Investigation of Immunomodulatory and Gut Microbiota-Altering Properties of Multicomponent Nutraceutical Prepared from Lactic Acid Bacteria, Bovine Colostrum, Apple Production By-Products and Essential Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Nutraceutical Composition
2.2. In Vivo Experiment Design
2.3. Sample Collection
2.4. Cholesterol, Immunoglobulin and Weight Gain Measurements
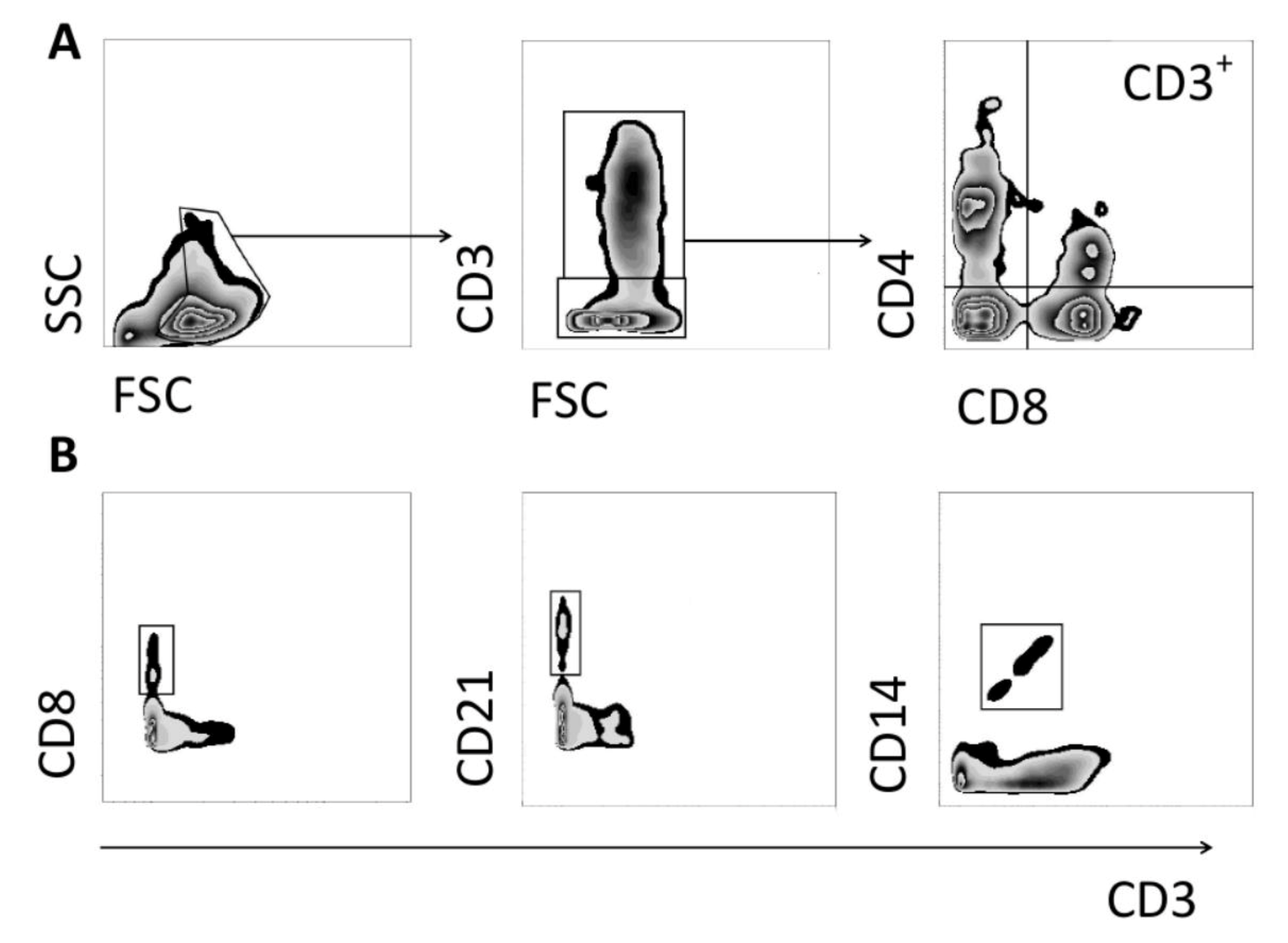
2.5. Peripheral Blood Mononuclear Cell Isolation, Cell Staining and Flow Cytometry
2.6. Metagenomics and Microbial Profiling Analysis
2.7. Statistical Analysis
3. Results
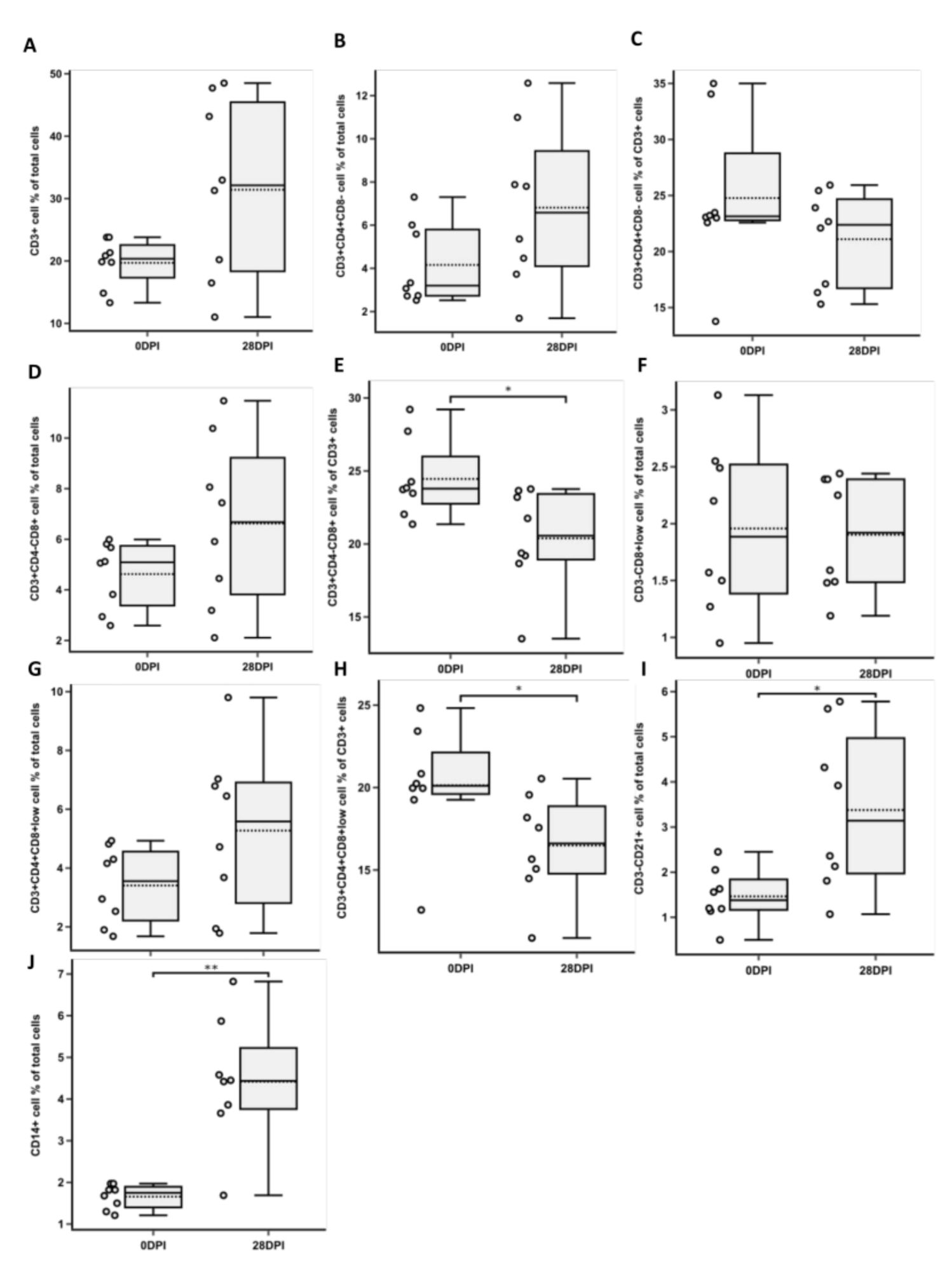
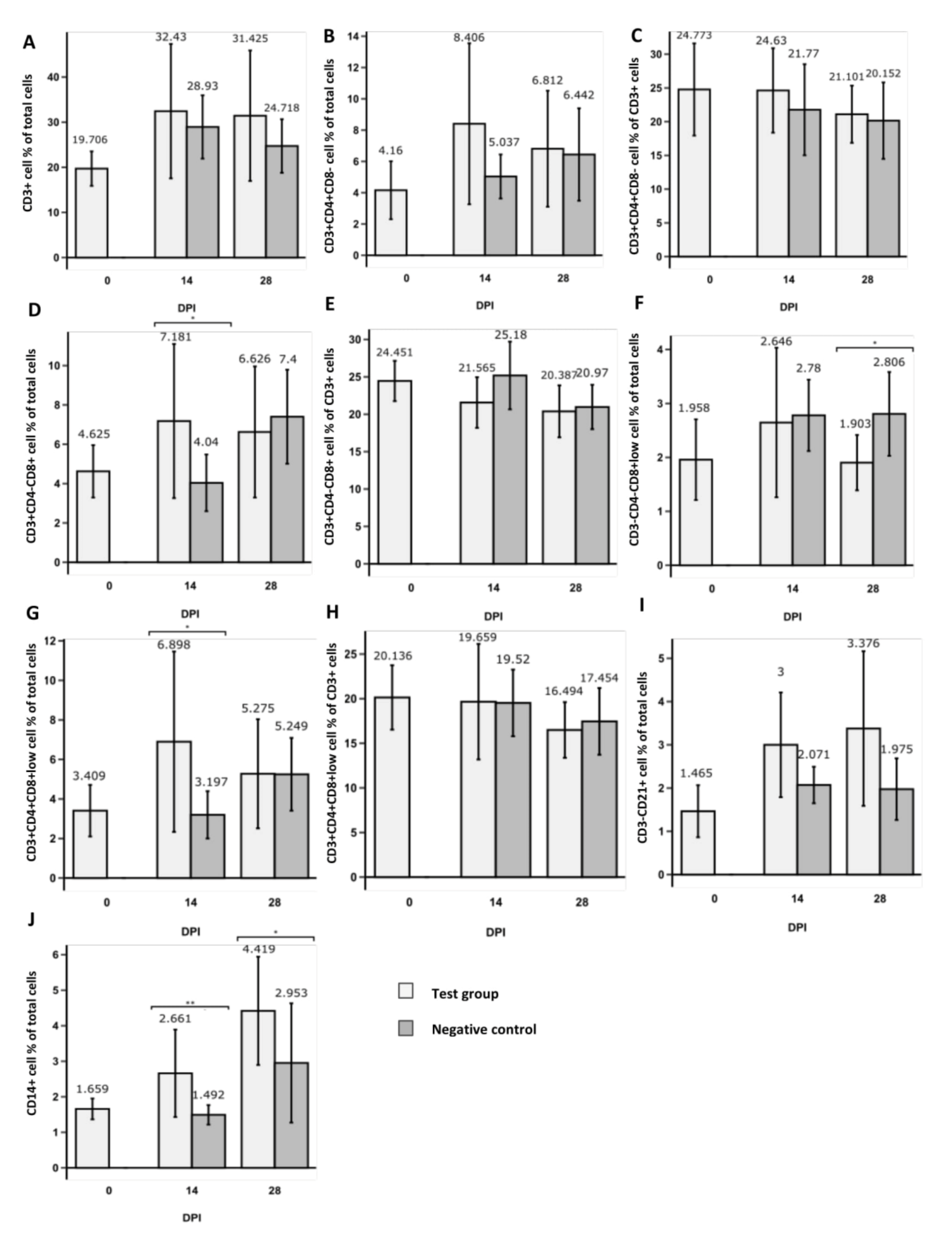
3.1. Changes in CD3+ T Cell Population Phenotypes
3.2. Changes in NK and B Cell Populations
3.3. Changes in CD14+ Population
3.4. Changes in Immunoglobulin, Cholesterol and Weight Gain Measurements
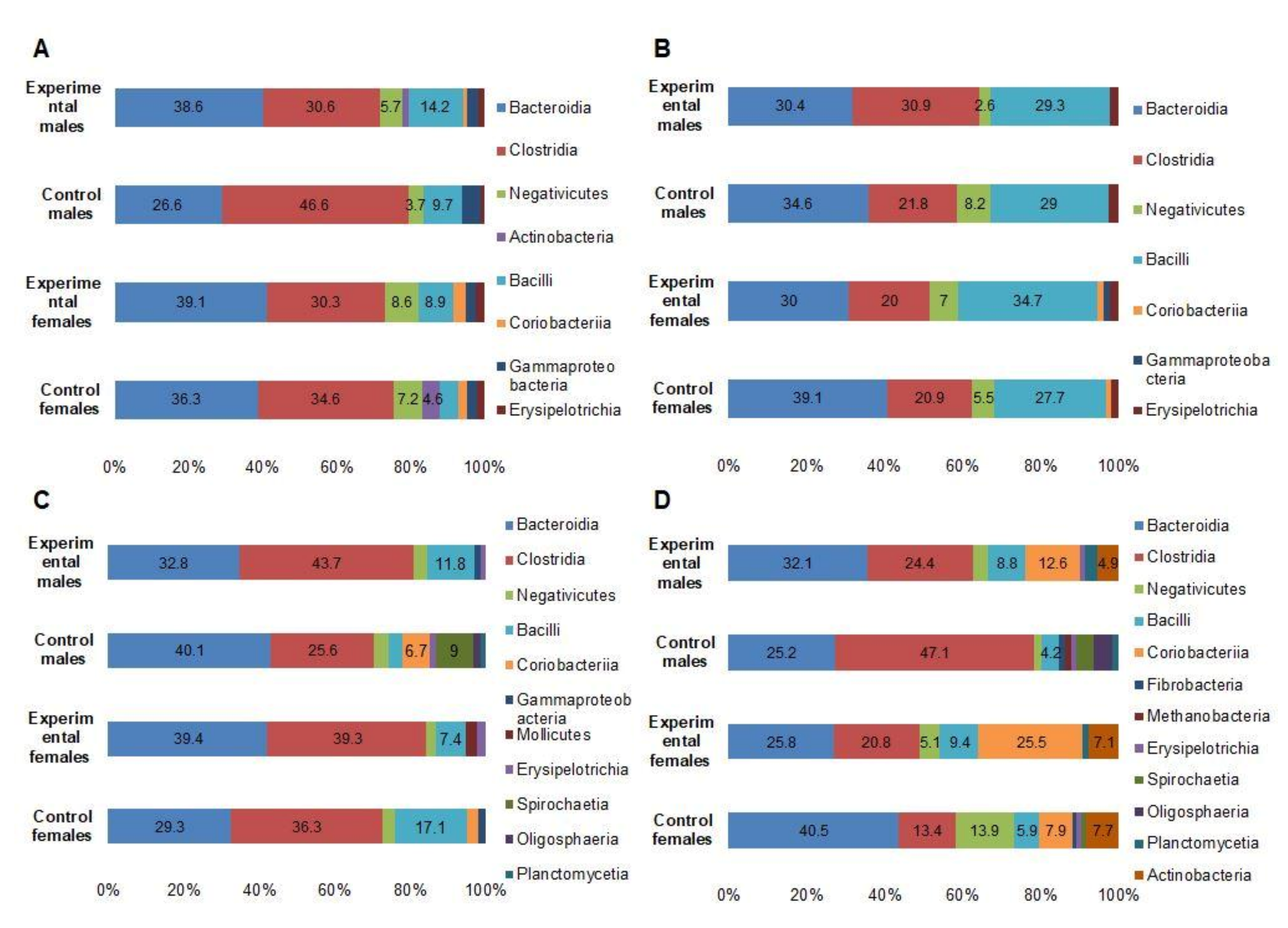
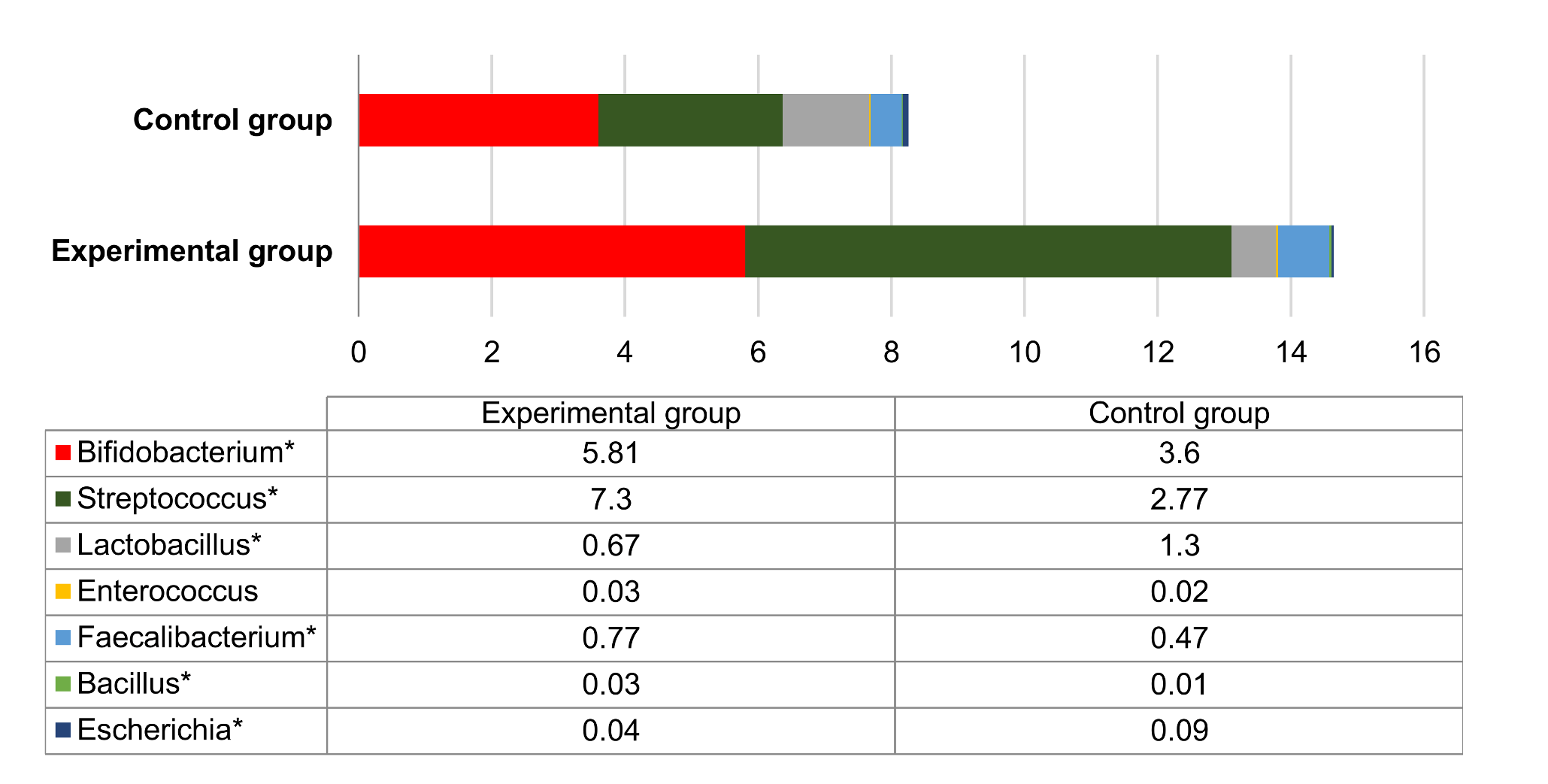
3.5. Microbial Profiles
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gul, K.; Singh, A.K.; Jabeen, R. Nutraceuticals and Functional Foods: The Foods for the Future World. Crit. Rev. Food Sci. Nutr. 2016, 56, 2617–2627. [Google Scholar] [CrossRef]
- Van Hees, H.M.J.; Davids, M.; Maes, D.; Millet, S.; Possemiers, S.; den Hartog, L.A.; van Kempen, T.A.T.G.; Janssens, G.P.J. Dietary fibre enrichment of supplemental feed modulates the development of the intestinal tract in suckling piglets. J. Anim. Sci. Biotechnol. 2019, 10, 83. [Google Scholar] [CrossRef]
- Hjelmsø, M.H.; Shah, S.A.; Thorsen, J.; Rasmussen, M.; Vestergaard, G.; Mortensen, M.S.; Brejnrod, A.; Brix, S.; Chawes, B.; Bønnelykke, K.; et al. Prenatal dietary supplements influence the infant airway microbiota in a randomized factorial clinical trial. Nat. Commun. 2020, 11, 426. [Google Scholar] [CrossRef] [PubMed]
- Reid, G. Probiotics: Definition, scope and mechanisms of action. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 17–25. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Raone, B.; Raboni, R.; Patrizi, A. Probiotics Reduce Gut Microbial Translocation and Improve Adult Atopic Dermatitis. J. Clin. Gastroenterol. 2014, 48, 95–96. [Google Scholar] [CrossRef]
- Kim, J.-E.; Chae, C.S.; Kim, G.-C.; Hwang, W.; Hwang, J.; Hwang, S.-M.; Kim, Y.; Ahn, Y.-T.; Park, S.-G.; Jun, C.-D.; et al. Lactobacillus helveticus suppresses experimental rheumatoid arthritis by reducing inflammatory T cell responses. J. Funct. Foods 2015, 13, 350–362. [Google Scholar] [CrossRef]
- Lavasani, S.; Dzhambazov, B.; Nouri, M.; Fåk, F.; Buske, S.; Molin, G.; Thorlacius, H.; Alenfall, J.; Jeppsson, B.; Weström, B. A Novel Probiotic Mixture Exerts a Therapeutic Effect on Experimental Autoimmune Encephalomyelitis Mediated by IL-10 Producing Regulatory T Cells. PLoS ONE 2010, 5, e9009. [Google Scholar] [CrossRef]
- Ghadimi, D.; Fölster-Holst, R.; de Vrese, M.; Winkler, P.; Heller, K.J.; Schrezenmeir, J. Effects of probiotic bacteria and their genomic DNA on TH1/TH2-cytokine production by peripheral blood mononuclear cells (PBMCs) of healthy and allergic subjects. Immunobiology 2008, 213, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Park, B.-K.; Park, H.-J.; Park, Y.-H.; Kim, B.-O.; Pyo, S. Atopic dermatitis-mitigating effects of new Lactobacillus strain, Lactobacillus sakei probio 65 isolated from Kimchi. J. Appl. Microbiol. 2013, 115, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Talpur, A.D.; Munir, M.B.; Mary, A.; Hashim, R. Dietary probiotics and prebiotics improved food acceptability, growth performance, haematology and immunological parameters and disease resistance against Aeromonas hydrophila in snakehead (Channa striata) fingerlings. Aquaculture 2014, 426–427, 14–20. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Mun, H.-S.; Islam, M.M.; Kim, S.-S.; Hwang, J.-A.; Kim, Y.-J.; Yang, C.-J. Effects of Citrus junos by-products fermented with multistrain probiotics on growth performance, immunity, caecal microbiology and meat oxidative stability in broilers. Br. Poult. Sci. 2014, 55, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, A.N.; Chattha, K.S.; Kandasamy, S.; Liu, Z.; Esseili, M.; Shao, L.; Rajashekara, G.; Saif, L.J. Lactobacilli and Bifidobacteria Promote Immune Homeostasis by Modulating Innate Immune Responses to Human Rotavirus in Neonatal Gnotobiotic Pigs. PLoS ONE 2013, 8, e76962. [Google Scholar] [CrossRef]
- Cox, M.J.; Huang, Y.J.; Fujimura, K.E.; Liu, J.T.; McKean, M.; Boushey, H.A.; Segal, M.R.; Brodie, E.L.; Cabana, M.D.; Lynch, S.V. Lactobacillus casei Abundance Is Associated with Profound Shifts in the Infant Gut Microbiome. PLoS ONE 2010, 5, e8745. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Pontin, M.C.F.; Kosmerl, E.; Jimenez-Flores, R.; Moretti, D.B.; Ziouzenkova, O. Assessment of adipogenic, antioxidant, and anti-inflammatory properties of whole and whey bovine colostrum. J. Dairy Sci. 2019, 102, 8614–8621. [Google Scholar] [CrossRef] [PubMed]
- Menchetti, L.; Traina, G.; Tomasello, G.; Casagrande-Proietti, P.; Leonardi, L.; Barbato, O.; Brecchia, G. Potential benefits of colostrum in gastrointestinal diseases. Front. Biosci. 2016, 8, 331–351. [Google Scholar]
- Steele, J.; Sponseller, J.; Schmidt, D.; Cohen, O.; Tzipori, S. Hyperimmune bovine colostrum for treatment of GI infections: A review and update on Clostridium difficile. Hum. Vaccines Immunother. 2013, 9, 1565–1568. [Google Scholar] [CrossRef]
- Actor, J.K.; Hwang, S.-A.; Kruzel, M.L. Lactoferrin as a Natural Immune Modulator. Curr. Pharm. Des. 2009, 15, 1956–1973. [Google Scholar] [CrossRef]
- Kanda, T.; Akiyama, H.; Yanagida, A.; Tanabe, M.; Goda, Y.; Toyoda, M.; Teshima, R.; Saito, Y. Inhibitory Effects of Apple Polyphenol on Induced Histamine Release from RBL-2H3 Cells and Rat Mast Cells. Biosci. Biotechnol. Biochem. 1998, 62, 1284–1289. [Google Scholar] [CrossRef]
- Akiyama, H.; Sato, Y.; Watanabe, T.; Nagaoka, M.H.; Yoshioka, Y.; Shoji, T.; Kanda, T.; Yamada, K.; Tosuka, M.; Teshima, R.; et al. Dietary unripe apple polyphenol inhibits the development of food allergies in murine models. FEBS Lett. 2005, 579, 4485–4491. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Akiyama, H.; Nakano, M.; Shoji, T.; Kanda, T.; Ohtake, Y.; Takita, T.; Matsuda, R.; Maitani, T. Orally administered apple procyanidins protect against experimental inflammatory bowel disease in mice. Int. Immunopharmacol. 2008, 8, 1802–1807. [Google Scholar] [CrossRef]
- Nakamura, K.; Matsuoka, H.; Nakashima, S.; Kanda, T.; Nishimaki-Mogami, T.; Akiyama, H. Oral administration of apple condensed tannins delays rheumatoid arthritis development in mice via downregulation of T helper 17 (Th17) cell responses. Mol. Nutr. Food Res. 2015, 59, 1406–1410. [Google Scholar] [CrossRef]
- Koutsos, A.; Lima, M.; Conterno, L.; Gasperotti, M.; Bianchi, M.; Fava, F.; Vrhovsek, U.; Lovegrove, J.A.; Tuohy, K.M. Effects of Commercial Apple Varieties on Human Gut Microbiota Composition and Metabolic Output Using an In Vitro Colonic Model. Nutrients 2017, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, K.; Ohashi, Y.; Kawasumi, K.; Terada, A.; Fujisawa, T. Effect of apple intake on fecal microbiota and metabolites in humans. Anaerobe 2010, 16, 510–515. [Google Scholar] [CrossRef]
- Garcia-Mazcorro, J.F.; Pedreschi, R.; Yuan, J.; Kawas, J.R.; Chew, B.; Dowd, S.E.; Noratto, G. Apple consumption is associated with a distinctive microbiota, proteomics and metabolomics profile in the gut of Dawley Sprague rats fed a high-fat diet. PLoS ONE 2019, 14, e0212586. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Tan, B.; Liu, Y.; Dunn, J.; Guerola, P.M.; Tortajada, M.; Cao, Z.; Ji, P. Chemical Composition and Antioxidant Properties of Essential Oils from Peppermint, Native Spearmint and Scotch Spearmint. Molecules 2019, 24, 2825. [Google Scholar] [CrossRef]
- Veenstra, J.P.; Johnson, J.J. Oregano (Origanum vulgare) extract for food preservation and improvement in gastrointestinal health. Int. J. Nutr. 2019, 3, 43–52. [Google Scholar] [CrossRef]
- Oniga, I.; Pușcaș, C.; Silaghi-Dumitrescu, R.; Olah, N.-K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E.; et al. Origanum vulgare ssp. vulgare: Chemical Composition and Biological Studies. Molecules 2018, 23, 2077. [Google Scholar] [CrossRef]
- Buleandra, M.; Oprea, E.; Popa, D.E.; David, I.G.; Moldovan, Z.; Mihai, I.; Badea, I.A. Comparative Chemical Analysis of Mentha piperita and M. spicata and a Fast Assessment of Commercial Peppermint Teas. Nat. Prod. Commun. 2016, 11, 1934578. [Google Scholar] [CrossRef]
- Borugă, O.; Jianu, C.; Mişcă, C.; Goleţ, I.; Gruia, A.; Horhat, F. Thymus vulgaris essential oil: Chemical composition and antimicrobial activity. J. Med. Life 2014, 7, 56–60. [Google Scholar]
- Gedikoğlu, A.; Sökmen, M.; Çivit, A. Evaluation of Thymus vulgaris and Thymbra spicata essential oils and plant extracts for chemical composition, antioxidant, and antimicrobial properties. Food Sci. Nutr. 2019, 7, 1704–1714. [Google Scholar] [CrossRef]
- Gonçalves, N.D.; Pena, F.D.L.; Sartoratto, A.; Derlamelina, C.; Duarte, M.C.T.; Antunes, A.E.C.; Prata, A.S. Encapsulated thyme (Thymus vulgaris) essential oil used as a natural preservative in bakery product. Food Res. Int. 2017, 96, 154–160. [Google Scholar] [CrossRef]
- Almanea, A.; El-Aziz, G.S.A.; Ahmed, M.M.M. The Potential Gastrointestinal Health Benefits of Thymus Vulgaris Essential Oil: A Review. Biomed. Pharmacol. J. 2019, 12, 1793–1799. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Ruzauskas, M.; Stankevicius, A.; Grigas, J.; Pautienius, A.; Bernatoniene, J.; Jakstas, V.; et al. Fermented, ultrasonicated, and dehydrated bovine colostrum: Changes in antimicrobial properties and immunoglobulin content. J. Dairy Sci. 2020, 103, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.J.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikiene, G.; et al. Lactic Acid Bacteria Isolation from Spontaneous Sourdough and Their Characterization Including Antimicrobial and Antifungal Properties Evaluation. Microorganisms 2020, 8, 64. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Ruzauskas, M.; Bernatoniene, J.; Jakstas, V.; Viskelis, P.; Zadeike, D.; Juodeikiene, G. Improvement of the antimicrobial activity of lactic acid bacteria in combination with berries/fruits and dairy industry by-products. J. Sci. Food Agric. 2019, 99, 3992–4002. [Google Scholar] [CrossRef]
- Bartkiene, E.; Ruzauskas, M.; Lele, V.; Zavistanaviciute, P.; Bernatoniene, J.; Jakstas, V.; Ivanauskas, L.; Zadeike, D.; Klupsaite, D.; Viskelis, P.; et al. Development of antimicrobial gummy candies with addition of bovine colostrum, essential oils and probiotics. Int. J. Food Sci. Technol. 2018, 53, 1227–1235. [Google Scholar] [CrossRef]
- Aindelis, G.; Tiptiri-Kourpeti, A.; Lampri, E.; Spyridopoulou, K.; Lamprianidou, E.; Kotsianidis, I.; Ypsilantis, P.; Pappa, A.; Chlichlia, K. Immune Responses Raised in an Experimental Colon Carcinoma Model Following Oral Administration of Lactobacillus casei. Cancers 2020, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Shing, C.M.; Peake, J.M.; Suzuki, K.; Jenkins, D.G.; Coombes, J.S. Bovine Colostrum Modulates Cytokine Production in Human Peripheral Blood Mononuclear Cells Stimulated with Lipopolysaccharide and Phytohemagglutinin. J. Interferon. Cytokine Res. 2008, 29, 37–44. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, A.-M.; Liu, Y.; Moore, K.W.; Mui, A.L.-F. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: Evidence for Stat3-dependent and -independent pathways. EMBO J. 1998, 17, 1006–1018. [Google Scholar] [CrossRef]
- Gerner, W.; Käser, T.; Saalmüller, A. Porcine T lymphocytes and NK cells—an update. Dev. Comp. Immunol. 2009, 33, 310–320. [Google Scholar] [CrossRef]
- Mañes-Lázaro, R.; Ferrer, S.; Rosselló-Mora, R.; Pardo, I. Lactobacillus uvarum sp. nov.—A new lactic acid bacterium isolated from Spanish Bobal grape must. Syst. Appl. Microbiol. 2008, 31, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Choe, W.; Durgannavar, T.A.; Chung, S.J. Fc-Binding Ligands of Immunoglobulin G: An Overview of High Affinity Proteins and Peptides. Materials 2016, 9, 994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wen, K.; Azevedo, M.S.P.; Gonzalez, A.; Saif, L.J.; Li, G.; Yousef, A.E.; Yuan, L. Lactic acid bacterial colonization and human rotavirus infection influence distribution and frequencies of monocytes/macrophages and dendritic cells in neonatal gnotobiotic pigs. Vet. Immunol. Immunopathol. 2008, 121, 222–231. [Google Scholar] [CrossRef]
- Park, M.-K.; Ngo, V.; Kwon, Y.-M.; Lee, Y.-T.; Yoo, S.; Cho, Y.-H.; Hong, S.-M.; Hwang, H.S.; Ko, E.-J.; Jung, Y.-J.; et al. Lactobacillus plantarum DK119 as a Probiotic Confers Protection against Influenza Virus by Modulating Innate Immunity. PLoS ONE 2013, 8, e75368. [Google Scholar] [CrossRef] [PubMed]
- Malefyt, R.d.W.; Abrams, J.; Bennett, B.; Figdor, C.G.; de Vries, J.E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: An autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991, 174, 1209–1220. [Google Scholar] [CrossRef]
- Hyson, D.A. A Comprehensive Review of Apples and Apple Components and Their Relationship to Human Health12. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef]
- Hendriks, J.J.A.; Alblas, J.; van der Pol, S.M.A.; van Tol, E.A.F.; Dijkstra, C.D.; de Vries, H.E. Flavonoids Influence Monocytic GTPase Activity and Are Protective in Experimental Allergic Encephalitis. J. Exp. Med. 2004, 200, 1667–1672. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2015, 6, 620. [Google Scholar] [CrossRef]
- Pajarillo, E.A.B.; Chae, J.-P.; Balolong, M.P.; Kim, H.B.; Kang, D.-K. Assessment of fecal bacterial diversity among healthy piglets during the weaning transition. J. Gen. Appl. Microbiol. 2014, 60, 140–146. [Google Scholar] [CrossRef]
- Wang, X.; Tsai, T.; Deng, F.; Wei, X.; Chai, J.; Knapp, J.; Apple, J.; Maxwell, C.V.; Lee, J.A.; Li, Y.; et al. Longitudinal investigation of the swine gut microbiome from birth to market reveals stage and growth performance associated bacteria. Microbiome 2019, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Hou, C.; Zeng, X.; Qiao, S. The Use of Lactic Acid Bacteria as a Probiotic in Swine Diets. Pathogens 2015, 4, 34–45. [Google Scholar] [CrossRef]
- Daillère, R.; Vétizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.P.; Flament, C.; Lepage, P.; Roberti, M.P.; et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity 2016, 45, 931–943. [Google Scholar] [CrossRef]
- Ding, X.; Lan, W.; Liu, G.; Ni, H.; Gu, J.-D. Exploring possible associations of the intestine bacterial microbiome with the pre-weaned weight gaining performance of piglets in intensive pig production. Sci. Rep. 2019, 9, 15534. [Google Scholar] [CrossRef] [PubMed]
- Kraatz, M.; Wallace, R.J.; Svensson, L. Olsenella umbonata sp. nov., a microaerotolerant anaerobic lactic acid bacterium from the sheep rumen and pig jejunum, and emended descriptions of Olsenella, Olsenella uli and Olsenella profusa. Int. J. Syst. Evol. Microbiol. 2011, 61, 795–803. [Google Scholar] [CrossRef]

| Parameter | Control Group | Test Group |
|---|---|---|
| Weight gain (kg) | 10.71 ± 0.99 | 11.65 ± 1.30 |
| IgA (g/L) | 0.078 ± 0.06 | 0.11 ± 0.04 |
| IgG (g/L) * | 3.90 ± 0.48 | 4.68 ± 0.42 |
| IgM (g/L) | 0.57 ± 0.17 | 0.73 ± 0.25 |
| Total cholesterol (mmol/l) * | 1.89 ± 0.38 | 2.34 ± 0.34 |
| HDL cholesterol (mmol/l) | 0.68 ± 0.26 | 0.85 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigas, J.; Ruzauskas, M.; Pautienius, A.; Bartkiene, E.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Zokaityte, E.; Bernatoniene, J.; Ivanauskas, L.; et al. Investigation of Immunomodulatory and Gut Microbiota-Altering Properties of Multicomponent Nutraceutical Prepared from Lactic Acid Bacteria, Bovine Colostrum, Apple Production By-Products and Essential Oils. Foods 2021, 10, 1313. https://doi.org/10.3390/foods10061313
Grigas J, Ruzauskas M, Pautienius A, Bartkiene E, Lele V, Starkute V, Zavistanaviciute P, Zokaityte E, Bernatoniene J, Ivanauskas L, et al. Investigation of Immunomodulatory and Gut Microbiota-Altering Properties of Multicomponent Nutraceutical Prepared from Lactic Acid Bacteria, Bovine Colostrum, Apple Production By-Products and Essential Oils. Foods. 2021; 10(6):1313. https://doi.org/10.3390/foods10061313
Chicago/Turabian StyleGrigas, Juozas, Modestas Ruzauskas, Arnoldas Pautienius, Elena Bartkiene, Vita Lele, Vytaute Starkute, Paulina Zavistanaviciute, Egle Zokaityte, Jurga Bernatoniene, Liudas Ivanauskas, and et al. 2021. "Investigation of Immunomodulatory and Gut Microbiota-Altering Properties of Multicomponent Nutraceutical Prepared from Lactic Acid Bacteria, Bovine Colostrum, Apple Production By-Products and Essential Oils" Foods 10, no. 6: 1313. https://doi.org/10.3390/foods10061313
APA StyleGrigas, J., Ruzauskas, M., Pautienius, A., Bartkiene, E., Lele, V., Starkute, V., Zavistanaviciute, P., Zokaityte, E., Bernatoniene, J., Ivanauskas, L., Jakstas, V., & Stankevicius, A. (2021). Investigation of Immunomodulatory and Gut Microbiota-Altering Properties of Multicomponent Nutraceutical Prepared from Lactic Acid Bacteria, Bovine Colostrum, Apple Production By-Products and Essential Oils. Foods, 10(6), 1313. https://doi.org/10.3390/foods10061313











