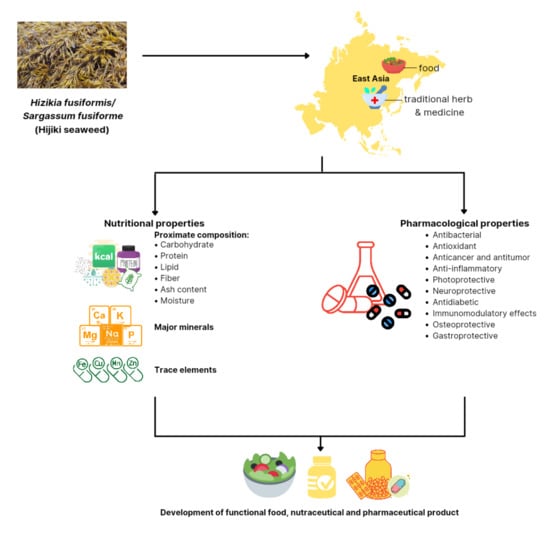
Hizikia fusiformis: Pharmacological and Nutritional Properties
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Nutritional Properties
3.1.1. Proximate Composition
3.1.2. Major Minerals and Trace Elements
3.1.3. Polysaccharide
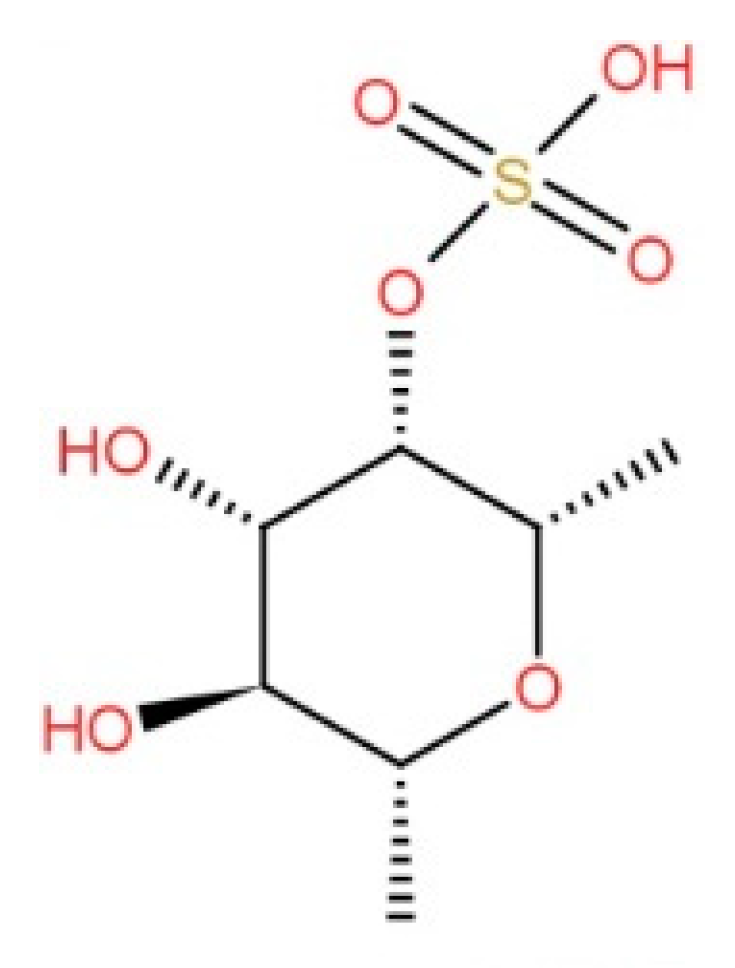
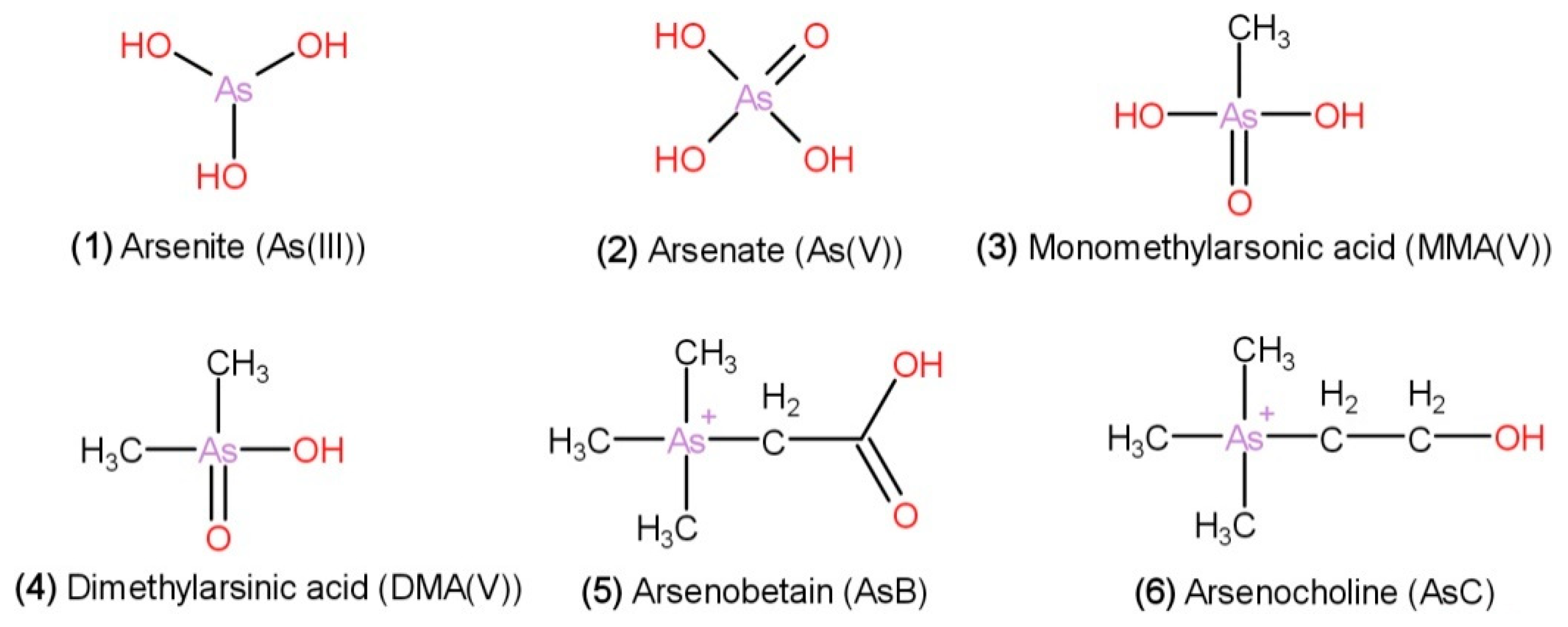
3.1.4. Bioactive Compounds
3.2. Pharmacological Properties
3.2.1. Antibacterial Activity
3.2.2. Antioxidant Activity
3.2.3. Anticancer and Antitumor Activity
3.2.4. Anti-Inflammatory Activity
3.2.5. Photoprotective Activity
3.2.6. Neuroprotective Activity
3.2.7. Antidiabetic Activity
3.2.8. Immunomodulatory Effects
3.2.9. Osteoprotective Activity
3.2.10. Gastroprotective Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zou, D.; Liu, S.; Du, H.; Xu, J. Growth and photosynthesis in seedlings of Hizikia fusiformis (Harvey) Okamura (Sargassaceae, Phaeophyta) cultured at two different temperatures. J. Appl. Phycol. 2012, 24, 1321–1327. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, M.; Lin, L.; Thring, R.W.; Yu, H.; Zhang, X.; Zhao, M. Allelopathic interactions between the macroalga Hizikia fusiformis (Harvey) and the harmful blooms-forming dinoflagellate Karenia mikimotoi. Harmful Algae 2017, 65, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). Genetic Resources for Farmed Seaweed; FAO: Rome, Italy, 2017; 72p. [Google Scholar]
- Liu, N.; Fu, X.; Duan, D.; Xu, J.; Gao, X.; Zhao, L. Evaluation of bioactivity of phenolic compounds from the brown seaweed of Sargassum fusiforme and development of their stable emulsion. J. Appl. Phycol. 2018, 30, 1955–1970. [Google Scholar] [CrossRef]
- Wang, W.; Lu, J.; Wang, C.; Wang, C.; Zhang, H.; Li, C.; Qian, G. Effects of Sargassum fusiforme polysaccharides on antioxidant activities and intestinal functions in mice. Int. J. Biol. Macromol. 2013, 58, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Huh, G.W.; Lee, D.Y.; In, S.J.; Lee, D.G.; Park, S.Y.; Yi, T.H.; Kang, H.C.; Seo, W.D.; Baek, N.I. Fucosterols from Hizikia fusiformis and their proliferation activities on osteosarcoma-derived cell MG63. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 551–555. [Google Scholar] [CrossRef]
- Dai, Y.L.; Jiang, Y.F.; Lee, H.G.; Jeon, Y.J.; Kang, M.C. Characterization and screening of anti-tumor activity of fucoidan from acid-processed hijiki (Hizikia fusiforme). Int. J. Biol. Macromol. 2019, 139, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Jiang, Y.; Lu, Y.; Yu, J.; Kang, M. Fucoxanthin-rich fraction from Sargassum fusiformis alleviates particulate matter-induced inflammation in vitro and in vivo. Toxicol. Rep. 2021, 8, 349–358. [Google Scholar] [CrossRef]
- Chen, P.; He, D.; Zhang, Y.; Yang, S.; Chen, L.; Wang, S.; Zou, H.; Liao, Z.; Zhang, X.; Wu, M. Sargassum fusiforme polysaccharides activate antioxidant defense by promoting Nrf2-dependent cytoprotection and ameliorate stress insult during aging. Food Funct. 2016, 7, 4576–4588. [Google Scholar] [CrossRef] [PubMed]
- Cong, Q.; Xiao, F.; Liao, W.; Dong, Q.; Ding, K. Structure and biological activities of an alginate from Sargassum fusiforme, and its sulfated derivative. Int. J. Biol. Macromol. 2014, 39, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.C.; Jeong, Y.T.; Lee, S.M.; Kim, J.H. Immune-modulating activities of polysaccharides extracted from brown algae Hizikia fusiforme. Biosci. Biotechnol. Biochem. 2015, 79, 1362–1365. [Google Scholar] [CrossRef]
- Kwon, H.O.; Lee, M.; Kim, O.K.; Ha, Y.; Jun, W.; Lee, J. Effect of Hijikia fusiforme extracts on degenerative osteoarthritis in vitro and in vivo models. Nutr. Res. Pract. 2016, 10, 265–273. [Google Scholar] [CrossRef]
- Lee, D.G.; Park, S.Y.; Chung, W.S.; Park, J.H.; Hwang, E.; Mavlonov, G.T.; Kim, I.H.; Kim, K.Y.; Yi, T.H. Fucoidan Prevents the Progression of Osteoarthritis in Rats. J. Med. Food 2015, 18, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Rana, Z.H.; Alam, M.K.; Akhtaruzzaman, M. Nutritional composition, total phenolic content, antioxidant and α-amylase inhibitory activities of different fractions of selected wild edible plants. Antioxidants 2019, 8, 203. [Google Scholar] [CrossRef]
- Alam, M.K.; Rana, Z.H.; Islam, S.N.; Akhtaruzzaman, M. Comparative assessment of nutritional composition, polyphenol profile, antidiabetic and antioxidative properties of selected edible wild plant species of Bangladesh. Food Chem. 2020, 320, 126646. [Google Scholar] [CrossRef]
- Wijesekara, I.; Kim, S.K.; Li, Y.; Li, Y.X. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Robledo, D. Bioactive Phenolic Compounds from Algae. Bioact. Compd. Mar. Foods 2013, 113–129. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed phenolics, From extraction to applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef] [PubMed]
- Siriwardhana, N.; Lee, K.W.; Kim, S.H.; Ha, J.W.; Jeon, Y.J. Antioxidant Activity of Hizikia fusiformis on Reactive Oxygen Species Scavenging and Lipid Peroxidation Inhibition. Food Sci. Technol. Int. 2003, 9, 339–346. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses, The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lee, S.R.; Oh, J.W. Effects of dietary fermented seaweed and seaweed fusiforme on growth performance, carcass parameters and immunoglobulin concentration in broiler chicks. Asian-Australasian J. Anim. Sci. 2014, 27, 862–870. [Google Scholar] [CrossRef]
- Zheng, T.; Liu, C.; Yang, J.; Liu, Q.; Li, J. Hijiki seaweed (Hizikia fusiformis), Nutritional value, safety concern and arsenic removal method. Adv. Mater. Res. 2013, 634–638, 1247–1252. [Google Scholar] [CrossRef]
- Hwang, E.; Park, S.-Y.; Sun, Z.; Shin, H.-S.; Lee, D.-G.; Yi, T.H. The Protective Effects of Fucosterol Against Skin Damage in UVB-Irradiated Human Dermal Fibroblasts. Mar. Biotechnol. 2013, 16, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.Y.; Hwang, H.J.; Nam, T.J. Protective effect of a polysaccharide from Hizikia fusiformis against ethanol-induced cytotoxicity in IEC-6 cells. Toxicol. In Vitro 2010, 24, 79–84. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kum, J.S.; Jeon, K.H.; Park, J.D.; Choi, H.W.; Hwang, K.E.; Jeong, T.J.; Kim, Y.B.; Kim, C.J. Effects of Edible Seaweed on Physicochemical and Sensory Characteristics of Reduced-salt Frankfurters. Korean J. Food Sci. Anim. Resour. 2015, 35, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-W.; Jang, J.-W.; Kim, S.-S.; Oh, D.-H.; Cha, J.-H.; Lee, K.-J. Effect of Dietary Supplementation with Alga (Hizikia fusiformis and Ecklonia cava) on the Non-specific Immune Responses of Parrot Fish Oplegnathus fasciatus. Korean J. Fish Aquat. Sci. 2011, 44, 332–338. [Google Scholar] [CrossRef][Green Version]
- Salehi, B.; Sharifi-rad, J.; Seca, A.M.L.; Pinto, D.C.G.A. Current Trends on Seaweeds, Looking at Chemical. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed, Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweed’s Bioactive Candidate Compounds to Food Industry and Global Food Security. Life 2020, 10, 140. [Google Scholar] [CrossRef]
- Karr-Lilienthal, L.K.; Bauer, L.L.; Utterback, P.L.; Zinn, K.E.; Frazier, R.L.; Parsons, C.M.; Fahey, G.C. Chemical composition and nutritional quality of soybean meals prepared by extruder/expeller processing for use in poultry diets. J. Agric. Food Chem. 2006, 54, 8108–8114. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Kraft, L.G.K.; van der Loos, L.M.; Kraft, G.T.; Virtue, P.; Nichols, P.D.; Hurd, C.L. Southern Australian seaweeds, A promising resource for omega-3 fatty acids. Food Chem. 2018, 265, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 1462. [Google Scholar] [CrossRef]
- Clark, M.J.; Slavin, J.L. The effect of fiber on satiety and food intake, A systematic review. J. Am. Coll. Nutr. 2013, 32, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Panel, E.; Nda, A. Scientific Opinion on the substantiation of health claims related to the replacement of mixtures of saturated fatty acids (SFAs) as present in foods or diets with mixtures of monounsaturated fatty acids (MUFAs) and/or mixtures of polyunsaturated fatty aci. EFSA J. 2011, 9, 2069. [Google Scholar] [CrossRef]
- Cherry, P.; O’hara, C.; Magee, P.J.; Mcsorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [PubMed]
- Rupérez, P.; Ahrazem, O.; Leal, J.A. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J. Agric. Food Chem. 2002, 50, 840–845. [Google Scholar] [CrossRef]
- Fouda, W.A.; Ibrahim, W.M.; Ellamie, A.M.; Ramadan, G. Biochemical and mineral compositions of six brown seaweeds collected from red sea at hurghada coast. Indian J. Geo-Mar. Sci. 2019, 48, 484–491. [Google Scholar]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K. Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J. Appl. Phycol. 2009, 21, 75–80. [Google Scholar] [CrossRef]
- Mendis, E.; Kim, S.K. Present and Future Prospects of Seaweeds in Developing Functional Foods. In Advances in Food and Nutrition Research; Academic Press: New York, NY, USA, 2011; Volume 64. [Google Scholar] [CrossRef]
- Rohani-Ghadikolaei, K.; Abdulalian, E.; Ng, W.K. Evaluation of the proximate, fatty acid and mineral composition of representative green, brown and red seaweeds from the Persian Gulf of Iran as potential food and feed resources. J. Food Sci. Technol. 2012, 49, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fanzo, J.; Miller, D.D.; Pingali, P.; Post, M.; Steiner, J.L.; Thalacker-Mercer, A.E. Production and supply of high-quality food protein for human consumption, Sustainability, challenges, and innovations. Ann. N. Y. Acad. Sci. 2014, 1321, 1–19. [Google Scholar] [CrossRef]
- Al-fartusie, F.S.; Mohssan, S.N. Essential Trace Elements and Their Vital Roles in Human Body. Indian J. Adv. Chem. Sci. 2017, 5, 127–136. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Rajauria, G.; Miranda, M.; Sweeney, T.; Lopez-Alonso, M.; O’Doherty, J. Seasonal Variation of the Proximate Composition, Mineral Content, Fatty Acid Profiles and Other Phytochemical Constituents of Selected Brown Macroalgae. Mar. Drugs 2021, 19, 204. [Google Scholar] [CrossRef] [PubMed]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Wang, L.; Jayawardena, T.U.; Yang, H.W.; Lee, H.G.; Kang, M.C.; Sanjeewa, K.K.A.; Oh, J.Y.; Jeon, Y.J. Isolation, characterization, and antioxidant activity evaluation of a fucoidan from an enzymatic digest of the edible seaweed, Hizikia fusiforme. Antioxidants 2020, 9, 363. [Google Scholar] [CrossRef]
- Hu, P.; Li, Z.; Chen, M.; Sun, Z.; Ling, Y.; Jiang, J.; Huang, C. Structural elucidation and protective role of a polysaccharide from Sargassum fusiforme on ameliorating learning and memory deficiencies in mice. Carbohydr. Polym. 2016, 139, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Lian, T.; Yang, B.; Yi, G.; Li, X. Ameliorative Effect of Sargassum fusiforme Polysaccharides on Oxidative Stress and Inflammation in Ethanol-induced Gastric Ulcer. Pharmacogn. Mag. 2019, 15, 244–252. [Google Scholar] [CrossRef]
- Jia, R.B.; Li, Z.R.; Ou, Z.R.; Wu, J.; Sun, B.; Lin, L.; Zhao, M. Physicochemical Characterization of Hizikia fusiforme Polysaccharide and Its Hypoglycemic Activity via Mediating Insulin-Stimulated Blood Glucose Utilization of Skeletal Muscle in Type 2 Diabetic Rats. Chem. Biodivers. 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Oh, J.Y.; Kim, H.S.; Lee, W.W.; Cui, Y.; Lee, H.G.; Kim, Y.T.; Ko, J.Y.; Jeon, Y.J. Protective effect of polysaccharides from Celluclast-assisted extract of Hizikia fusiforme against hydrogen peroxide-induced oxidative stress in vitro in Vero cells and in vivo in zebrafish. Int. J. Biol. Macromol. 2018, 112, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, P.; Liu, J.; Hu, C.; Yang, S.; He, D.; Yu, P.; Wu, M.; Zhang, X. Sargassum fusiforme polysaccharide SFP-F2 activates the NF-κB signaling pathway via CD14/IKK and p38 axes in RAW264.7 cells. Mar. Drugs 2018, 16, 264. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zhang, J.; Nie, W.; Zhou, W.; Jin, L.; Chen, X.; Lu, J. Antitumor Effects of Polysaccharide from Sargassum Fusiforme against Human Hepatocellular Carcinoma HepG2 Cells. Food Chem Toxicol. 2017, 102, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cong, Q.; Du, Z.; Liao, W.; Zhang, L. Sulfated fucoidan FP08S2 inhibits lung cancer cell growth in vivo by disrupting angiogenesis via targeting VEGFR2/VEGF and blocking VEGFR2/Erk/VEGF signaling. Cancer Lett. 2016, 382, 44–52. [Google Scholar] [CrossRef]
- Ye, Y.; Ji, D.; You, L.; Zhou, L.; Zhao, Z.; Brennan, C. Structural properties and protective effect of Sargassum fusiforme polysaccharides against ultraviolet B radiation in hairless Kun Ming mice. J. Funct. Foods 2018, 43, 8–16. [Google Scholar] [CrossRef]
- Cheng, Y.; Sibusiso, L.; Hou, L.; Jiang, H.; Chen, P.; Zhang, X.; Wu, M.; Tong, H. Sargassum fusiforme fucoidan modifies the gut microbiota during alleviation of streptozotocin-induced hyperglycemia in mice. Int. J. Biol. Macromol. 2019, 131, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Heo, H.J.; Row, K.H. Optimization of crude polysaccharides extraction from Hizikia fusiformis using response surface methodology. Carbohydr. Polym. 2010, 82, 106–110. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral utilization of red seaweed for bioactive production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef] [PubMed]
- Pierre, G.; Delattre, C.; Dubessay, P.; Jubeau, S.; Vialleix, C.; Cadoret, J.P.; Probert, I.; Michaud, P. What is in store for EPS microalgae in the next decade? Molecules 2019, 24, 4296. [Google Scholar] [CrossRef] [PubMed]
- Stiger-Pouvreau, V.; Bourgougnon, N.; Deslandes, E. Carbohydrates from Seaweeds; Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Vendri, I.; Abdelkafi, S.; Michaud, P. Bioactive polysaccharides from seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan, Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological activities of fucoidan and the factors mediating its therapeutic effects, A review of recent studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef]
- García-Ríos, V.; Ríos-Leal, E.; Robledo, D.; Freile-Pelegrin, Y. Polysaccharides composition from tropical brown seaweeds. Phycol. Res. 2012, 60, 305–315. [Google Scholar] [CrossRef]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds, An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef]
- Wagle, A.; Seong, S.H.; Zhao, B.T.; Woo, M.H.; Jung, H.A.; Choi, J.S. Comparative study of selective in vitro and in silico BACE1 inhibitory potential of glycyrrhizin together with its metabolites, 18α- and 18β-glycyrrhetinic acid, isolated from Hizikia fusiformis. Arch. Pharm. Res. 2018, 41, 409–418. [Google Scholar] [CrossRef]
- Yang, E.-J.; Moon, J.-Y.; Kim, M.-J.; Kim, D.S.; Kim, C.-S.; Lee, W.J.; Lee, N.H.; Hyun, C.-G. Inhibitory effect of Jeju endemic seaweeds on the production of pro-inflammatory mediators in mouse macrophage cell line RAW 264.7. J. Zhejiang Univ. Sci. B 2010, 11, 315–322. [Google Scholar] [CrossRef]
- Park G young Kang D eun Davaatseren, M.; Shin, C.; Kang, G.J.; Chung, M.S. Reduction of total, organic, and inorganic arsenic content in Hizikia fusiforme (Hijiki). Food Sci. Biotechnol. 2019, 28, 615–622. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Wu, J.F.; Shang, D.R.; Ning, J.S.; Ding, H.Y.; Zhai, Y.X. Arsenic species in edible seaweeds using in vitro biomimetic digestion determined by high-performance liquid chromatography inductively coupled plasma mass spectrometry. Int. J. Food Sci. 2014, 2014, 436347. [Google Scholar] [CrossRef]
- Yang, W.C.; Zhang, Y.Y.; Li, Y.J.; Nie, Y.Y.; Liang, J.Y.; Liu, Y.Y.; Liu, J.S.; Zhang, Y.P.; Song, C.; Qian, Z.J.; et al. Chemical Composition and Anti-Alzheimer’s Disease-Related Activities of a Functional Oil from the Edible Seaweed Hizikia fusiforme. Chem. Biodivers. 2020, 17. [Google Scholar] [CrossRef]
- Park, S.Y.; Hwang, E.; Shin, Y.K.; Lee, D.G.; Yang, J.E.; Park, J.H.; Yi, T.H. Immunostimulatory Effect of Enzyme-Modified Hizikia fusiforme in a Mouse Model In Vitro and Ex Vivo. Mar. Biotechnol. 2017, 19, 65–75. [Google Scholar] [CrossRef]
- Seong, S.H.; Nguyen, D.H.; Wagle, A.; Woo, M.H.; Jung, H.A.; Choi, J.S. Experimental and Computational Study to Reveal the Potential of Non-Polar Constituents from Hizikia fusiformis as Dual Protein Tyrosine Phosphatase 1B and α-Glucosidase Inhibitors. Mar. Drugs 2019, 17, 302. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Tong, C.; Wu, Y.; Liu, S.; Li, W. A novel thyroglobulin-binding lectin from the brown alga Hizikia fusiformis and its antioxidant activities. Food Chem. 2016, 201, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Akter, K.F.; Owens, G.; Davey, D.E.; Naidu, R. Arsenic speciation and toxicity in biological systems. Rev. Environ. Contam. Toxicol. 2006, 184, 97–149. [Google Scholar] [CrossRef]
- Abernathy, C.O.; Thomas, D.J.; Calderon, R.L. Toxicity and Risk Assessment of Trace Elements. J. Nutr. 2003, 133, 1536S–1538S. [Google Scholar] [CrossRef] [PubMed]
- Rispin, A.; Farrar, D.; Margosches, E.; Gupta, K.; Stitzel, K.; Carr, G.; Greene, M.; Meyer, W.; McCall, D. Alternative methods for the median lethal dose (LD(50)) test, The up-and-down procedure for acute oral toxicity. ILAR J. 2002, 43, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Poma, P.; Labbozzetta, M.; Notarbartolo, M.; Bruno, M.; Maggio, A.; Rosselli, S.; Sajeva, M.; Zito, P. Chemical composition, in vitro antitumor and pro-oxidant activities of Glandora rosmarinifolia (Boraginaceae) essential oil. PLoS ONE 2018, 13, e0196947. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Lee, S.-Y.; Kim, B.-Y.; Lee, E.-K.; Ryu, J.-H.; Lim, G.-B. Effects of modifiers on the supercritical CO2 extraction of glycyrrhizin from licorice and the morphology of licorice tissue after extraction. Biotechnol. Bioprocess Eng. 2004, 9, 447–453. [Google Scholar] [CrossRef]
- Batiha, G.E.S.; Beshbishy, A.M.; El-Mleeh, A.; Abdel-Daim, M.M.; Devkota, H.P. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Son, D.H.; Chung, T.H.; Lee, Y.J. A Review of the Pharmacological Efficacy and Safety of Licorice Root from Corroborative Clinical Trial Findings. J. Med. Food 2020, 23, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.J.; Yin, A.C.Y. Therapeutic effects of glycyrrhizic acid. Nat. Prod. Commun. 2013, 8, 415–418. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Olafsdóttir, G. Antioxidant capacities of phlorotannins extracted from the brown algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883. [Google Scholar] [CrossRef]
- Meslet-Cladière, L.; Delage, L.; Goulitquer, S.; Leblanc, C.; Creis, E.; Gall, E.A.; Stiger-pouvreau, V.; Czjzek, M.; Potin, P. Structure/Function Analysis of a Type III Polyketide Synthase in the Brown Alga Ectocarpus siliculosus Reveals a Biochemical Pathway in Phlorotannin Monomer Biosynthesis. Plant Cell 2013, 25, 3089–3103. [Google Scholar] [CrossRef]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and Identification of Phlorotannins from the Brown Alga, Sargassum fusiforme. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef]
- Martens, N.; Schepers, M.; Zhan, N.; Leijten, F.; Voortman, G.; Tiane, A.; Rombaut, B.; Poisquet, J.; van de Sande, N.; Kerksiek, A.; et al. 24(S)-Saringosterol Prevents Cognitive Decline in a Mouse Model for Alzheimer’s Disease. Mar. Drugs 2021, 19, 190. [Google Scholar] [CrossRef]
- Schultz, J.R.; Tu, H.; Luk, A.; Repa, J.J.; Medina, J.C.; Li, L.; Schwendner, S.; Wang, S.; Thoolen, M.; Mangelsdorf, D.J.; et al. Role of LXRs in control of lipogenesis. Genes Dev. 2000, 14, 2831–2838. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, J.; Fu, Z.; Ye, C.; Zhang, R.; Song, Y.; Zhang, Y.; Li, H.; Ying, H.; Liu, H. 24(S)-saringosterol from edible marine seaweed Sargassum fusiforme is a novel selective LXRβ agonist. J. Agric. Food Chem. 2014, 62, 6130–6137. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, Y.; Zhang, Y.; Zhang, S.; Qu, J.; Wang, X.; Kong, R.; Han, C.; Liu, Z. Fucoxanthin, A Promising Medicinal and Nutritional Ingredient. Evid. Based Complement Altern. Med. 2015, 2015, 723515. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.K.; Sams, S.; Rana, Z.H.; Akhtaruzzaman, M.; Islam, S.N. Minerals, vitamin C, and effect of thermal processing on carotenoids composition in nine varieties orange-fleshed sweet potato (Ipomoea batatas L.). J. Food Compos. Anal. 2020, 92, 103582. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Miyashita, K. Antiobesity effect of fucoxanthin from edible seaweeds and its multibiological functions. ACS Symp. Ser. 2008, 993, 376–388. [Google Scholar] [CrossRef]
- de Quirós, A.R.B.; Frecha-Ferreiro, S.; Vidal-Pérez, A.M.; López-Hernández, J. Antioxidant compounds in edible brown seaweeds. Eur. Food Res. Technol. 2010, 231, 495–498. [Google Scholar] [CrossRef]
- Kim, S.M.; Jung, Y.J.; Kwon, O.N.; Cha, K.H.; Um, B.H.; Chung, D.; Pan, C.H. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Main properties and current applications of some polysaccharides as biomaterials. Polym. Int. 2008, 57, 397–430. [Google Scholar] [CrossRef]
- Hreggviðsson, G.Ó.; Nordberg-Karlsson, E.M.; Tøndervik, A.; Aachmann, F.L.; Dobruchowska, J.M.; Linares-Pastén, J.; Daugbjerg-Christensen, M.; Moneart, A.; Kristjansdottir, T.; Sletta, H.; et al. Chapter 16. Biocatalytic refining of polysaccharides from brown seaweeds. In Advanced in Green Chemistry, Sustainable Seaweed Technologies; Torres, M.D., Kraan, S., Dominguez, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 447–504. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs, Alginate and chitosan—A review. J. Control Release 2006, 114, 1–14. [Google Scholar] [CrossRef]
- Weis, W.I.; Drickamer, K. Structural Basis of Recognition Lectin-Carb Ohydrate. Anna. Rev. Biochen 1996, 65, 441–473. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowska, N.E.; Wlodawer, A. Structural studies of algal lectins with anti-HIV activity. Acta Biochim. Pol. 2006, 53, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; Fitzgerald, R.J. Bioactive proteins, peptides, and amino acids from macroalgae. J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-J.; Wu, Y.; Tong, C.-Q.; Jin, Q.; Li, W. Antibacterial Total Phenolic Compounds from a Brown Alga Hizikia fusiformis. DEStech Trans. Environ. Energy Earth Sci. 2017, 418–425. [Google Scholar] [CrossRef]
- Tang, J.; Wang, W.; Chu, W. Antimicrobial and Anti-Quorum Sensing Activities of Phlorotannins From Seaweed (Hizikia fusiforme). Front. Cell. Infect. Microbiol. 2020, 10, 586750. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef]
- Arshad, M.A.; Khurshid, U.; Ahmad, S.; Ijaz, S.; Rashid, F.; Azam, R. Review on methods used to determine Antioxidant activity. Intern. Ional. J. Mul. Tidisc. Iplinary Res. Dev. 2014, 1, 41–46. [Google Scholar]
- Dai, Y.; Jiang, Y.; Lu, Y.; Kang, M.; Jeon, Y. Fucoidan from acid-processed Hizikia fusiforme attenuates oxidative damage and regulate apoptosis. Int. J. Biol. Macromol. 2020, 160, 390–397. [Google Scholar] [CrossRef]
- Wu, M.; Wu, Y.; Qu, M.; Li, W.; Yan, X. Evaluation of antioxidant activities of water-soluble polysaccharides from brown alga Hizikia fusiformis. Int. J. Biol. Macromol. 2013, 56, 28–33. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Y.; Xu, M.; Chen, H.; Zou, H.; Zhang, X.; Tong, H.; You, C.; Wu, M. Proteomic landscape of liver tissue in old male mice that are long-term treated with Polysaccharides from Sargassum fusiforme. Food Funct. 2020, 11, 3632–3644. [Google Scholar] [CrossRef]
- Oktaviani, D.F.; Bae, Y.; Dyah, M.; Meinita, N.; Moon, I.S. An Ethanol Extract of the Brown Seaweed Hizikia fusiformis and Its Active Constituent, Fucosterol, Extend the Lifespan of the Nematode Caenorhabditis elegans. J. Life Sci. 2019, 29, 1120–1125. [Google Scholar] [CrossRef]
- Park, C.; Lee, H.; Hwangbo, H.; Ji, S.Y.; Kim, M.Y.; Kim, S.Y.; Hong, S.H.; Kim, G.Y.; Choi, Y.H. Ethanol extract of Hizikia fusiforme induces apoptosis in B16F10 mouse melanoma cells through ROS-dependent inhibition of the PI3K/Akt signaling pathway. Asian Pac. J. Cancer Prev. 2020, 21, 1275–1282. [Google Scholar] [CrossRef]
- Choi, E.O.; Lee, H.; Park, C.; Kim, G.Y.; Cha, H.J.; Kim, H.S.; Jeon, Y.J.; Hwang, H.J.; Choi, Y.H. Ethanol extracts of Hizikia fusiforme induce apoptosis in human prostate cancer PC3 cells via modulating a ROS-dependent pathway. Asian Pac. J. Trop. Biomed. 2020, 10, 78–86. [Google Scholar] [CrossRef]
- Dai, Y.L.; Jiang, Y.F.; Nie, Y.H.; Lu, Y.A.; Kang, M.C.; Jeon, Y.J. Hepato-protective effect of fucoidan extracted from acid-processed Sargassum fusiformis in ethanol-treated Chang liver cells and in a zebrafish model. J. Appl. Phycol. 2020, 32, 4289–4298. [Google Scholar] [CrossRef]
- Lee, S.G.; Karadeniz, F.; Oh, J.H.; Yu, G.H.; Kong, C.S. Inhibitory effect of Hizikia fusiformis solvent-partitioned fractions on invasion and MMP activity of HT1080 human fibrosarcoma cells. Prev. Nutr. Food Sci. 2017, 22, 184–190. [Google Scholar] [CrossRef]
- Fuchs, Y.; Steller, H. Programmed cell death in animal development and disease. Cell 2011, 147, 742–758. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.Y. Apoptosis in cancer, From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N.; Stockinger, B.; Tak, P.P. The resolution of inflammation. Nat. Rev. Immunol. 2013, 13, 59–66. [Google Scholar] [CrossRef]
- Sharma, B.R.; Park, C.S.; Ma, S.J.; Rhyu, D.Y. Anti-inflammatory effects and mechanisms of Hizikia fusiformis via multicellular signaling pathways in lipopolysaccharide-induced RAW 264.7 cells. Pak. J. Pharm. Sci. 2017, 30, 43–48. [Google Scholar]
- Hwang, E.; Park, S.Y.; Shin, H.S.; Lee, D.G.; Yi, T.H. Effect of oral administration of fucosterol from Hizikia fusiformis on DNCB-induced atopic dermatitis in NC/Nga mice. Food Sci. Biotechnol. 2014, 23, 593–599. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, H.J.; Kim, H.B.; Kim, S.T.; Choi, Y.R.; Seo, D.W.; Yu, J.M.; Kil Jang, S.; Kim, S.M.; Lee, D.-I.; et al. Hizikia fusiformis fractions successfully improve atopic dermatitis indices in anti-CD3-stimulated splenocytes and 2, 4-dinitrochlorobenzene-treated BALB/c mice. J. Pharm. Pharmacol. 2013, 66, 466–476. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Shin, H.J.; Lee, J.H.; Lee, J. Antiallergic effect of Hizikia fusiformis in an ovalbumin-induced allergic rhinitis mouse model. Clin. Exp. Otorhinolaryngol. 2019, 12, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.M.; Bieber, T. Atopic dermatitis. Lancet 2003, 361, 151–160. [Google Scholar] [CrossRef]
- Solano, F. Photoprotection and skin pigmentation, Melanin-related molecules and some other new agents obtained from natural sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Oh, J.Y.; Lee, W.W.; Jeon, Y.J. Fucoidan isolated from Hizikia fusiforme suppresses ultraviolet B-induced photodamage by down-regulating the expressions of matrix metalloproteinases and pro-inflammatory cytokines via inhibiting NF-κB, AP-1, and MAPK signaling pathways. Int. J. Biol. Macromol. 2021, 166, 751–759. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Jayawardena, T.U.; Jeon, Y.; Ryu, B. Anti-inflammatory and anti-melanogenesis activities of sulfated polysaccharides isolated from Hizikia fusiforme, Short communication. Int. J. Biol. Macromol. 2019, 142, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Oh, J.Y.; Kim, Y.S.; Lee, H.G.; Lee, J.S.; Jeon, Y.J. Anti-photoaging and anti-melanogenesis effects of fucoidan isolated from Hizikia fusiforme and its underlying mechanisms. Mar. Drugs 2020, 18, 427. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Kang, H. Evaluation of antioxidant and anti-neuroinflammatory activities of Hizikia fusiformis (Harvey) okamura extract. Trop. J. Pharm. Res. 2015, 14, 463–468. [Google Scholar] [CrossRef]
- Kang, C.H.; Kim, M.J.; Seo, M.J.; Choi, Y.H.; Jo, W.S.; Lee, K.T.; Jeong, Y.K.; Kim, G.Y. 5-Hydroxy-3,6,7,8,3040-hexamethoxyflavone inhibits nitric oxide production in lipopolysaccharide-stimulated BV2 microglia via NF-κB suppression and Nrf-2-dependent heme oxygenase-1 induction. Food Chem. Toxicol. 2013, 57, 119–125. [Google Scholar] [CrossRef]
- Bogie, J.; Hoeks, C.; Schepers, M.; Tiane, A.; Cuypers, A.; Leijten, F.; Chintapakhorn, Y.; Suttiyut, T.; Pornpakakul, S.; Struik, D.; et al. Dietary Sargassum fusiforme improves memory and reduces amyloid plaque load in an Alzheimer’s disease mouse model. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef]
- DiSabato, D.; Quan, N.; Godbout, J.P. Neuroinflammation, The Devil Is in the Details. Ann. Surg. Oncol. 2021, 28, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kim, E.; Kang, I.; Lee, M.; Lee, Y. Anti-diabetic effects and anti-inflammatory effects of Laminaria japonica and Hizikia fusiforme in skeletal muscle, In vitro and in vivo model. Nutrients 2018, 10, 491. [Google Scholar] [CrossRef]
- Jia, R.; Li, Z.R.; Wu, J.; Ou, Z.R.; Liao, B.; Sun, B.; Lin, L.; Zhao, M. Mitigation mechanisms of Hizikia fusifarme polysaccharide consumption on type 2 diabetes in rats. Int. J. Biol. Macromol. 2020, 164, 2659–2670. [Google Scholar] [CrossRef]
- Polak, E.; Stępień, A.E.; Gol, O.; Tabarkiewicz, J. Potential immunomodulatory effects from consumption of nutrients in whole foods and supplements on the frequency and course of infection, Preliminary results. Nutrients 2021, 13, 1157. [Google Scholar] [CrossRef]
- Avorn, J.M.D. Learning abot the Safety of Drugs. A Half-Century of Evolution. N. Engl. J. Med. 2011, 365, 2151–2153. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.E.; Cho, J.H.; Jung, I.; Kim, H.K.; Lee, J.S. Hizikia fusiforme extract enhances dendritic cell maturation in vitro and in vivo. Biosci. Biotechnol. Biochem. 2020, 84, 1861–1869. [Google Scholar] [CrossRef]
- Yoon, Y.D.; Lee, E.S.; Park, J.P.; Kim, M.R.; Lee, J.W.; Kim, T.H.; Na, M.K.; Kim, J.H. Immunostimulatory effect by aqueous extract of Hizikia fusiforme in RAW 264.7 macrophage and whole spleen cells. Biotechnol. Bioprocess. Eng. 2011, 16, 1099–1105. [Google Scholar] [CrossRef]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis, Classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.T.; Baek, S.H.; Jeong, S.C.; Yoon, Y.D.; Kim, O.H.; Oh, B.C.; Jung, J.W.; Kim, J.H. Osteoprotective Effects of Polysaccharide-Enriched Hizikia fusiforme Processing Byproduct In Vitro and In Vivo Models. J. Med. Food 2016, 19, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Kim, I.; Nam, T. Protective Effect of Polysaccharide from Hizikia fusiformis against Ethanol-Induced Toxicity. In Advances in Food and Nutrition Research; Academic Press: New York, NY, USA, 2011; Volume 64, pp. 143–161. [Google Scholar] [CrossRef] [PubMed]
- Higham, J.; Kang, J.Y.; Majeed, A. Recent trends in admissions and mortality due to peptic ulcer in England, Increasing frequency of haemorrhage among older subjects. Gut 2002, 50, 460–464. [Google Scholar] [CrossRef] [PubMed]



| Primary Metabolite (% DW) | Reference | |||||
|---|---|---|---|---|---|---|
| Carbohydrates | Protein | Lipid | Fiber | Ash Content | Moisture | |
| 40.73 | 18.41 | nd | nd | 16.63 | nd | [21] |
| nd | 12.2 | 1.8 | 11.3 | 14 | nd | [22] |
| 61.85 ± 3.56 | 12.94 ± 3.61 | 1.76 ± 0.07 | nd | 19.18 ± 0.09 | 4.27 ± 0.12 | [23,24] |
| nd | 10.4 0 ± 0.59 | 1.38 ± 0.08 | nd | 17.89 ± 0.05 | 5.71 ± 0.34 | [25] |
| nd | 9.9 | 1.2 | nd | 40 | 9.5 | [26] |
| Metabolite Class | Dietary Content (%DW) |
|---|---|
| Major minerals | |
| Ca | 0.87–1.17 |
| Mg | 0.01–0.63 |
| K | 0.32–1.14 |
| Na | 0.16–0.83 |
| P | 0.11 |
| Trace elements | |
| Fe | 14.3–47.6 |
| Cu | 0.7 |
| Mn | 1.7 |
| Zn | 1.5–1.6 |
| Type of Polysaccharides | Mw (kDa) | Chemical Composition (%DW) | Monosaccharide Composition (Weight Ratio) | Ref. | |||
|---|---|---|---|---|---|---|---|
| Total Carbohydrate | Sulfate | Uronic Acid | Protein | ||||
| Fucoidan | 102.67 | 71.79 ± 0.56 | 27.22 ± 0.05 | nd | nd | Fuc:Rha:Glc:Man:Ara = 79.2:2.1:0.2:18.1:0.4 | [46] |
| Crude polysaccharide | 75 | 97.9 | 9.2 | 51.2 | nd | Fuc:Rha:Glc:Man:Xyl:Gal:GluA:ManA:GulA = 28.9:5.3:1:6.1:5.2:9.1:3.8:8.8:38.9 | [9] |
| Fucoidan | 90 | 67.5 | 17.5 | 41.04 | 5.22 | Fuc:Man:Xyl:Gal:Glc:GlcA = 19.2:2.6:6.6:9.6:1.0:6.5 | [47] |
| Crude polysaccharide | 229 | 42.69 | 25.69 | nd | nd | Fuc = 19.5 | [48] |
| Crude polysaccharide | 58.28 and 7.46 | 73.86 ± 0.85 | 5.17 ± 0.57 | 32.62 ± 1.43 | 0.51 ± 0.08 | Fuc:Rha:Glc:Man:Gal:GlcA = 43.9:2.5:6.5:16.3:18.7:12.1 | [49] |
| Fucoidan | 30–50 | nd | 11.60 | nd | nd | Fuc:Rha:Glc:Man:Ara:Xyl:Gal = 61.5:1.2:0.7:5:0.1:7.1:24.5 | [13] |
| Crude polysaccharide | nd | nd | 63.56 ± 0.32 | nd | nd | Fuc:Glc:Man:Xyl:Gal = 53.5:5.9:17.4:23.1 | [50] |
| Crude polysaccharide | 24 | 62.9 | 27.7 | 14.7 | 0.4 | Fuc:Man:Xyl:Gal:GalA = 80.6:2.4:3.0:13.3:0.7 | [51] |
| Crude polysaccharide | 299 | nd | 10.74 | 6.48 | nd | Fuc:Man:Xyl:Gal = 5.9:2.3:1.0:2.2 | [52] |
| Fucoidan | 47.5 | 16.8 | 20.8 | 34.6 | Nd | Fuc:Man:Xyl:Gal:GlcA = 36.6:7.0:18.3:19.1:19.1 | [53] |
| Crude polysaccharide | 224 | 58.10 ± 2.12 | 9.85 ± 0.96 | 17.66 ± 0.54 | 1.01 ± 0.15 | Fuc:Rha:Glc:Man:Xyl:Gal:Fru = 28.8:2.3:1.0:6.0:3.9:12.3:12.3 | [54] |
| Fucoidan | 205.8 | 68.33 | 14.55 | nd | 4.13 | Fuc:Rha:Glc:Man:Xyl:Gal = 16.7:1.0:1.6:1.3:1.1:6.2 | [55] |
| Chemical | Characteristics | Reference |
|---|---|---|
| Glycyrrhizin | Its metabolites, 18α-glycyrrhetinic acid and 18β-glycyrrhetinic acid | [65] |
| Phlorotannins | Total content 88.48 ± 0.30 (mg/100 mg DW) | [4] |
| Total content 43.3 µg/mL | [66] | |
| Arsenic compounds | Inorganic arsenic: arsenite [As(III)] and arsenate [As(V)], and organic arsenic: dimethylarsinic acid (DMA), monomethylarsonic acid (MMA), arsenobetaine (AsB), and arsenocholine (AsC) | [67] |
| H. fusiformis has the highest total As content compared with that of L. japonica, P. yezoensis, U. pinnatifida, E. prolifera. Contains dimethylarsinate (DMA), arsenite [As (III)], arsenate [As (V)] | [68] | |
| H. fusiformis functional oil (HFFO) | Its main components are tetradecanoic acid (11%), 9-hexadecenoic acid (2.67%), palmitic acid (37.80%), phytol (33.21%), and arachidonic acid (15.32%) | [69] |
| Fucosterol | The total concentration in normal HF extract (NH) and modified HF extract (EH) are 0.249 mg/g and 1.067 mg/g, respectively | [70] |
| Non-polar components which are extracted from methanol | [71] | |
| Saringosterol | 24R-saringosterol is an uncommon sterol in algae, with potential role in the inhibition of MG63 cell proliferation | [6] |
| Non-polar components which are extracted from methanol | [71] | |
| Fucoxanthin | Comprised of five pigments: Fx, chlorophyll-a, β-carotene, cis-fucoxanthin, and pheophytin-a. | [8] |
| Non-polar components which are extracted from methanol | [71] | |
| Alginate | Rich in M blocks and the average molecular weights of 04S2P-S and commercial alginate (Alg-S) were 55.5 kDa and 557 kDa, respectively. Sulfation modification in Alg-S produced higher molecular weights | [10] |
| Lectin (HFL) | Molecular weights (16.1 kDa) and the monosaccharide units of HFL are glucose, galactose and fucose. HFL may be linked by N-glucosidic bonds | [72] |
| Experimental Models | Extract or Constituent | Study Type | Microorganism | Effects | Ref. |
|---|---|---|---|---|---|
| nd | Phenolic Content | In vitro | Escherichia coli Staphylococcus aureus Bacillus subtilis Enterobacter aerogenes Shewanella sp. | Methanol extracts of H. fusiformis from Zhoushan and Mazu against E. coli showed moderate inhibitory activity (11–16 mm) Ethanol extract of H. fusiformis from Naozhou showed moderate inhibitory activity (11–16 mm) against B. subtilis | [98] |
| Caenorhabditis elegans | Phlorotannins | In vitro and in vivo | Chromobacterium violaceum E. coli S. aureus Aeromonas hydrophilia Vibrio parahaemolyticus Pseudomonas aeruginosa | In vitro: inhibited the anti-quorum sensing (QS) activities at 0.04858 g/mL, reduced virulence factor production and biofilm formation. In vivo: increased survival rate of P. aeruginosa-infected C. elegans to >80% during the first 4 days of treatment | [99] |
| Experimental Models | Extract or Constituent | Antioxidant Assay | Scavenging Activity (%) | Study Type | Effects | Ref |
|---|---|---|---|---|---|---|
| Sheep erythrocytes | Lectin | Hemagglutination, DPPH, hydroxyl, and ABTS+ | Hydroxyl: 33.65% and DPPH: 77.23% | In vitro | Showed free radical scavenging activity against hydroxyl, DPPH, and ABTS+ radicals | [72] |
| Vero cells and zebrafish embryos | Fucoidan | DPPH, alkyl, and hydroxyl | >80% | In vitro and in vivo | In vitro: reduced ROS level, increased cell viability, and inhibited cleavage caspase-3. In vivo: reduced ROS generation and lipid peroxidation | [102] |
| Vero cells and zebrafish embryos | Fucoidan | DPPH, hydroxyl, and alkyl | ≤80% | In vitro and in vivo | In vitro: Reduced apoptosis. In vivo: increased the survival rate and decreased the heart rate | [46] |
| Liver tissues and ICR mice | Sulfated polysaccharides | DPPH and hydroxyl | 100% | In vitro and in vivo | In vitro: Exhibited free radical scavenging activity and enhanced cell viability. In vivo: enhanced cytoprotective potential via upregulation of the Nrf2 signaling pathway | [9] |
| BALB/c mice and Liver tissues | Water-soluble polysaccharides | DPPH and hydroxyl | DPPH: >20 to ≤70% and hydroxyl: ≥20 to ≤100% | In vitro and in vivo | In vitro: showed free radical scavenging activity against hydroxyl and DPPH radicals. In vivo: reduced the MDA level and elevation of hepatic SOD activity | [103] |
| RAW 264.7 macrophages | Phenolic compounds | DPPH | <100% | In vitro | Protective effect against oxidant and inflammatory activity | [4] |
| ICR male mice | Polysaccharide | nd | nd | In vivo | Stimulated antioxidant enzymes against free radicals | [104] |
| C. elegans | Fucosterol | nd | nd | In vivo | Prolonged the lifespan of C. elegans | [105] |
| Experimental Models | Extract or Constituent | Study Type | Optimum Dose | Effects | Ref. |
|---|---|---|---|---|---|
| B16F10 mouse melanoma cells | Ethanol extract | In vitro | 400 µg/mL | Activated the intrinsic and extrinsic apoptotic pathways and the ROS-dependent pathway inactivated the PI3K/Akt signaling | [106] |
| Human prostate cancer PC3 cells | Ethanol extracts | In vitro | 100 µg/mL | Suppressed PC3 cells growth and apoptosis via regulating a ROS-dependent pathway | [107] |
| Hep3B human liver cancer cell line | Fucoidan | In vitro | 50 µg/mL | Reduced Hep3B cell growth | [7] |
| Bel7402, SMMC7721, Huh7, HT-29 and Caco-2 cells | Alginate | In vitro | nd | Inhibited the cell growth of Bel7402, SMMC7721, and HT-29 cell lines | [10] |
| Chang liver cells and zebrafish embryos | Fucoidan | In vitro and in vivo | 100 µg/mL | In vitro: increased the viability of cells, decreased ROS levels, and inhibited apoptosis. In vivo: suppressed cell death and ROS production | [108] |
| Human microvascular endothelial cells (HMEC-1) and mice | Fucoidan | In vitro and in vivo | nd | Interfered VEGF-induced angiogenesis | [53] |
| HT1080 Human fibrosarcoma cells | H. fusiformis crude extract | In vitro | 50 µg/mL | Inhibited MMP activity and intracellular MMP pathways via regulation of TIMP expression | [109] |
| Human hepatocellular carcinoma (HepG2) cells and mice | Polysaccharide | In vitro and in vivo | 2000 µg/mL | In vitro: demonstrated a high level of cytotoxicity against HepG2 cells. In vivo: significantly decreased the tumor growth | [52] |
| Experimental Models | Extract or Constituent | Study Type | Optimum Dose | Effects | Ref. |
|---|---|---|---|---|---|
| RAW264.7 macrophages, HaCaT keratinocytes, and zebrafish embryos | Fucoxanthin | In vitro and in vivo | 100 µg/mL | In vitro: decreased cell viability and cytokine levels. In vivo: decreased nitric oxide (NO), ROS, and cell death | [8] |
| RAW 264.7 cells | HF extract | In vitro | 250 µg/mL | Inhibited iNOS expression, NF-κB translocation, activated MAPKs, and STAT1 phosphorylation | [114] |
| RAW 264.7 cells and NC/Nga male mice | Fucosterol | In vitro and in vivo | 50 µg/mL | In vitro: reduced NO production. In vivo: regulated the Th1/Th2 immune balance and reduced systemic inflammation | [115] |
| Male BALB/c mice | Ethyl acetate (EA) extract | In vitro and in vivo | 100 µg/mL | In vitro: inhibited activation of T cell activation by eliminating NFAT dephosphorylation. In vivo: inhibited activation of T cell activation by suppressing Th cell-dependent cytokines | [116] |
| BALB/c mice | HF extract | In vivo | nd | Suppressed T-helper type 2 cytokine production (IL-13) | [117] |
| Macrophage cell line RAW 264.7 | Phlorotannin | In vitro | 43.3 µg/mL | Inhibited the production of pro-inflammatory mediators | [66] |
| Experimental Models | Extract or Constituent | Study Type | Optimum Dose | Effects | Ref. |
|---|---|---|---|---|---|
| Human dermal fibroblast (HDF) cells exposed to UVB (50 mJ/cm2) and zebrafish larvae | Fucoidan | In vitro and in vivo | ≤50 μg/mL | In vitro: suppressed cell death, MMPs, PGE2, and pro-inflammatory cytokines and elevated collagen. In vivo: reduced ROS levels and inflammatory responses | [120] |
| RAW 264.7 cell line and B16F10 cell line | Crude sulfated polysaccharides | In vitro | 100 μg/mL | Inhibited lipopolysaccharide (LPS)-induced inflammation, and reduced α-MSH-stimulated melanogenesis. | [121] |
| Normal human dermal fibroblasts (NHDFs) | Fucosterol | In vitro | nd | Reduced the UVB- induced expression of MMP-1, IL-6, p-c-Jun, and p-c-Fos, and increased type I procollagen expression | [23] |
| Human keratinocytes (HaCaT cells) and B16F10 melanoma cells | Fucoidan | In vitro | 100 μg/mL | Reduced ROS levels, enhanced cell viability, suppressed UVB-induced apoptosis in HaCaT cells and inhibited melanin biosynthesis | [122] |
| Human dermal fibroblasts | Sulfated polysaccharides | In vitro | ≤50 μg/mL | In vitro: suppressed cell death, MMPs, PGE2, and pro-inflammatory cytokines and elevated collagen. In vivo: reduced ROS levels and inflammatory responses | [50] |
| Kun Ming Mice | Polysaccharide | In vivo | 600 mg/kg/day | Exhibited protection against UVB due to decreased oxidative stress | [54] |
| Experimental Models | Extract or Chemical or Constituent | Study Type | Optimum Dose | Effects | Ref. |
|---|---|---|---|---|---|
| BV-2 cells (mouse microglia) | H. fusiformis functional oil (HFFO) | In vitro and in silico | 20 μg/mL | Inhibited acetylcholinesterase (AChE) and nitric oxide (NO) production, reduced ROS levels | [69] |
| Cell sample | Glycyrrhizin | In vitro and in silico | nd | Inhibited BACE1 activity | [65] |
| Murine BV-2 microglial cells | HF extract | In vitro | 2 mg/mL | Increased the NO levels. Inhibited iNOS expression, pro-inflammatory cytokines, and expression of NF-κB activation | [123] |
| Murine BV2 microglia | Nd | In vitro | 500 ng/mL | Suppressed LPS-induced iNOS expression | [124] |
| Male ICR mice | Polysaccharide | In vivo | nd | Improved cognitive abilities | [47] |
| Male ICR mice | H. fusiformis or its extract | In vivo | 5 µg/mL | Enhanced cognition and alleviated disease | [125] |
| Experimental Models | Extract or Chemical or Constituent | Study Type | Optimum Dose | Effects | Ref. |
|---|---|---|---|---|---|
| C57BL/6N muscle tissue (mice) and C2C12 myotube cells (mice) | Polyphenols | In vitro and in vivo | 100 µg/mL | In vitro: reduced α-glucosidase activity. In vivo: enhanced muscle glucose uptake, activated insulin signaling-related proteins | [127] |
| Male SD rats | Polysaccharide Extract | In vivo | nd | Improved hypoglycemic activity via restoration of insulin resistance and mitochondrial function of skeletal muscle | [49] |
| Male SD rats | Polysaccharide | In vivo | nd | Enhanced storage of glycogen in liver and skeletal muscle and suppressed gluconeogenesis | [128] |
| Human recombinant PTP1B | Methanol extract | In vitro | nd | PTP1B and α-glucosidase inhibitors | [71] |
| Mice | Fucoidan | In vivo | nd | Reduced fasting blood glucose levels, diet, and water intake | [55] |
| Experimental Models | Extract or Constituent | Study Type | Optimum Dose | Effects | Ref. |
|---|---|---|---|---|---|
| Dendritic cells (DCs) and C57BL/6 (8 weeks) mice | HF extract | In Vitro and in vivo | 5 μg/mL | In vitro: induced functional and phenotypical maturation of DCs. In vivo: activated CD8+ T cells | [131] |
| Murine macrophages Raw 264.7 cells and splenocytes | Polysaccharide | In vitro | 1 mg/mL | Increased NO production and pro-inflammatory cytokine levels | [11] |
| Murine macrophages and C57BL/6 mice | Fucoidan and fucosterol | In Vitro and in vivo | 100 μg/mL | In vitro: increased production of NO, secretion of tumor necrosis factor-α (TNF-α), and phagocytosis activity. In vivo: stimulated splenocyte proliferation and restored the level of cytokines | [70] |
| RAW 264.7 macrophages and C57/BL6 mice | Lipopolysac- charide | In vitro and in vivo | 5 μg/mL | In vitro: increased cytokine expression. In vivo: regulated the immune function | [132] |
| RAW264.7 cells | Polysaccharide | In vitro | nd | Upregulated cytokine production and activation of the NF-κB signaling pathway | [51] |
| Experimental Models | Extract or Constituent | Study Type | Optimum Dose | Effects | Ref. |
|---|---|---|---|---|---|
| Cartilage cells and Male SD rats | HF extract | In vitro and in vivo | 600–1000 μg/mL | In vitro: reduced pro-inflammatory cytokine responses. In vivo: reduced the articular cartilage damage and development of OA | [12] |
| Male SD rats | Fucoidan | In vivo | 100 mg/kg | Prevented OA progression by decreasing bone loss, prevented articular cartilage inflammation, and increased cytokine levels | [13] |
| Mouse C2C12 cells, bone marrow cells, zebrafish embryos, ovariectomized (OVX) mice, mouse calvarial bone | Polysaccharide | In vitro and in vivo | 200 μg/mL | In vitro: enhanced osteoblast differentiation via alkaline phosphatase (ALP) and bone morphogenetic protein-2 (BMP-2) stimulation, suppressed osteoclast differentiation. In vivo: increased BMP2a and 2b levels, protected bone mass loss, and increased bone regeneration | [134] |
| MG63 cells | Fucosterol | In vitro | nd | Protective activity through bone-resorbent metabolic bone disorders | [6] |
| Experimental Models | Extract or Constituent | Study Type | Optimum Dose | Effects | Ref. |
|---|---|---|---|---|---|
| IEC-6 cells and SD rats | Polysaccharide Extract | In vitro and in vivo | 500 μg/mL | Decreased the phosphorylation of Shc and JNK | [24] |
| IEC-6 cells and SD rats | Polysaccharide Extract | In vitro and in vivo | 300 mg/kg | In vitro: reduced phosphorylation of Shc. In vivo: reduced total glutathione levels and enhanced JNK phosphorylation | [135] |
| Wistar albino adult male rats | Polysaccharide | In vivo | 300 mg/kg | Suppressed oxidative stress and showed anti-inflammatory and antioxidant activity | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meinita, M.D.N.; Harwanto, D.; Sohn, J.-H.; Kim, J.-S.; Choi, J.-S. Hizikia fusiformis: Pharmacological and Nutritional Properties. Foods 2021, 10, 1660. https://doi.org/10.3390/foods10071660
Meinita MDN, Harwanto D, Sohn J-H, Kim J-S, Choi J-S. Hizikia fusiformis: Pharmacological and Nutritional Properties. Foods. 2021; 10(7):1660. https://doi.org/10.3390/foods10071660
Chicago/Turabian StyleMeinita, Maria Dyah Nur, Dicky Harwanto, Jae-Hak Sohn, Jin-Soo Kim, and Jae-Suk Choi. 2021. "Hizikia fusiformis: Pharmacological and Nutritional Properties" Foods 10, no. 7: 1660. https://doi.org/10.3390/foods10071660
APA StyleMeinita, M. D. N., Harwanto, D., Sohn, J.-H., Kim, J.-S., & Choi, J.-S. (2021). Hizikia fusiformis: Pharmacological and Nutritional Properties. Foods, 10(7), 1660. https://doi.org/10.3390/foods10071660