Recent Developments in Seafood Packaging Technologies
Abstract
1. Introduction
2. Composition and Structure of Seafood
3. Seafood Spoilage
3.1. Spoilage Due to ENZYMIC Autolysis
3.2. Microbial Spoilage
3.3. Oxidation and Hydrolysis
4. Seafood Packaging Technologies
4.1. Modified Atmosphere Packaging
4.1.1. Use of MAP for Fish Preservation
4.1.2. Use of Modified Atmosphere Packaging for the Preservation of Fishery Products
4.1.3. Use of Modified Atmosphere Packaging to Control Food Pathogens in Seafood
4.2. Vacuum Packaging
4.2.1. Use of Vacuum Packaging for Fish Preservation
4.2.2. Use of Vacuum Packaging for the Preservation of Fishery Products
4.3. Active Packaging
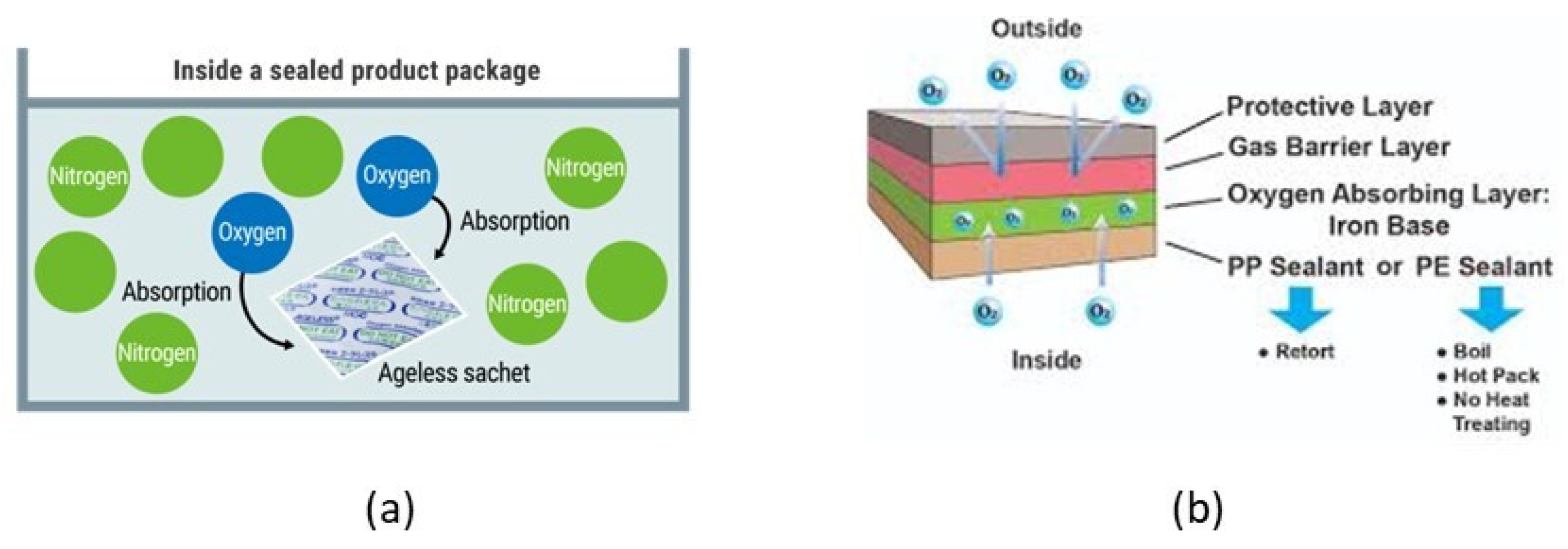
4.3.1. O2-Scavengers
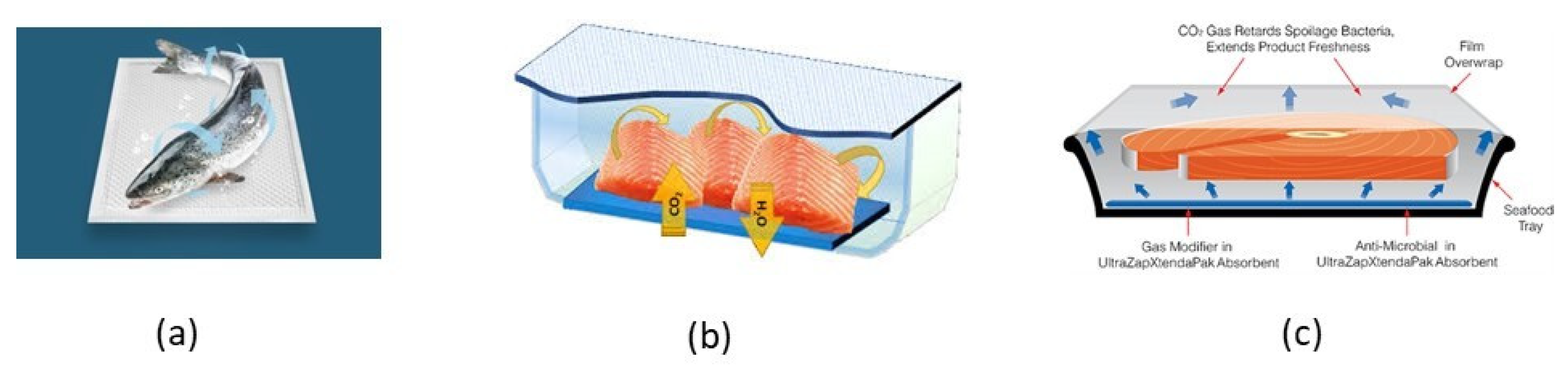
4.3.2. CO2-Emitters
4.3.3. Moisture Regulators
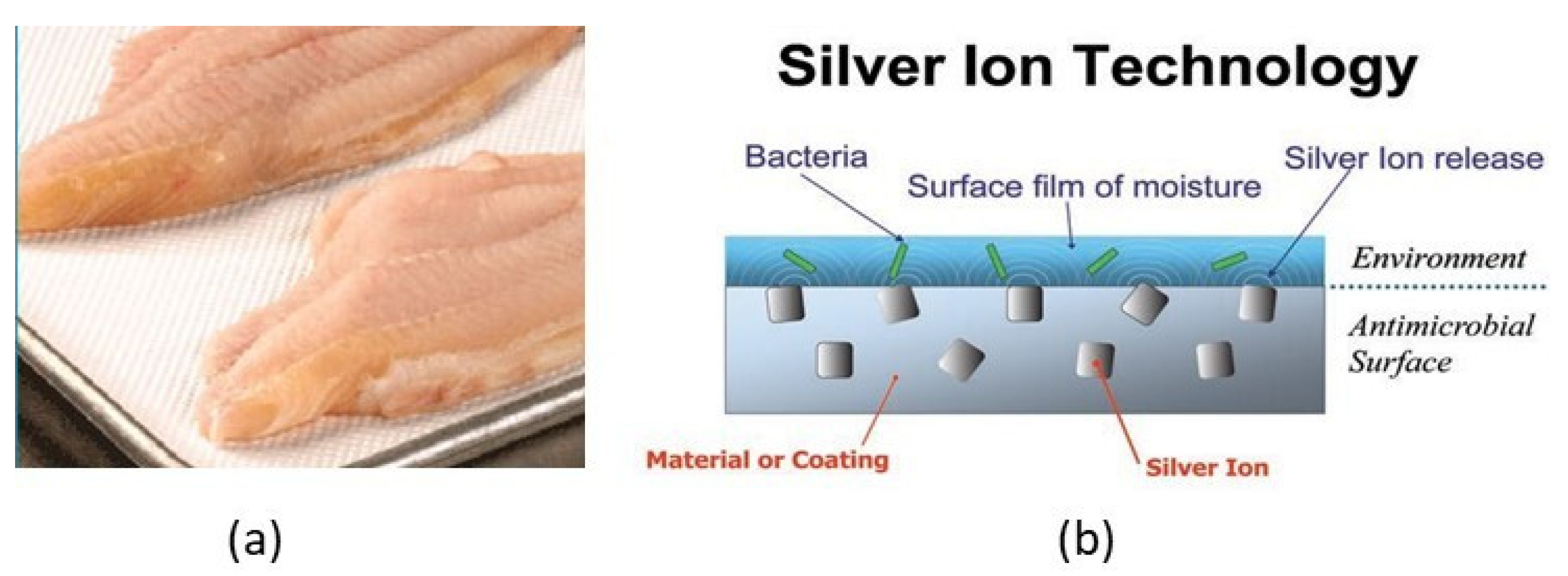
4.3.4. Antimicrobial Packaging
4.3.5. Antioxidant Packaging
4.3.6. Active Packaging Systems with Multiple Functionality
4.3.7. Use of Active Packaging to Seafood Products Preservation
4.4. Intelligent Packaging
4.4.1. Freshness Indicators
4.4.2. Time–Temperature Indicators (TTI)
- be activated in a simple way;
- provide a measurable change, which is a function of time and temperature;
- provide a short and irreversible response;
- correlate well with the degree of food deterioration.
4.4.3. Leakage Indicators
4.4.4. Use of Intelligent Packaging in Seafood Preservation
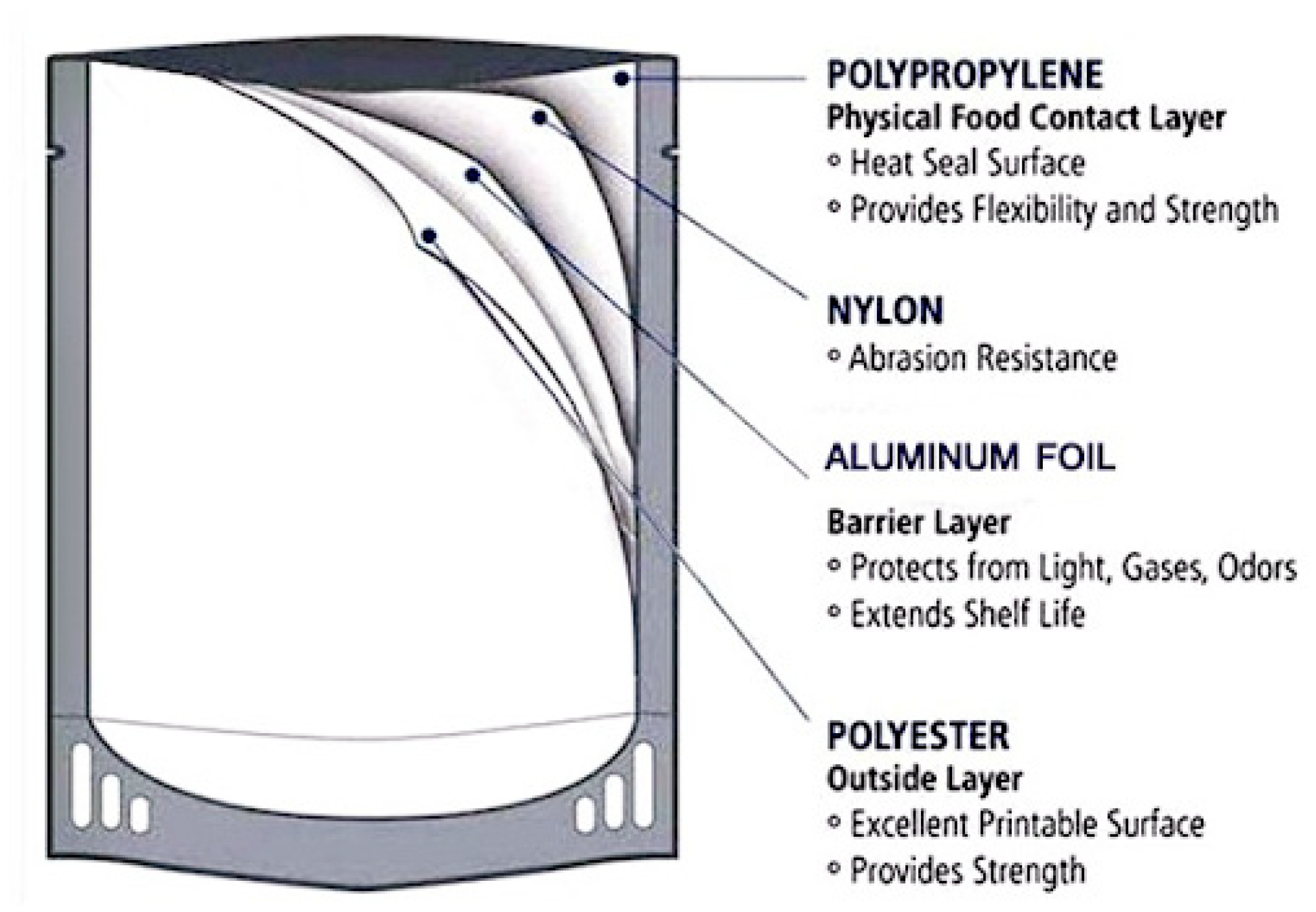
4.5. Retort Pouch Processing
- The PET layer contributes with its mechanical strength and printability.
- The Alu F protects the product from the effect of light and transport of gases, moisture and odors.
- The PA layer protects from abrasion.
- The CPP provides heat sealability and acts as a food contact surface.
- The patented construction of the pouch enables high heat transfer rates for sterilization, resulting in a substantially lower processing time and respective energy consumption.
- Retention of product nutrient and sensory attributes due to the reduced heat exposure the product undergoes.
- Reduced preparation time for serving the product (immersion of the pouch in boiling water for 3–5 min. or microwave oven heating).
- Comparable shelf life of RP products to those in metal containers.
- No need for refrigeration or freezing by processors, retailers, or consumers.
- Minimum product–container interaction, without the risk of external corrosion.
- Easy opening of the pouch
- Reduction in storage space for empty RP for processors. Empty retort pouches occupy 85% less space compared to empty tin cans and are significantly lighter.
- Less energy required to manufacture pouches compared to metal cans.
4.5.1. Use of RP Packaging in Fish Preservation
4.5.2. Use of RP Packaging in Fishery Product Preservation
4.5.3. Edible Films and Coatings/Biodegradable Polymers
4.5.4. Use of EFCs/Biodegradable Polymers in Seafood Preservation
4.6. Safety Concerns and Legal Aspects of Packaging
4.6.1. Vacuum Packaging and Modified Atmosphere Packaging
4.6.2. Active (AP) and Intelligent Packaging (IP)
- (a)
- with the words ‘DO NOT EAT’ and
- (b)
- always where technically possible, with the following symbol (Figure 6):
4.6.3. Edible Films and Coatings
5. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. Facts and Figures on the Common Fisheries Policy; Publications Office of the EU: Luxembourg, 2006; ISBN 9789279227400. [Google Scholar]
- Adams, M.R.; Moss, M.O. Microbiology of primary food commodities. In Food Microbiology; Adams, M.R., Moss, M.O., Eds.; Royal Society of Chemistry: Cambridge, UK, 1999; pp. 103–135. [Google Scholar]
- Shewan, J.M. The microbiology of fish and fishery products—A progress report. J. Appl. Bacteriol. 1971, 34, 299–315. [Google Scholar] [CrossRef]
- Jackson, T.C.; Marshal, D.L.; Acuff, G.R.; Dickson, J.S. Meat, poultry, and seafood. In Food Microbiology: Fundamentals and Frontiers; Doyle, M.P., Beuchat, L.R., Montville, T.J., Eds.; ASM Press: Washington, DC, USA, 2001; pp. 91–105. [Google Scholar]
- Olafsdottir, G.; Lauzon, H.L.; Martinsdottir, E.; Kristbergsson, K. Influence of storage temperature on microbial spoilage characteristics of haddock fillets (Melanogrammus aeglefinus) evaluated by multivariate quality prediction. Int. J. Food Microbiol. 2006, 111, 112–125. [Google Scholar] [CrossRef] [PubMed]
- FAO. Post-harvest Changes in Fish. Available online: http://www.fao.org/fishery/topic/12320/en (accessed on 15 October 2019).
- Fraser, O.; Sumar, S. Compositional changes and spoilage in fish—An introduction. Nutr. Food Sci. 1998, 98, 275–279. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Dave, D.; Budge, S.; Brooks, M.S. Fish spoilage mechanisms and preservation techniques: Review. Am. J. Appl. Sci. 2010, 7, 846–864. [Google Scholar] [CrossRef]
- Liston, J. Microbiology in fishery science. In Advances in Fish Science and Technology; Connell, J.J., Ed.; Fishing News Books Ltd.: Farnham, UK, 1980; pp. 138–157. [Google Scholar]
- Cheng, J.H.; Sun, D.W.; Pu, H.; Zhu, Z. Development of hyperspectral imaging coupled with chemometric analysis to monitor K value for evaluation of chemical spoilage in fish fillets. Food Chem. 2015, 185, 245–253. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S.; Sumpavapol, P.; Nirmal, N.P. Quality changes of sea bass slices wrapped with gelatin film incorporated with lemongrass essential oil. Int. J. Food Microbiol. 2012, 155, 171–178. [Google Scholar] [CrossRef]
- Gram, L.; Huss, H.H. Fresh and processed fish and shellfish. In The Microbiological Safety and Quality of Foods; Lund, B.M., Baird-Parker, A.C., Gould, G.W., Eds.; Chapman and Hall: London, UK, 2000; pp. 472–506. [Google Scholar]
- Dalgaard, P.; Madsen, H.L.; Samieian, N.; Emborg, J. Biogenic amine formation and microbial spoilage in chilled garfish (Belone belone belone)—Effect of modified atmosphere packaging and previous frozen storage. J. Appl. Microbiol. 2006, 101, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Gram, L.; Dalgaard, P. Fish spoilage bacteria—Problems and solutions. Curr. Opin. Biotechnol. 2002, 13, 262–266. [Google Scholar] [CrossRef]
- Connell, J.J. Control of Fish Quality, 4th ed.; John Willey and Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Boziaris, I.S. Current trends on the study of microbiological spoilage of fresh fish. Fish. Aquac. J. 2014, 6, 1000e115. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Mallouchos, A.; Haroutounian, S.A.; Boziaris, I.S. Microbiological spoilage and investigation of volatile profile during storage of sea bream fillets under various conditions. Int. J. Food Microbiol. 2014, 189, 153–163. [Google Scholar] [CrossRef]
- Abbas, K.A.; Saleh, A.M.; Mohamed, A.; Lasekan, O. The relationship between water activity and fish spoilage during cold storage: A review. J. Food, Agric. Environ. 2009, 7, 86–90. [Google Scholar]
- Ishimaru, M.; Muto, Y.; Nakayama, A.; Hatate, H.; Tanaka, R. Determination of biogenic amines in fish meat and fermented foods using column-switching high-performance liquid chromatography with fluorescence detection. Food Anal. Methods 2019, 12, 166–175. [Google Scholar] [CrossRef]
- Biji, K.B.; Ravishankar, C.N.; Venkateswarlu, R.; Mohan, C.O.; Srinivasa Gopal, T.K. Biogenic amines in seafood: A review. J. Food Sci. Technol. 2016, 53, 2210–2218. [Google Scholar] [CrossRef]
- Hungerford, J.M. Scombroid poisoning: A review. Toxicon 2010, 56, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Visciano, P.; Schirone, M.; Tofalo, R.; Suzzi, G. Histamine poisoning and control measures in fish and fishery products. Front. Microbiol. 2014, 5, 500. [Google Scholar] [CrossRef]
- Secci, G.; Parisi, G. From farm to fork: Lipid oxidation in fish products. A review. Ital. J. Anim. Sci. 2016, 15, 124–136. [Google Scholar] [CrossRef]
- Hultin, H.O. Oxidation of lipids in seafoods. In Seafoods Chemistry, Processing Technology and Quality; Shahidi, F., Botta, J.R., Eds.; Blackie Academic and Professional: London, UK, 1994; pp. 49–74. [Google Scholar]
- Huis In’t Veld, J.H.J. Microbial and biochemical spoilage of foods: An overview. Int. J. Food Microbiol. 1996, 33, 1–18. [Google Scholar] [CrossRef]
- Undeland, I.; Hall, G.; Wendin, K.; Gangby, I.; Rutgersson, A. Preventing lipid oxidation during recovery of functional proteins from herring (Clupea harengus) fillets by an acid solubilization process. J. Agric. Food Chem. 2005, 53, 5625–5634. [Google Scholar] [CrossRef]
- Lampila, L.E.; McMillin, K.W. Major microbial hazards associated with packaged seafood. In Advances in Meat, Poultry and Seafood Packaging; Kerry, J.P., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2012; pp. 59–85. ISBN 9781845697518. [Google Scholar]
- Schwarz, J.R. Rapid chilling of oyster shell stock: A postharvest process to reduce Vibrio. In Proceedings of the 25th Annual Meeting of the Seafood Science & Technology Society of the Americas, Longboat, FL, USA, 9–11 October 2000. [Google Scholar]
- Papadopoulou, C.; Economou, E.; Zakas, G.; Salamoura, C.; Dontorou, C.; Apostolou, J. Microbiological and pathogenic contaminants of seafood in Greece. J. Food Qual. 2007, 30, 28–42. [Google Scholar] [CrossRef]
- International Packaging Institude. Glossary of Packaging Terms, 6th ed.; The Packaging Institute International Publ.: Stamford, CT, USA, 1988. [Google Scholar]
- Robertson, G.L. Food Packaging, Principles and Practice, 3rd ed.; Robertson, G.L., Ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Jay, J.; Loessner, M.L.; Golden, D.A. Modern Food Microbiology; Springer Science & Business Media: New York, NY, USA, 2008. [Google Scholar]
- Kontominas, M.G. Packaging: Modified atmosphere packaging of foods. In Encyclopedia of Food Microbiology; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 1012–1016. ISBN 9780123847331. [Google Scholar]
- Phillips, C.A. Review: Modified atmosphere packaging and its effects on the microbiological quality and safety of produce. Int. J. Food Sci. Technol. 1996, 31, 463–479. [Google Scholar] [CrossRef]
- Speranza, B.; Corbo, M.R.; Conte, A.; Sinigaglia, M.; Del Nobile, M.A. Microbiological and sensorial quality assessment of ready-to-cook seafood products packaged under modified atmosphere. J. Food Sci. 2009, 74, 473–478. [Google Scholar] [CrossRef]
- Yesudhason, P.; Lalitha, K.V.; Gopal, T.K.S.; Ravishankar, C.N. Retention of shelf life and microbial quality of seer fish stored in modified atmosphere packaging and sodium acetate pretreatment. Food Packag. Shelf Life 2014, 1, 123–130. [Google Scholar] [CrossRef]
- Kykkidou, S.; Giatrakou, V.; Papavergou, A.; Kontominas, M.G.; Savvaidis, I.N. Effect of thyme essential oil and packaging treatments on fresh Mediterranean swordfish fillets during storage at 4 °C. Food Chem. 2009, 115, 169–175. [Google Scholar] [CrossRef]
- Del Nobile, M.A.; Corbo, M.R.; Speranza, B.; Sinigaglia, M.; Conte, A.; Caroprese, M. Combined effect of MAP and active compounds on fresh blue fish burger. Int. J. Food Microbiol. 2009, 135, 281–287. [Google Scholar] [CrossRef]
- Fernández, K.; Aspe, E.; Roeckel, M. Shelf-life extension on fillets of Atlantic Salmon (Salmo salar) using natural additives, superchilling and modified atmosphere packaging. Food Control. 2009, 20, 1036–1042. [Google Scholar] [CrossRef]
- Torrieri, E.; Carlino, P.A.; Cavella, S.; Fogliano, V.; Attianese, I.; Buonocore, G.G.; Masi, P. Effect of modified atmosphere and active packaging on the shelf-life of fresh bluefin tuna fillets. J. Food Eng. 2011, 105, 429–435. [Google Scholar] [CrossRef]
- Babic, J.; Milijasevic, M.; Vranic, D.; Veskovic-Moracanin, S.; Djinovic-Stojanovic, J. Effect of modified atmosphere pakaging on the shelf-life of common carp (Cyprinus carpio) steaks. Procedia Food Sci. 2015, 5, 2–5. [Google Scholar] [CrossRef][Green Version]
- Messina, C.M.; Bono, G.; Renda, G.; La Barbera, L.; Santulli, A. Effect of natural antioxidants and modified atmosphere packaging in preventing lipid oxidation and increasing the shelf-life of common dolphinfish (Coryphaena hippurus) fillets. LWT—Food Sci. Technol. 2015, 62, 271–277. [Google Scholar] [CrossRef]
- Hansen, A.A.; Moen, B.; Rødbotten, M.; Berget, I.; Pettersen, M.K. Effect of vacuum or modified atmosphere packaging (MAP) in combination with a CO2 emitter on quality parameters of cod loins (Gadus morhua). Food Packag. Shelf Life 2016, 9, 29–37. [Google Scholar] [CrossRef]
- Kuuliala, L.; Al Hage, Y.; Ioannidis, A.G.; Sader, M.; Kerckhof, F.M.; Vanderroost, M.; Boon, N.; De Baets, B.; De Meulenaer, B.; Ragaert, P.; et al. Microbiological, chemical and sensory spoilage analysis of raw Atlantic cod (Gadus morhua) stored under modified atmospheres. Food Microbiol. 2018, 70, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Tsironi, T.; Stamatiou, A.; Giannoglou, M.; Velliou, E.; Taoukis, P.S. Predictive modelling and selection of Time Temperature Integrators for monitoring the shelf life of modified atmosphere packed gilthead seabream fillets. LWT—Food Sci. Technol. 2011, 44, 1156–1163. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Kormas, K.A.; Boziaris, I.S. Microbiological changes, shelf life and identification of initial and spoilage microbiota of sea bream fillets stored under various conditions using 16S rRNA gene analysis. J. Sci. Food Agric. 2015, 95, 2386–2394. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, B.L.; Alvares, T.S.; Sampaio, G.S.L.; Cabral, C.C.; Araujo, J.V.A.; Franco, R.M.; Mano, S.B.; Conte Junior, C.A. Influence of vacuum and modified atmosphere packaging in combination with UV-C radiation on the shelf life of rainbow trout (Oncorhynchus mykiss) fillets. Food Control. 2016, 60, 596–605. [Google Scholar] [CrossRef]
- Bono, G.; Badalucco, C. Combining ozone and modified atmosphere packaging (MAP) to maximize shelf-life and quality of striped red mullet (Mullus surmuletus). LWT—Food Sci. Technol. 2012, 47, 500–504. [Google Scholar] [CrossRef]
- Kostaki, M.; Giatrakou, V.; Savvaidis, I.N.; Kontominas, M.G. Combined effect of MAP and thyme essential oil on the microbiological, chemical and sensory attributes of organically aquacultured sea bass (Dicentrarchus labrax) fillets. Food Microbiol. 2009, 26, 475–482. [Google Scholar] [CrossRef]
- Hassoun, A.; Karoui, R. Monitoring changes in whiting (Merlangius merlangus) fillets stored under modified atmosphere packaging by front face fluorescence spectroscopy and instrumental techniques. Food Chem. 2016, 200, 343–353. [Google Scholar] [CrossRef]
- Silbande, A.; Adenet, S.; Smith-Ravin, J.; Joffraud, J.J.; Rochefort, K.; Leroi, F. Quality assessment of ice-stored tropical yellowfin tuna (Thunnus albacares) and influence of vacuum and modified atmosphere packaging. Food Microbiol. 2016, 60, 62–72. [Google Scholar] [CrossRef]
- Milne, D.; Powell, S.M. Limited microbial growth in Atlantic salmon packed in a modified atmosphere. Food Control. 2014, 42, 29–33. [Google Scholar] [CrossRef]
- Alfaro, B.; Hernandez, I. Evolution of the indigenous microbiota in modified atmosphere packaged Atlantic horse mackerel (Trachurus trachurus) identified by conventional and molecular methods. Int. J. Food Microbiol. 2013, 167, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, A.; Fernández-Segovia, I.; Barat, J.M.; Serra, J.A. Influence of sodium replacement and packaging on quality and shelf life of smoked sea bass (Dicentrarchus labrax L.). LWT—Food Sci. Technol. 2011, 44, 917–923. [Google Scholar] [CrossRef]
- Noseda, B.; Islam, M.T.; Eriksson, M.; Heyndrickx, M.; De Reu, K.; Van Langenhove, H.; Devlieghere, F. Microbiological spoilage of vacuum and modified atmosphere packaged Vietnamese Pangasius hypophthalmus fillets. Food Microbiol. 2012, 30, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, B.C.; Heiberg, R.; Eie, T.; Møretrø, T.; Maugesten, T.; Carlehøg, M.; Langsrud, S. A novel packaging method with a dissolving CO2 headspace combined with organic acids prolongs the shelf life of fresh salmon. Int. J. Food Microbiol. 2009, 133, 154–160. [Google Scholar] [CrossRef]
- Van Haute, S.; Raes, K.; Devlieghere, F.; Sampers, I. Combined use of cinnamon essential oil and MAP/vacuum packaging to increase the microbial and sensorial shelf life of lean pork and salmon. Food Packag. Shelf Life 2017, 12, 51–58. [Google Scholar] [CrossRef]
- El-Sayed, M.H.; Khaled, Z.; El-Aziz, A.; Elbadawy, H.H. Evaluation the microbial spoilage of Atlantic Salmon (Salmo salar) fillets during the packaging processes and its control by preservatives. IJSRSET 2015, 1, 134–141. [Google Scholar]
- Gunsen, U.; Ozcan, A.; Aydin, A. The effect of modified atmosphere packaging on extending shelf-lifes of cold storaged marinated seafood salad. J. Anim. Vet. Adv. 2010, 9, 2017–2024. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Danza, A.; Conte, A.; Muratore, G.; Del Nobile, M.A. Shelf life of ready to use peeled shrimps as affected by thymol essential oil and modified atmosphere packaging. Int. J. Food Microbiol. 2010, 144, 250–256. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Benjakul, S. Retardation of quality changes of Pacific white shrimp by green tea extract treatment and modified atmosphere packaging during refrigerated storage. Int. J. Food Microbiol. 2011, 149, 247–253. [Google Scholar] [CrossRef]
- Qian, Y.F.; Xie, J.; Yang, S.P.; Huang, S.; Wu, W.H.; Li, L. Inhibitory effect of a quercetin-based soaking formulation andmodified atmospheric packaging (MAP) on muscle degradation ofPacific white shrimp (Litopenaeus vannamei). LWT—Food Sci. Technol. 2015, 63, 1339–1346. [Google Scholar] [CrossRef]
- Gornik, S.G.; Albalat, A.; Theethakaew, C.; Neil, D.M. Shelf life extension of whole Norway lobster Nephrops norvegicus using modified atmosphere packaging. Int. J. Food Microbiol. 2013, 167, 369–377. [Google Scholar] [CrossRef]
- Ulusoy, Ş.; Özden, Ö. Preservation of stuffed mussels at 4 °C in modified atmosphere packaging. J. Aquat. Food Prod. Technol. 2011, 20, 319–330. [Google Scholar] [CrossRef]
- Masniyom, P.; Benjama, O.; Maneesri, J. Extending the shelf-life of refrigerated green mussel (Perna viridis) under modified atmosphere packaging. Songklanakarin J. Sci. Technol. 2011, 33, 171–179. [Google Scholar]
- Fall, P.A.; Leroi, F.; Cardinal, M.; Chevalier, F.; Pilet, M.F. Inhibition of Brochothrix thermosphacta and sensory improvement of tropical peeled cooked shrimp by Lactococcus piscium CNCM I-4031. Lett. Appl. Microbiol. 2010, 50, 357–361. [Google Scholar] [CrossRef]
- Church, N. Developments in modified-atmosphere packaging and related technologies. Trends Food Sci. Technol. 1994, 5, 345–352. [Google Scholar] [CrossRef]
- Provincial, L.; Guillén, E.; Alonso, V.; Gil, M.; Roncalés, P.; Beltrán, J.A. Survival of Vibrio parahaemolyticus and Aeromonas hydrophila in sea bream (Sparus aurata) fillets packaged under enriched CO2 modified atmospheres. Int. J. Food Microbiol. 2013, 166, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Newell, C.R.; Doyle, M.; Ma, L. Inability of non-proteolytic Clostridium botulinum to grow in mussels inoculated via immersion and packaged in high oxygen atmospheres. Food Microbiol. 2015, 46, 204–209. [Google Scholar] [CrossRef]
- DeWitt, C.; Oliveira, A. Modified atmosphere systems and shelf life extension of fish and fishery products. Foods 2016, 5, 48. [Google Scholar] [CrossRef]
- Walsh, H.; Kerry, J.P. Packaging of ready-to-serve and retail-ready meat, poultry and seafood products. In Advances in Meat, Poultry and Seafood Packaging; Elsevier Ltd.: Amsterdam, The Netherlands, 2012; pp. 406–436. ISBN 9781845697518. [Google Scholar]
- Mohan, C.O.; Ravishankar, C.N.; Srinivasa Gopal, T.K. Effect of vacuum packaging and sous vide processing on the quality of Indian white shrimp (Fenneropenaeus indicus) during chilled storage. J. Aquat. Food Prod. Technol. 2017, 26, 1280–1292. [Google Scholar] [CrossRef]
- Gokoglu, N. Innovations in seafood packaging technologies: A review. Food Rev. Int. 2020, 36, 340–366. [Google Scholar] [CrossRef]
- Packaging Europe. Available online: https://packagingeurope.com/sealed-air-on-sustainability-through-waste-reduction/ (accessed on 14 April 2021).
- Masniyom, P.; Benjama, O.; Maneesri, J. Effect of modified atmosphere and vacuum packaging on quality changes of refrigerated tilapia (Oreochromis niloticus) fillets. Int. Food Res. J. 2013, 20, 1401–1408. [Google Scholar]
- Erkan, N. The effect of active and vacuum packaging on the quality of Turkish traditional salted dried fish “çiroz”. J. Food Heal. Sci. 2017, 21, 29–35. [Google Scholar] [CrossRef]
- Silbande, A.; Adenet, S.; Chopin, C.; Cornet, J.; Smith-Ravin, J.; Rochefort, K.; Leroi, F. Effect of vacuum and modified atmosphere packaging on the microbiological, chemical and sensory properties of tropical red drum (Sciaenops ocellatus) fillets stored at 4 °C. Int. J. Food Microbiol. 2018, 266, 31–41. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Dong, S.; Zhao, Y.; Zeng, M. Effects of vacuum and modified atmosphere packaging on microbial flora and shelf-life of pacific white shrimp (Litopenaeus vannamei) during controlled freezing-point storage at −0.8 °C. Food Sci. Technol. Res. 2014, 20, 1141–1152. [Google Scholar] [CrossRef][Green Version]
- Tsogas, A.; Vatavali, K.; Dimitriou, E.; Badeka, A.; Kontakos, S.; Kontominas, M.G. Combined effect of light salting and vacuum packaging on the microbiological, chemical, and sensory attributes of mullet fillets (Mugil cephalus) during refrigerated and frozen/refrigerated storage. J. Food Process. Preserv. 2019, 43, e14009. [Google Scholar] [CrossRef]
- Ramírez-Suárez, J.C.; Pacheco-Aguilar, R.; Scheuren-Acevedo, S.M.; García-Sánchez, G.; Carvallo-Ruiz, G.; Lugo-Sánchez, M.E.; Cumplido-Barbeitia, G.; Jiménez-Ruiz, E.I. Microbiological and physicochemical quality changes in frankfurters made from jumbo squid (Dosidicus gigas) mantle muscle in the presence and absence of a natural antimicrobial agent. J. Food Saf. 2015, 35, 473–481. [Google Scholar] [CrossRef]
- Özogul, F.; Kuley, E.; Kenar, M. Effects of rosemary and sage tea extract on biogenic amines formation of sardine (Sardina pilchardus) fillets. Int. J. Food Sci. Technol. 2011, 46, 761–766. [Google Scholar] [CrossRef]
- Houicher, A.; Kuley, E.; Bendeddouche, B.; Özogul, F. Effect of Mentha spicata L. and Artemisia campestris extracts on the shelf life and quality of vacuum-packed refrigerated sardine (Sardina pilchardus) fillets. J. Food Prot. 2013, 76, 1719–1725. [Google Scholar] [CrossRef]
- Attouchi, M.; Sadok, S. The effects of essential oils addition on the quality of wild and farmed sea bream (Sparus aurata) stored in ice. Food Bioprocess. Technol. 2012, 5, 1803–1816. [Google Scholar] [CrossRef]
- Ibrahim, F.; Vesterlund, S. Lactococcus lactis ssp. lactis as protective culture in vacuum-packed raw salmon (Salmo salar). J. Aquat. Food Prod. Technol. 2014, 23, 601–607. [Google Scholar] [CrossRef]
- Speranza, B.; Bevilacqua, A.; Sinigaglia, M.; Corbo, M.R. Shelf life definition for Italian anchovies inoculated with Lactobacillus plantarum and Bifidobacterium animalis subsp. lactis. Innov. Food Sci. Emerg. Technol. 2012, 16, 171–180. [Google Scholar] [CrossRef]
- Leroi, F.; Cornet, J.; Chevalier, F.; Cardinal, M.; Coeuret, G.; Chaillou, S.; Joffraud, J.J. Selection of bioprotective cultures for preventing cold-smoked salmon spoilage. Int. J. Food Microbiol. 2015, 213, 79–87. [Google Scholar] [CrossRef]
- Duan, J.; Jiang, Y.; Cherian, G.; Zhao, Y. Effect of combined chitosan-krill oil coating and modified atmosphere packaging on the storability of cold-stored lingcod (Ophiodon elongates) fillets. Food Chem. 2010, 122, 1035–1042. [Google Scholar] [CrossRef]
- Matějková, K.; Křížek, M.; Vácha, F.; Dadáková, E. Effect of high-pressure treatment on biogenic amines formation in vacuum-packed trout flesh (Oncorhynchus mykiss). Food Chem. 2013, 137, 31–36. [Google Scholar] [CrossRef]
- Günlü, A.; Sipahioǧlu, S.; Alpas, H. The effect of chitosan-based edible film and high hydrostatic pressure process on the microbiological and chemical quality of rainbow trout (Oncorhynchus mykiss Walbaum) fillets during cold storage (4 ± 1 °C). High. Press. Res. 2014, 34, 110–121. [Google Scholar] [CrossRef]
- Navarro-Segura, L.; Ros-Chumillas, M.; Martínez-Hernández, G.B.; López-Gómez, A. A new advanced packaging system for extending the shelf life of refrigerated farmed fish fillets. J. Sci. Food Agric. 2020, 100, 4601–4611. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.; Skare, M.; Rotabakk, B.T.; Sivertsvik, M.; Lerfall, J.; Løvdal, T.; Roth, B. Evaluation of physical and instrumentally determined sensory attributes of Atlantic salmon portions packaged in modified atmosphere and vacuum skin. LWT—Food Sci. Technol. 2021, 146, 111404. [Google Scholar] [CrossRef]
- Dordević, D.; Buchtová, H.; Borkovcová, I. Estimation of amino acids profile and escolar fish consumption risks due to biogenic amines content fluctuations in vacuum skin packaging/VSP during cold storage. LWT—Food Sci. Technol. 2016, 66, 657–663. [Google Scholar] [CrossRef]
- Esteves, E.; Guerra, L.; Aníbal, J. Effects of vacuum and modified atmosphere packaging on the quality and shelf-life of gray triggerfish (Balistes capriscus) fillets. Foods 2021, 10, 250. [Google Scholar] [CrossRef]
- Atrea, I.; Papavergou, A.; Amvrosiadis, I.; Savvaidis, I.N. Combined effect of vacuum-packaging and oregano essential oil on the shelf-life of Mediterranean octopus (Octopus vulgaris) from the Aegean Sea stored at 4 °C. Food Microbiol. 2009, 26, 166–172. [Google Scholar] [CrossRef]
- Matamoros, S.; Leroi, F.; Cardinal, M.; Gigout, F.; Chadli, F.K.; Cornet, J.; Prévost, H.; Pilet, M.F. Psychrotrophic lactic acid bacteria used to improve the safety and quality of vacuum-packaged cooked and peeled tropical shrimp and cold-smoked salmon. J. Food Prot. 2009, 72, 365–374. [Google Scholar] [CrossRef]
- Regulation (EC) 450/2009. Regulation (EC) No 450/2009 of 29 May 2009 on active and intelligent materials and articles intended to come into contact with food. Off. J. Eur. Union 2009, L135, 3–11. [Google Scholar]
- Suppakul, P.; Miltz, J.; Sonneveld, K.; Bigger, S.W. Active packaging technologies with an emphasis on antimicrobial packaging and its applications. J. Food Sci. 2003, 68, 408–420. [Google Scholar] [CrossRef]
- Kerry, J.P.; O’Grady, M.N.; Hogan, S.A. Past, current and potential utilisation of active and intelligent packaging systems for meat and muscle-based products: A review. Meat Sci. 2006, 74, 113–130. [Google Scholar] [CrossRef]
- Mexis, S.F.; Kontominas, M.G. Packaging: Active food packaging. In Encyclopedia of Food Microbiology: Second Edition; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 999–1005. ISBN 9780123847331. [Google Scholar]
- Mohan, C.O.; Ravishankar, C.N. Active and intelligent packaging aystems-Application in seafood. World J. Aquac. Res. Dev. 2019, 1, 10–16. [Google Scholar]
- ResearchGate. Available online: www.researchgate.net (accessed on 10 April 2021).
- Sivertsvik, M. Active packaging in practice: Fish. In Novel Food Packaging Techniques; Elsevier: Amsterdam, The Netherlands, 2003; pp. 384–400. [Google Scholar]
- Packaging Digest. Available online: www.packagingdigest.com (accessed on 10 April 2021).
- Pharmaceutical Microbiology Resources. Available online: www.pharmamicroresources.com (accessed on 10 April 2021).
- Pokorný, J. Are natural antioxidants better—and safer—Than synthetic antioxidants? Eur. J. Lipid Sci. Technol. 2007, 109, 629–642. [Google Scholar] [CrossRef]
- Zeid, A.; Karabagias, I.K.; Nassif, M.; Kontominas, M.G. Preparation and evaluation of antioxidant packaging films made of polylactic acid containing thyme, rosemary, and oregano essential oils. J. Food Process. Preserv. 2019, 43. [Google Scholar] [CrossRef]
- Mohan, C.O.; Ravishankar, C.N.; Srivansa Gopal, T.K.; Ashok Kumar, K. Nucleotide breakdown products of seer fish (Scomberomorus commerson) steaks stored in O2 scavenger packs during chilled storage. Innov. Food Sci. Emerg. Technol. 2009, 10, 272–278. [Google Scholar] [CrossRef]
- Mohan, C.O.; Ravishankar, C.N.; Srinivasa Gopal, T.K.; Ashok Kumar, K.; Lalitha, K.V. Biogenic amines formation in seer fish (Scomberomorus commerson) steaks packed with O2 scavenger during chilled storage. Food Res. Int. 2009, 42, 411–416. [Google Scholar] [CrossRef]
- López-De-Dicastillo, C.; Gómez-Estaca, J.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Active antioxidant packaging films: Development and effect on lipid stability of brined sardines. Food Chem. 2012, 131, 1376–1384. [Google Scholar] [CrossRef]
- Pereira de Abreu, D.A.; Losada, P.P.; Maroto, J.; Cruz, J.M. Evaluation of the effectiveness of a new active packaging film containing natural antioxidants (from barley husks) that retard lipid damage in frozen Atlantic salmon (Salmo salar L.). Food Res. Int. 2010, 43, 1277–1282. [Google Scholar] [CrossRef]
- Mexis, S.F.; Chouliara, E.; Kontominas, M.G. Combined effect of an O2 absorber and oregano essential oil on shelf-life extension of Greek cod roe paste (tarama salad) stored at 4 °C. Innov. Food Sci. Emerg. Technol. 2009, 10, 572–579. [Google Scholar] [CrossRef]
- Neetoo, H.; Mahomoodally, F. Use of antimicrobial films and edible coatings incorporating chemical and biological preservatives to control growth of Listeria monocytogenes on cold smoked salmon. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.A.; Mørkøre, T.; Rudi, K.; Rødbotten, M.; Bjerke, F.; Eie, T. Quality changes of prerigor filleted atlantic salmon (salmo salar L.) packaged in modified atmosphere using CO2 emitter, traditional MAP, and vacuum. J. Food Sci. 2009, 74. [Google Scholar] [CrossRef] [PubMed]
- Alboofetileh, M.; Rezaei, M.; Hosseini, H.; Abdollahi, M. Efficacy of activated alginate-based nanocomposite films to control Listeria monocytogenes and spoilage flora in rainbow trout slice. J. Food Sci. Technol. 2016, 53, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Jalali, N.; Ariiai, P.; Fattahi, E. Effect of alginate/carboxyl methyl cellulose composite coating incorporated with clove essential oil on the quality of silver carp fillet and Escherichia coli O157:H7 inhibition during refrigerated storage. J. Food Sci. Technol. 2016, 53, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Tokur, B.K.; Sert, F.; Aksun, E.T.; Özoğul, F. The effect of whey protein isolate coating enriched with thyme essential oils on trout quality at refrigerated storage (4 ± 2 °C). J. Aquat. Food Prod. Technol. 2016, 25, 585–596. [Google Scholar] [CrossRef]
- Shakila, R.J.; Jeevithan, E.; Arumugam, V.; Jeyasekaran, G. Suitability of antimicrobial grouper bone gelatin films as edible coatings for vacuum-packaged fish steaks. J. Aquat. Food Prod. Technol. 2016, 25, 724–734. [Google Scholar] [CrossRef]
- Pereira De Abreu, D.A.; Paseiro Losada, P.; Maroto, J.; Cruz, J.M. Lipid damage during frozen storage of Atlantic halibut (Hippoglossus hippoglossus) in active packaging film containing antioxidants. Food Chem. 2011, 126, 315–320. [Google Scholar] [CrossRef]
- Pereira De Abreu, D.A.; Paseiro Losada, P.; Maroto, J.; Cruz, J.M. Lipid damage inhibition in hake by active packaging film with natural antioxidants. Packag. Technol. Sci. 2011, 24, 353–360. [Google Scholar] [CrossRef]
- Remya, S.; Mohan, C.O.; Bindu, J.; Sivaraman, G.K.; Venkateshwarlu, G.; Ravishankar, C.N. Effect of chitosan based active packaging film on the keeping quality of chilled stored barracuda fish. J. Food Sci. Technol. 2016, 53, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Remya, S.; Mohan, C.O.; Venkateshwarlu, G.; Sivaraman, G.K.; Ravishankar, C.N. Combined effect of O2 scavenger and antimicrobial film on shelf life of fresh cobia (Rachycentron canadum) fish steaks stored at 2 °C. Food Control. 2017, 71, 71–78. [Google Scholar] [CrossRef]
- Mexis, S.F.; Chouliara, E.; Kontominas, M.G. Combined effect of an oxygen absorber and oregano essential oil on shelf life extension of rainbow trout fillets stored at 4 °C. Food Microbiol. 2009, 26, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Nagarajarao, R.C. Recent advances in processing and packaging of fishery products: A review. Aquat. Procedia 2016, 7, 201–213. [Google Scholar] [CrossRef]
- Htun, N.N. State-of-the-art Technologies in Intelligent Packaging. Available online: https://matisiceland.org/state-of-the-art-technologies-in-intelligent-packaging/ (accessed on 12 April 2021).
- Kuswandi, B.; Wicaksono, Y.; Abdullah, A.; Heng, L.Y.; Ahmad, M. Smart packaging: Sensors for monitoring of food quality and safety. Sens. Instrum. Food Qual. Saf. 2011, 5, 137–146. [Google Scholar] [CrossRef]
- Pacquit, A.; Lau, K.T.; Mclaughlin, H.; Frisby, J.; Quilty, B.; Diamond, D. Development of a volatile amine sensor for the monitoring of fish spoilage. Talanta 2006, 69, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Pacquit, A.; Frisby, J.; Diamond, D.; Lau, K.T.; Farrell, A.; Quilty, B.; Diamond, D. Development of a smart packaging for the monitoring of fish spoilage. Food Chem. 2007, 102, 466–470. [Google Scholar] [CrossRef]
- 3M Science Applied to Life, Microbiology, FoodSafety. Available online: http://solutions.3m.com/wps/portal/3M/en_US/Microbiology/FoodSafety/product-information/product-catalog (accessed on 18 October 2010).
- Taoukis, P.; Tsironi, T. Smart packaging for monitoring and managing food and beverage shelf life. In The Stability and Shelf Life of Food; Elsevier: Amsterdam, The Netherlands, 2016; pp. 141–168. ISBN 9780081004357. [Google Scholar]
- Ahvenainen, R. Active and inteligent packaging: An introduction. In Novel Food Packaging Techniques; Ahvenainen, R., Ed.; Woodhead Publ. Series in food Science, Technology and Nutrition: Cambridge, UK, 2003; pp. 5–21. [Google Scholar]
- Sadeghi, S.; Fooladi, E.; Malekaneh, M. A nanocomposite/crude extract enzyme-based xanthine biosensor. Anal. Biochem. 2014, 464, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.; Yadav, S.; Nehra, R.; Pundir, C.S. An amperometric hypoxanthine biosensor based on Au@FeNPs for determination of hypoxanthine in meat samples. Int. J. Biol. Macromol. 2013, 62, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Dervisevic, M.; Custiuc, E.; Çevik, E.; Şenel, M. Construction of novel xanthine biosensor by using polymeric mediator/MWCNT nanocomposite layer for fish freshness detection. Food Chem. 2015, 181, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Dervisevic, M.; Custiuc, E.; Çevik, E.; Durmus, Z.; Şenel, M.; Durmus, A. Electrochemical biosensor based on REGO/Fe3O4 bionanocomposite interface for xanthine detection in fish sample. Food Control. 2015, 57, 402–410. [Google Scholar] [CrossRef]
- Kuswandi, B.; Larasati, L.; Abdullah, A.; Heng, L.Y. Real-time monitoring of shrimp spoilage using on-package sticker sensor based on natural dye of curcumin. Food Anal. Methods 2012, 5, 881–889. [Google Scholar] [CrossRef]
- Cierpiszewski, R.; Tichoniuk, M.; Radomska, N. Application of freshness indicator for monitoring of fish spoilage in model and commercial packaging. Waroznawcze Probl. Jakości 2017, 4, 103–118. [Google Scholar]
- Kuswandi, B.; Jayus, R.A.; Abdullah, A.; Heng, L.Y.; Ahmad, M. A novel colorimetric food package label for fish spoilage based on polyaniline film. Food Control 2012, 25, 184–189. [Google Scholar] [CrossRef]
- Chun, H.N.; Kim, B.; Shin, H.S. Evaluation of a freshness indicator for quality of fish products during storage. Food Sci. Biotechnol. 2014, 23, 1719–1725. [Google Scholar] [CrossRef]
- Giannoglou, M.; Touli, A.; Platakou, E.; Tsironi, T.; Taoukis, P.S. Predictive modeling and selection of TTI smart labels for monitoring the quality and shelf-life of frozen seafood. Innov. Food Sci. Emerg. Technol. 2014, 26, 294–301. [Google Scholar] [CrossRef]
- Tsironi, T.; Ronnow, P.; Giannoglou, M.; Taoukis, P. Developing suitable smart TTI labels to match specific monitoring requirements: The case of Vibrio spp. growth during transportation of oysters. Food Control. 2017, 73, 51–56. [Google Scholar] [CrossRef]
- Tsironi, T.; Giannoglou, M.; Platakou, E.; Taoukis, P. Evaluation of Time Temperature Integrators for shelf-life monitoring of frozen seafood under real cold chain conditions. Food Packag. Shelf Life 2016, 10, 46–53. [Google Scholar] [CrossRef]
- Koutsoumanis, K.P.; Gougouli, M. Use of time temperature integrators in food safety management. Trends Food Sci. Technol. 2015, 43, 236–244. [Google Scholar] [CrossRef]
- FDA. Guidance for the industry. In Fish and Fishery Products Hazards and Controls Guidance; Department of Health and Human Services, Public Health Service Food and Drug Administration Center for Food Safety and Applied Nutrition Office of Food Safety: White Oak, MA, USA, 2011; pp. 245–292. [Google Scholar]
- Ronnow, P.; Tsironi, T.; Giannoglou, M.; Taoukis, P.S. Developing suitable smart TTI labels to match specific shelf life monitoring requirements: The case of different seafood products. In Proceedings of the 29th EFFoST International Conference, Athens, Greece, 10–12 November 2015; pp. 663–668. [Google Scholar]
- Pennanen, K.; Focas, C.; Kumpusalo-Sanna, V.; Keskitalo-Vuokko, K.; Matullat, I.; Ellouze, M.; Pentikäinen, S.; Smolander, M.; Korhonen, V.; Ollila, M. European consumers’ perceptions of time-temperature indicators in food packaging. Packag. Technol. Sci. 2015, 28, 303–323. [Google Scholar] [CrossRef]
- Flair Packaging. Available online: www.flairpackaging.com (accessed on 10 April 2021).
- Holdsworth, D.; Simpson, R. Introduction. In Thermal Processing of Packaged Foods; Holdsworth, D., Simpson, R., Eds.; Springer: New York, NY, USA, 2007; pp. 1–13. [Google Scholar]
- Featherstone, S. Retortable flexible containers for food packaging. In A Complete Course in Canning and Related Processes; Featherstone, S., Ed.; Woodhead Publ.: Cambridge, UK, 2015; pp. 137–146. [Google Scholar]
- Byun, Y.; Bae, H.J.; Cooksey, K.; Whiteside, S. Comparison of the quality and storage stability of salmon packaged in various retort pouches. LWT—Food Sci. Technol. 2010, 43, 551–555. [Google Scholar] [CrossRef]
- Bindu, J.; Ravishankar, C.N.; Srinivasa Gopal, T.K.; Mallick, A.K. Investigation of shelf life and heat penetration attributes of ready-to-eat “fish peera” from anchovy (Stolephorous commersoni) in retort pouches. J. Food Process. Preserv. 2010, 34, 207–222. [Google Scholar] [CrossRef]
- Majumdar, R.K.; Dhar, B.; Roy, D.; Saha, A. Optimization of process conditions for Rohu fish in curry medium in retortable pouches using instrumental and sensory characteristics. J. Food Sci. Technol. 2015, 52, 5671–5680. [Google Scholar] [CrossRef]
- Majumdar, R.K.; Dhar, B.; Saha, A.; Roy, D.; Parhi, J.; Singh, A.S. Evaluation of textural quality as a parameter to optimize thermal process during retort pouch processing of boneless rohu balls in curry medium. J. Food Process. Preserv. 2017, 41. [Google Scholar] [CrossRef]
- Sreelakshmi, K.R.; Manjusha, L.; Nagalakshmi, K.; Chouksey, M.K.; Venkateshwarlu, G. Ready-to-serve crab sandwich spread in retort pouch: Product development and process optimization. J. Aquat. Food Prod. Technol. 2015, 24, 315–329. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef]
- Embuscado, M.E.; Huber, K.C. Edible Films and Coatings for Food Applications; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Cordeiro De Azeredo, H.M. Edible coatings. In Advances in Fruit Processing Technologies; Rodrigues, S., Fernandes, F.A.N., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 345–362. ISBN 9781439851531. [Google Scholar]
- Costa, C.; Conte, A.; Del Nobile, M.A. Effective preservation techniques to prolong the shelf life of ready-to-eat oysters. J. Sci. Food Agric. 2014, 94, 2661–2667. [Google Scholar] [CrossRef]
- Cai, L.; Wu, X.; Dong, Z.; Li, X.; Yi, S.; Li, J. Physicochemical responses and quality changes of red sea bream (Pagrosomus major) to gum arabic coating enriched with ergothioneine treatment during refrigerated storage. Food Chem. 2014, 160, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Aider, M. Chitosan application for active bio-based films production and potential in the food industry: Review. LWT—Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Cai, L.; Li, X.; Wu, X.; Lv, Y.; Liu, X.; Li, J. Effect of chitosan coating enriched with ergothioneine on quality changes of Japanese sea bass (Lateolabrax japonicas). Food Bioprocess. Technol. 2014, 7, 2281–2290. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging chitosan-based films for food packaging applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Motalebi, A.A.; Seifzadeh, M. Effects of whey protein edible coating on bacterial, chemical and sensory characteristics of frozen common Kilka. Iran. J. Fish. Sci. 2012, 11, 132–144. [Google Scholar]
- Rodriguez-Turienzo, L.; Cobos, A.; Diaz, O. Effects of microbial transglutaminase added edible coatings based on heated or ultrasound-treated whey proteins in physical and chemical parameters of frozen Atlantic salmon (Salmo salar). J. Food Eng. 2013, 119, 433–438. [Google Scholar] [CrossRef]
- Seyfzadeh, M.; Motalebi, A.A.; Kakoolaki, S.; Gholipour, H. Chemical, microbiological and sensory evaluation of gutted kilka coated with whey protein based edible film incorporated with sodium alginate during frozen storage. Iran. J. Fish. Sci. 2013, 12, 140–153. [Google Scholar]
- Lin, L.S.; Wang, B.J.; Weng, Y.M. Preservation of commercial fish ball quality with edible antioxidant-incorporated zein coatings. J. Food Process. Preserv. 2009, 33, 605–617. [Google Scholar] [CrossRef]
- Lin, L.S.; Wang, B.J.; Weng, Y.M. Quality preservation of commercial fish balls with antimicrobial zein coatings. J. Food Qual. 2011, 34, 81–87. [Google Scholar] [CrossRef]
- Dursun, S.; Erkan, N. The effect of edible coating on the quality of smoked fish. Ital. J. Food Sci. 2014, 26, 370–382. [Google Scholar]
- Gómez-Estaca, J.; López de Lacey, A.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P. Biodegradable gelatin-chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microbiol. 2010, 27, 889–896. [Google Scholar] [CrossRef]
- Sánchez-Ortega, I.; García-Almendárez, B.E.; Santos-López, E.M.; Amaro-Reyes, A.; Barboza-Corona, J.E.; Regalado, C. Antimicrobial edible films and coatings for meat and meat products preservation. Sci. World J. 2014, 2014, 248935. [Google Scholar] [CrossRef]
- Min, B.J.; Oh, J.H. Antimicrobial activity of catfish gelatin coating containing origanum (Thymus capitatus) oil against gram-negative pathogenic bacteria. J. Food Sci. 2009, 74, 143–148. [Google Scholar] [CrossRef]
- Chamanara, V.; Shabanpour, B.; Gorgin, S.; Khomeiri, M. An investigation on characteristics of rainbow trout coated using chitosan assisted with thyme essential oil. Int. J. Biol. Macromol. 2012, 50, 540–544. [Google Scholar] [CrossRef]
- Tammineni, N.; Ünlü, G.; Min, S.C. Development of antimicrobial potato peel waste-based edible films with oregano essential oil to inhibit Listeria monocytogenes on cold-smoked salmon. Int. J. Food Sci. Technol. 2013, 48, 211–214. [Google Scholar] [CrossRef]
- Nowzari, F.; Shábanpour, B.; Ojagh, S.M. Comparison of chitosan-gelatin composite and bilayer coating and film effect on the quality of refrigerated rainbow trout. Food Chem. 2013, 141, 1667–1672. [Google Scholar] [CrossRef]
- Soares, N.M.; Mendes, T.S.; Vicente, A.A. Effect of chitosan-based solutions applied as edible coatings and water glazing on frozen salmon preservation—A pilot-scale study. J. Food Eng. 2013, 119, 316–323. [Google Scholar] [CrossRef]
- ICMSF. Microorganisms in Foods 2. Sampling for Microbiological Analysis: Principles and Scientific Applications, 2nd ed.; University of Toronto Press: Toronto, ON, Canada, 1986. [Google Scholar]
- Commission Directive 95/149/EC. Commission Decision of 8 March 1995 fixing the total volatile basic nitrogen (TVB-N) limit values for certain categories of fishery products and specifying the analysis methods to be used. Off. J. Eur. Commun. 1995, 97, 84–87. [Google Scholar]
- Günlü, A.; Koyun, E. Effects of vacuum packaging and wrapping with chitosan-based edible film on the extension of the shelf life of sea bass (Dicentrarchus labrax) fillets in cold storage (4 °C). Food Bioprocess. Technol. 2013, 6, 1713–1719. [Google Scholar] [CrossRef]
- Khemir, M.; Besbes, N.; Ben Khemis, I.; Di Bella, C.; Lo Monaco, D.; Sadok, S. Determination of shelf-life of vacuum-packed sea bream (Sparus aurata) fillets using chitosan-microparticles-coating. CyTA-J. Food 2020, 18, 51–60. [Google Scholar] [CrossRef]
- Jiang, Z.; Neetoo, H.; Chen, H. Control of Listeria monocytogenes on cold-smoked salmon using chitosan-based antimicrobial coatings and films. J. Food Sci. 2011, 76, M22–M26. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, J.; Hu, W.; Li, X. Quality enhancement in refrigerated red drum (Sciaenops ocellatus) fillets using chitosan coatings containing natural preservatives. Food Chem. 2013, 138, 821–826. [Google Scholar] [CrossRef]
- Qiu, X.; Chen, S.; Liu, G.; Yang, Q. Quality enhancement in the Japanese sea bass (Lateolabrax japonicas) fillets stored at 4 °C by chitosan coating incorporated with citric acid or licorice extract. Food Chem. 2014, 162, 156–160. [Google Scholar] [CrossRef]
- Souza, B.W.S.; Cerqueira, M.A.; Ruiz, H.A.; Martins, J.T.; Casariego, A.; Teixeira, J.A.; Vicente, A.A. Effect of Chitosan-based coatings on the shelf life of Salmon (Salmo salar). J. Agric. Food Chem. 2010, 58, 11456–11462. [Google Scholar] [CrossRef]
- Duan, J.; Cherian, G.; Zhao, Y. Quality enhancement in fresh and frozen lingcod (Ophiodon elongates) fillets by employment of fish oil incorporated chitosan coatings. Food Chem. 2010, 119, 524–532. [Google Scholar] [CrossRef]
- Choulitoudi, E.; Ganiari, S.; Tsironi, T.; Ntzimani, A.; Tsimogiannis, D.; Taoukis, P.; Oreopoulou, V. Edible coating enriched with rosemary extracts to enhance oxidative and microbial stability of smoked eel fillets. Food Packag. Shelf Life 2017, 12, 107–113. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, S.; Wang, Y. Effects of antimicrobial coating from catfish skin gelatin on quality and shelf life of fresh white shrimp (Penaeus vannamei). J. Food Sci. 2011, 76, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Bansal, N.; Yang, H. Fish gelatin combined with chitosan coating inhibits myofibril degradation of golden pomfret (Trachinotus blochii) fillet during cold storage. Food Chem. 2016, 200, 283–292. [Google Scholar] [CrossRef]
- Kim, H.J.; Son, K.T.; Lee, S.G.; Park, S.Y.; Heu, M.S.; Kim, J.S. Suppression of lipid deterioration in boiled-dried anchovy by coating with fish gelatin hydrolysates. J. Food Biochem. 2017, 41. [Google Scholar] [CrossRef]
- Prudêncio, C.V.; dos Santos, M.T.; Vanetti, M.C.D. Strategies for the use of bacteriocins in Gram-negative bacteria: Relevance in food microbiology. J. Food Sci. Technol. 2015, 52, 5408–5417. [Google Scholar] [CrossRef]
- Shokri, S.; Ehsani, A.; Jasour, M.S. Efficacy of lactoperoxidase system-whey protein coating on shelf-life extension of rainbow trout fillets during cold storage (4 °C). Food Bioprocess. Technol. 2015, 8, 54–62. [Google Scholar] [CrossRef]
- Jasour, M.S.; Ehsani, A.; Mehryar, L.; Naghibi, S.S. Chitosan coating incorporated with the lactoperoxidase system: An active edible coating for fish preservation. J. Sci. Food Agric. 2015, 95, 1373–1378. [Google Scholar] [CrossRef]
- Shokri, S.; Ehsani, A. Efficacy of whey protein coating incorporated with lactoperoxidase and α-tocopherol in shelf life extension of Pike-Perch fillets during refrigeration. LWT—Food Sci. Technol. 2017, 85, 225–231. [Google Scholar] [CrossRef]
- Farshidi, M.; Yousefi, M.; Ehsani, A. The combined effects of lactoperoxidase system and whey protein coating on microbial, chemical, textural, and sensory quality of shrimp (Penaeus merguiensis) during cold storage (4 ± 1 °C). Food Sci. Nutr. 2018, 6, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Barkhori, M.S.; Khanzadi, S.; Hashemi, M.; Azizzadeh, M. The effect of sodium alginate coating incorporated with lactoperoxidase system and Zataria multiflora boiss essential oil on shelf life extension of rainbow trout fillets during refrigeration. Iran. J. Chem. Chem. Eng. 2019, 38, 163–172. [Google Scholar]
- Lu, F.; Ding, Y.; Ye, X.; Liu, D. Cinnamon and nisin in alginate-calcium coating maintain quality of fresh northern snakehead fish fillets. LWT—Food Sci. Technol. 2010, 43, 1331–1335. [Google Scholar] [CrossRef]
- Andevari, G.T.; Rezaei, M. Effect of gelatin coating incorporated with cinnamon oil on the quality of fresh rainbow trout in cold storage. Int. J. Food Sci. Technol. 2011, 46, 2305–2311. [Google Scholar] [CrossRef]
- Aşik, E.; Candoǧan, K. Effects of chitosan coatings incorporated with garlic oil on quality characteristics of shrimp. J. Food Qual. 2014, 37, 237–246. [Google Scholar] [CrossRef]
- Heydari, R.; Bavandi, S.; Seyed, R.J. Effect of sodium alginate coating enriched with horsemint (Mentha longifolia) essential oil on the quality of bighead carp fillets during storage at 4 °C. Food Sci. Nutr. 2015, 3, 188–194. [Google Scholar] [CrossRef]
- Karami, N.; Kamkar, A.; Shahbazi, Y.; Misaghi, A. Edible films based on chitosan-flaxseed mucilage: In vitro antimicrobial and antioxidant properties and their application on survival of food-borne pathogenic bacteria in raw minced trout fillets. Pharm. Biomed. Res. 2019, 5, 10–16. [Google Scholar] [CrossRef]
- Kim, I.H.; Yang, H.J.; Noh, B.S.; Chung, S.J.; Min, S.C. Development of a defatted mustard meal-based composite film and its application to smoked salmon to retard lipid oxidation. Food Chem. 2012, 133, 1501–1509. [Google Scholar] [CrossRef]
- Guo, M.; Jin, T.Z.; Scullen, O.J.; Sommers, C.H. Effects of antimicrobial coatings and cryogenic freezing on survival and growth of Listeria innocua on frozen ready-to-eat shrimp during thawing. J. Food Sci. 2013, 78, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- García-Soto, B.; Miranda, J.M.; Rodríguez-Bernaldo de Quirós, A.; Sendón, R.; Rodríguez-Martínez, A.V.; Barros-Velázquez, J.; Aubourg, S.P. Effect of biodegradable film (lyophilised alga Fucus spiralis and sorbic acid) on quality properties of refrigerated megrim (Lepidorhombus whiffiagonis). Int. J. Food Sci. Technol. 2015, 50, 1891–1900. [Google Scholar] [CrossRef]
- Javidi, Z.; Hosseini, S.F.; Rezaei, M. Development of flexible bactericidal films based on poly(lactic acid) and essential oil and its effectiveness to reduce microbial growth of refrigerated rainbow trout. LWT—Food Sci. Technol. 2016, 72, 251–260. [Google Scholar] [CrossRef]
- Cardoso, L.G.; Pereira Santos, J.C.; Camilloto, G.P.; Miranda, A.L.; Druzian, J.I.; Guimarães, A.G. Development of active films poly (butylene adipate co-terephthalate)—PBAT incorporated with oregano essential oil and application in fish fillet preservation. Ind. Crops Prod. 2017, 108, 388–397. [Google Scholar] [CrossRef]
- Kakaei, S.; Shahbazi, Y. Effect of chitosan-gelatin film incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil on survival of Listeria monocytogenes and chemical, microbial and sensory properties of minced trout fillet. LWT—Food Sci. Technol. 2016, 72, 432–438. [Google Scholar] [CrossRef]
- Merlo, T.C.; Contreras-Castillo, C.J.; Saldaña, E.; Barancelli, G.V.; Dargelio, M.D.B.; Yoshida, C.M.P.; Ribeiro Junior, E.E.; Massarioli, A.; Venturini, A.C. Incorporation of pink pepper residue extract into chitosan film combined with a modified atmosphere packaging: Effects on the shelf life of salmon fillets. Food Res. Int. 2019, 125, 108633. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.O.; Ravishankar, C.N.; Lalitha, K.V.; Srinivasa Gopal, T.K. Effect of chitosan edible coating on the quality of double filleted Indian oil sardine (Sardinella longiceps) during chilled storage. Food Hydrocoll. 2012, 26, 167–174. [Google Scholar] [CrossRef]
- Fan, W.; Sun, J.; Chen, Y.; Qiu, J.; Zhang, Y.; Chi, Y. Effects of chitosan coating on quality and shelf life of silver carp during frozen storage. Food Chem. 2009, 115, 66–70. [Google Scholar] [CrossRef]
- Alak, G. The effect of chitosan prepared in different solvents on the quality parameters of brown trout fillets (Salmo trutta fario). Food Nutr. Sci. 2012, 3, 1303–1306. [Google Scholar] [CrossRef]
- Carrión-Granda, X.; Fernández-Pan, I.; Jaime, I.; Rovira, J.; Maté, J.I. Improvement of the microbiological quality of ready-to-eat peeled shrimps (Penaeus vannamei) by the use of chitosan coatings. Int. J. Food Microbiol. 2016, 232, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Yanar, Y.; Küçükgülmez, A.; Gokcin, M.; Gelibolu, S.; Dikel, C. Antioxidant effects of chitosan in European eel (Anguilla anguilla L.) fillets during refrigerated storage. CYTA—J. Food 2013, 11, 328–333. [Google Scholar] [CrossRef]
- Vatavali, K.; Karakosta, L.; Nathanailides, C.; Georgantelis, D.; Kontominas, M.G. Combined effect of chitosan and oregano essential oil dip on the microbiological, chemical, and sensory attributes of red porgy (Pagrus pagrus) stored in ice. Food Bioprocess. Technol. 2013, 6, 3510–3521. [Google Scholar] [CrossRef]
- Bazargani-Gilani, B. Activating sodium alginate-based edible coating using a dietary supplement for increasing the shelf life of rainbow trout fillet during refrigerated storage (4 ± 1 °C). J. Food Saf. 2018, 38, e12395. [Google Scholar] [CrossRef]
- Cai, L.; Wang, Y.; Cao, A.; Lv, Y.; Li, J. Effect of alginate coating enriched with 6-gingerol on the shelf life and quality changes of refrigerated red sea bream (Pagrosomus major) fillets. RSC Adv. 2015, 5, 36882–36889. [Google Scholar] [CrossRef]
- Concha-Meyer, A.; Schöbitz, R.; Brito, C.; Fuentes, R. Lactic acid bacteria in an alginate film inhibit Listeria monocytogenes growth on smoked salmon. Food Control. 2011, 22, 485–489. [Google Scholar] [CrossRef]
- USDA. Food Safety and Inspection Service, National Advisory Committee on Microbiological Criteria for Foods. Assessment and Management of Seafood Safety and Quality-HACCP; USDA: Washington, DC, USA, 1992.
- Regulation (EC) No 852/2004. Regulation (EC) 852/2004 of the European Parliament and of the Council of 29 April, 2004 on the hygiene of foodstuffs. Off. J. Eur. Union 2004, L139, 1–54. [Google Scholar]
- Food Standards Agency. Guidance on the Safety and Shelf-Life of Vacuum and Modified Atmosphere Packed Chilled Foods with Respect to Non-proteolytic Clostridium botulinum; Food Standards Agency: London, UK, 2017; pp. 1–29.
- Anonymous. Active and intelligent packaging news. Pira Int. 2007, 5, 13. [Google Scholar]
- Commision Directive 2002/72/EC. Commission Directive 2002/72/EC of 6 August 2002 relating to plastic materials and articles intended to come into contact with foodstuffs. Off. J. Eur. Union 2002, L220, 18–58. [Google Scholar]
- Regulation (EC) No 1935/2004. Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on materials and articles intended to come into contact with food and repealing Directives 80/590/EEC and 89/109/EEC. Off. J. Eur. Union 2004, L338/4, 4–17. [Google Scholar]
- Dainelli, D.; Gontard, N.; Spyropoulos, D.; Zondervan-van den Beuken, E.; Tobback, P. Active and intelligent food packaging: Legal aspects and safety concerns. Trends Food Sci. Technol. 2008, 19, S103–S112. [Google Scholar] [CrossRef]
- Regulation (EC) No 882/2004. Regulation (EC) No 882/2004 of the European Parliament and of the Council of 29 April 2004 on official controls performed to ensure the verification of compliance with feed and food law, animal health and animal welfare rule. Off. J. Eur. Union 2004, L165, 1–141. [Google Scholar]
- Council Directive 82/711/EEC. Council Directive 82/711/EEC of 18 October 1982 laying down the basic rules necessary for testing migration of the constituents of plastic materials and articles intended to come into contact with foodstuffs. Off. J. Eur. Commun. 1982, L297, 26–30. [Google Scholar]
- Council Directive 85/572/EEC. Council Directive 85/572/EEC laying down the list of simulants to be used for testing migration of constituents of plastic materials and articles intended to come into contact with foodstuffs. Off. J. Eur. Commun. 1985, L372, 14–21. [Google Scholar]
- Commission Regulation (EC) No 2023/2006. Commission Regulation (EC) No 2023/2006 of 22 December 2006 on good manufacturing practice for materials and articles intended to come into contact with food. Off. J. Eur. Union 2006, L384, 75–78. [Google Scholar]
- Rossman, J.M. Commercial manufacture of edible films. In Edible Films and Coatings for Food Applications; Embuscado, M.E., Hubern, K.C., Eds.; Springer Publ.: New York, NY, USA, 2009; pp. 367–390. ISBN 9780387928241. [Google Scholar]
- Martín-Belloso, O.; Rojas-Graü, M.A.; Soliva-Fortuny, R. Edible films and coatings for food applications. In Edible Films and Coatings for Food Applications; Embuscado, M.E., Huber, K.C., Eds.; Springer Publ.: New York, NY, USA, 2009; pp. 295–313. ISBN 9780387928241. [Google Scholar]
- López-Cervantes, L.; Sánchez-Machado, D.I.; Pastorelli, S.; Rijk, R.; Paseiro-Losada, P. Evaluating the migration of ingredients from active packaging and development of dedicated methods: A study of two iron-based oxygen absorbers. Food Addit. Contam. 2003, 20, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Pérez Espitia, P.J.; Du, W.X.; Avena-Bustillos, R.J.; Soares, N.F.F.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties—A review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]

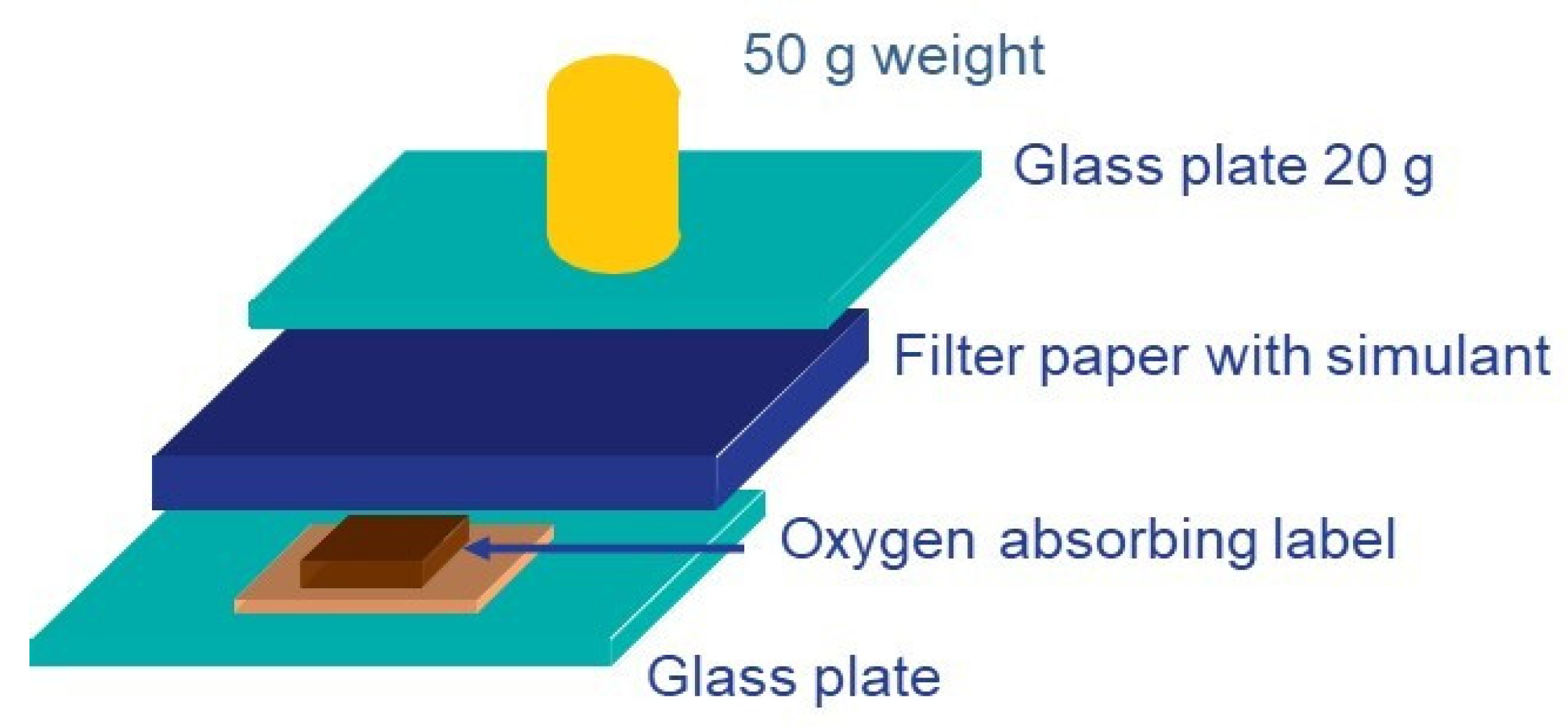
| Τype of Active Packaging | Type of Food | Potential Benefit |
|---|---|---|
| Active scavenging systems | ||
| Oxygen absorber | Fatty fish | Prevention of rancidity and discoloration |
| Moisture absorber | Fresh fish | Shelf life extension, reduction of moisture condensation within the package |
| Active releasing systems | ||
| Antioxidant releaser | Fresh fatty fish | Enhancement of oxidative stability |
| Carbon dioxide emitter | Fresh fish | Shelf life extension, reduction in head space volume of MAP |
| Antimicrobial releaser | Fresh and smoked fish, fresh seafood | Retardation of microbial growth, shelf life extension |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontominas, M.G.; Badeka, A.V.; Kosma, I.S.; Nathanailides, C.I. Recent Developments in Seafood Packaging Technologies. Foods 2021, 10, 940. https://doi.org/10.3390/foods10050940
Kontominas MG, Badeka AV, Kosma IS, Nathanailides CI. Recent Developments in Seafood Packaging Technologies. Foods. 2021; 10(5):940. https://doi.org/10.3390/foods10050940
Chicago/Turabian StyleKontominas, Michael G., Anastasia V. Badeka, Ioanna S. Kosma, and Cosmas I. Nathanailides. 2021. "Recent Developments in Seafood Packaging Technologies" Foods 10, no. 5: 940. https://doi.org/10.3390/foods10050940
APA StyleKontominas, M. G., Badeka, A. V., Kosma, I. S., & Nathanailides, C. I. (2021). Recent Developments in Seafood Packaging Technologies. Foods, 10(5), 940. https://doi.org/10.3390/foods10050940








