. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Mayol, I.; Guerra-Valle, M.; Casas-Forero, N.; Sobral, M.M.C.; Viegas, O.; Alarcón-Enos, J.; Ferreira, I.M.; Pinho, O. Western dietary pattern antioxidant intakes and oxidative stress: Importance during the SARS-CoV-2/COVID-19 pandemic. Adv. Nutr. 2021, 12, 670–681. [Google Scholar] [CrossRef]
- Dwyer, J.T.; Saldanha, L.; Bailen, R.; Durazzo, A.; Le Donne, C.; Piccinelli, R.; Andrews, K.; Pehrsson, P.; Gusev, P.; Calvillo, A.; et al. Commentary: An impossible dream? Integrating dietary supplement label databases: Needs, challenges, next steps. J. Food Compos. Anal. 2021, 102, 103882. [Google Scholar] [CrossRef]
- Wang, Y.; Harrington, P.D.B.; Chen, P. Analysis of phenolic compositions in cranberry dietary supplements using UHPLC-HRMS. J. Food Compos. Anal. 2020, 86, 103362. [Google Scholar] [CrossRef]
- Malta, L.; Liu, R. Analyses of Total Phenolics, Total Flavonoids, and Total Antioxidant Activities in Foods and Dietary Supplements. In Encyclopedia of Agriculture and Food Systems; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 305–314. [Google Scholar]
- Celik, C.; Gencay, A.; Ocsoy, I. Can food and food supplements be deployed in the fight against the COVID-19 pandemic? BBA-Gen. Subj. 2021, 1895, 129801. [Google Scholar] [CrossRef]
- Islam, S.S.; Midya, S.; Sinha, S.; Saadi, S.M.A.I. Natural medicinal plant products as an immune-boosters: A possible role to lessen the impact of Covid-19. Case Stud. Chem. Environ. Eng. 2021, 4, 100105. [Google Scholar] [CrossRef]
- Aanouz, I.; Belhassan, A.; El-Khatabi, K.; Lakhlifi, T.; El-ldrissi, M.; Bouachrine, M. Moroccan Medicinal plants as inhibitors against SARS-CoV-2 main protease: Computational investigations. J. Biomol. Struct. Dyn. 2021, 39, 2971–2979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.-W.; Wang, L.; Bao, L.-D. Exploring the active compounds of traditional Mongolian medicine in intervention of novel coronavirus (COVID-19) based on molecular docking method. J. Funct. Foods 2020, 71, 104016. [Google Scholar] [CrossRef]
- Feng, Z.; Yang, J.; Xu, M.; Lin, R.; Yang, H.; Lai, L.; Wang, Y.; Wahner-Roedler, D.L.; Zhou, X.; Shin, K.-M.; et al. Dietary supplements and herbal medicine for COVID-19: A systematic review of randomized control trials. Clin. Nutr. ESPEN 2021, 44, 50–60. [Google Scholar] [CrossRef]
- George, T.T.; Obilana, A.O.; Oyenihi, A.B.; Rautenbach, F.G. Moringa oleifera through the years: A bibliometric analysis of scientific research (2000-2020). S. Afr. J. Bot. 2021, 141, 12–24. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Zhang, J.; Wang, S. Advances in the chemical constituents and chemical analysis of Ginkgo biloba leaf, extract, and phytopharmaceuticals. J. Pharm. Biomed. Anal. 2021, 193, 113704. [Google Scholar] [CrossRef]
- Jain, A.S.; Sushma, P.; Dharmashekar, C.; Beelagi, M.S.; Prasad, S.K.; Shivamallu, C.; Prasad, A.; Syed, A.; Marraiki, N.; Prasad, K.S. In silico evaluation of flavonoids as effective antiviral agents on the spike glycoprotein of SARS-CoV-2. Saudi J. Biol. Sci. 2021, 28, 1040–1051. [Google Scholar] [CrossRef]
- Balkrishna, A.; Ben Bhatt, A.; Singh, P.; Haldar, S.; Varshney, A. Comparative retrospective open-label study of ayurvedic medicines and their combination with allopathic drugs on asymptomatic and mildly-symptomatic COVID-19 patients. J. Herb. Med. 2021, 29, 100472. [Google Scholar] [CrossRef] [PubMed]
- Günalan, E.; Cebioğlu, İ.K.; Çonak, Ö. The Popularity of the Biologically-Based Therapies During Coronavirus Pandemic Among the Google Users in the USA, UK, Germany, Italy and France. Complement. Ther. Med. 2021, 58, 102682. [Google Scholar] [CrossRef] [PubMed]
- Mehany, T.; Khalifa, I.; Barakat, H.; Althwab, S.A.; Alharbi, Y.M.; El-Sohaimy, S. Polyphenols as promising biologically active substances for preventing SARS-CoV-2: A review with research evidence and underlying mechanisms. Food Biosci. 2021, 40, 100891. [Google Scholar] [CrossRef]
- Witkamp, R.F.; Norren, K. Let thy food be thy medicine when possible. Eur. J. Pharmacol. 2018, 836, 102–114. [Google Scholar] [CrossRef]
- Khabour, O.F.; Hassanein, S.F. Use of vitamin/zinc supplements, medicinal plants, and immune boosting drinks during COVID-19 pandemic: A pilot study from Benha city, Egypt. Heliyon 2021, 7, e06538. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, R.; Sooriyaarachchi, P.; Chourdakis, M.; Jeewandara, C.; Ranasinghe, P. Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Cobre, A.D.F.; Surek, M.; Vilhena, R.D.O.; Böger, B.; Fachi, M.M.; Momade, D.R.O.; Tonin, F.S.; Sarti, F.M.; Pontarolo, R. Influence of foods and nutrients on COVID-19 recovery: A multivariate analysis of data from 170 countries using a generalized linear model. Clin. Nutr. 2021. [Google Scholar] [CrossRef]
- Greene, M.W.; Roberts, A.P.; Frugé, A.D. Negative Association Between Mediterranean Diet Adherence and COVID-19 Cases and Related Deaths in Spain and 23 OECD Countries: An Ecological Study. Front. Nutr. 2021, 8, 591964. [Google Scholar] [CrossRef]
- Scoditti, E.; Calabriso, N.; Massaro, M.; Pellegrino, M.; Storelli, C.; Martines, G.; De Caterina, R.; Carluccio, M.A. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: A potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch. Biochem. Biophys. 2012, 527, 81–89. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Félix, J.D.; Gonçalves, A.C.; Alves, G.; Silva, L.R. Consumption of Phenolic-Rich Food and Dietary Supplements as a Key Tool in SARS-CoV-19 Infection. Foods 2021, 10, 2084. https://doi.org/10.3390/foods10092084
Flores-Félix JD, Gonçalves AC, Alves G, Silva LR. Consumption of Phenolic-Rich Food and Dietary Supplements as a Key Tool in SARS-CoV-19 Infection. Foods. 2021; 10(9):2084. https://doi.org/10.3390/foods10092084
Chicago/Turabian StyleFlores-Félix, José David, Ana C. Gonçalves, Gilberto Alves, and Luís R. Silva. 2021. "Consumption of Phenolic-Rich Food and Dietary Supplements as a Key Tool in SARS-CoV-19 Infection" Foods 10, no. 9: 2084. https://doi.org/10.3390/foods10092084
APA StyleFlores-Félix, J. D., Gonçalves, A. C., Alves, G., & Silva, L. R. (2021). Consumption of Phenolic-Rich Food and Dietary Supplements as a Key Tool in SARS-CoV-19 Infection. Foods, 10(9), 2084. https://doi.org/10.3390/foods10092084










