Influence of Two Innovative Packaging Materials on Quality Parameters and Aromatic Fingerprint of Extra-Virgin Olive Oils
Abstract
1. Introduction
2. Materials and Methods
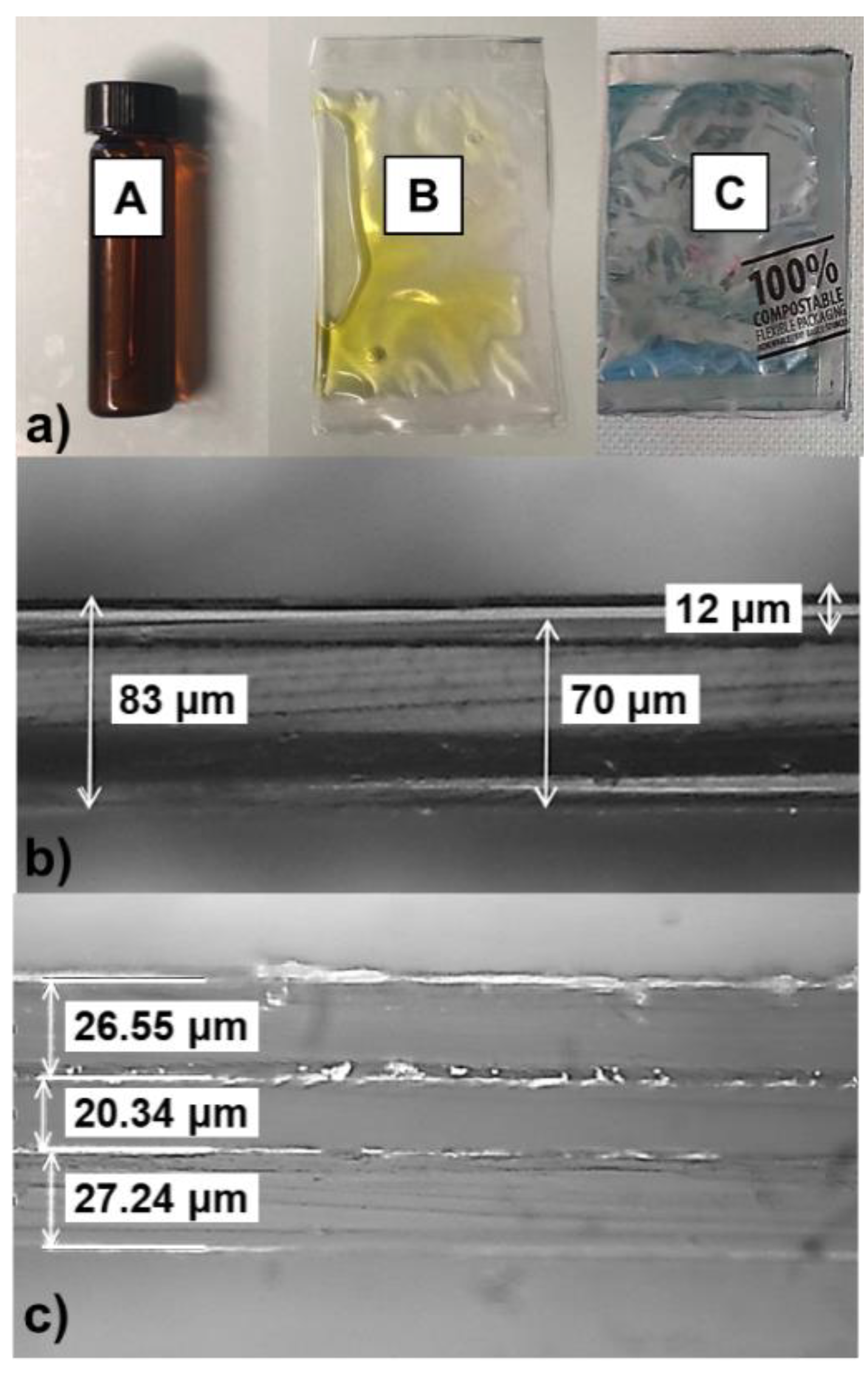
2.1. Packaging Materials
2.2. Olive Oil Samples
2.3. Oxygen Barrier Properties of Packaging Films
2.4. Olive Oil Quality Parameters
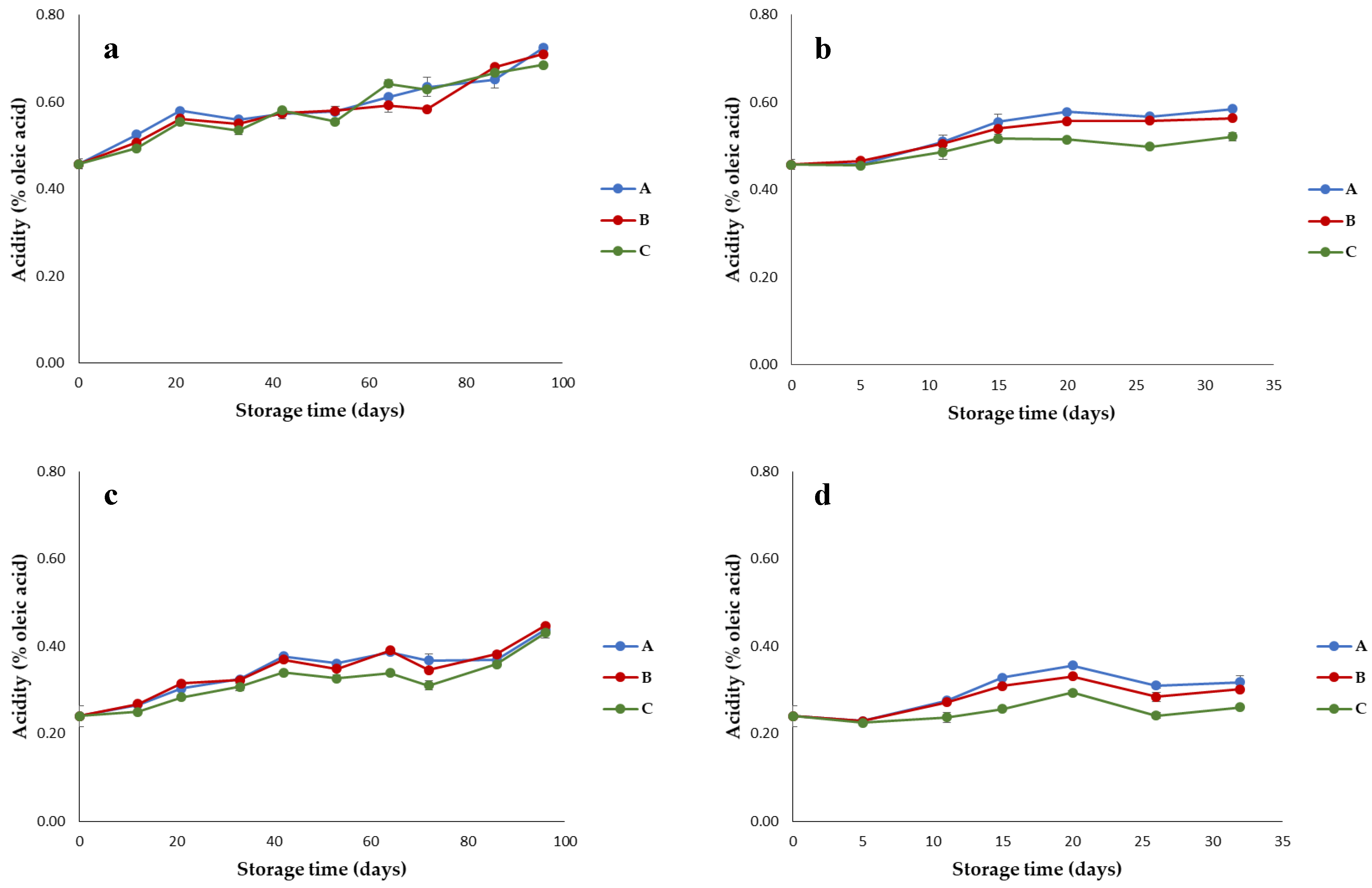
- Acidity, indicative of the free fatty acid content and expressed as oleic acid (% oleic acid);
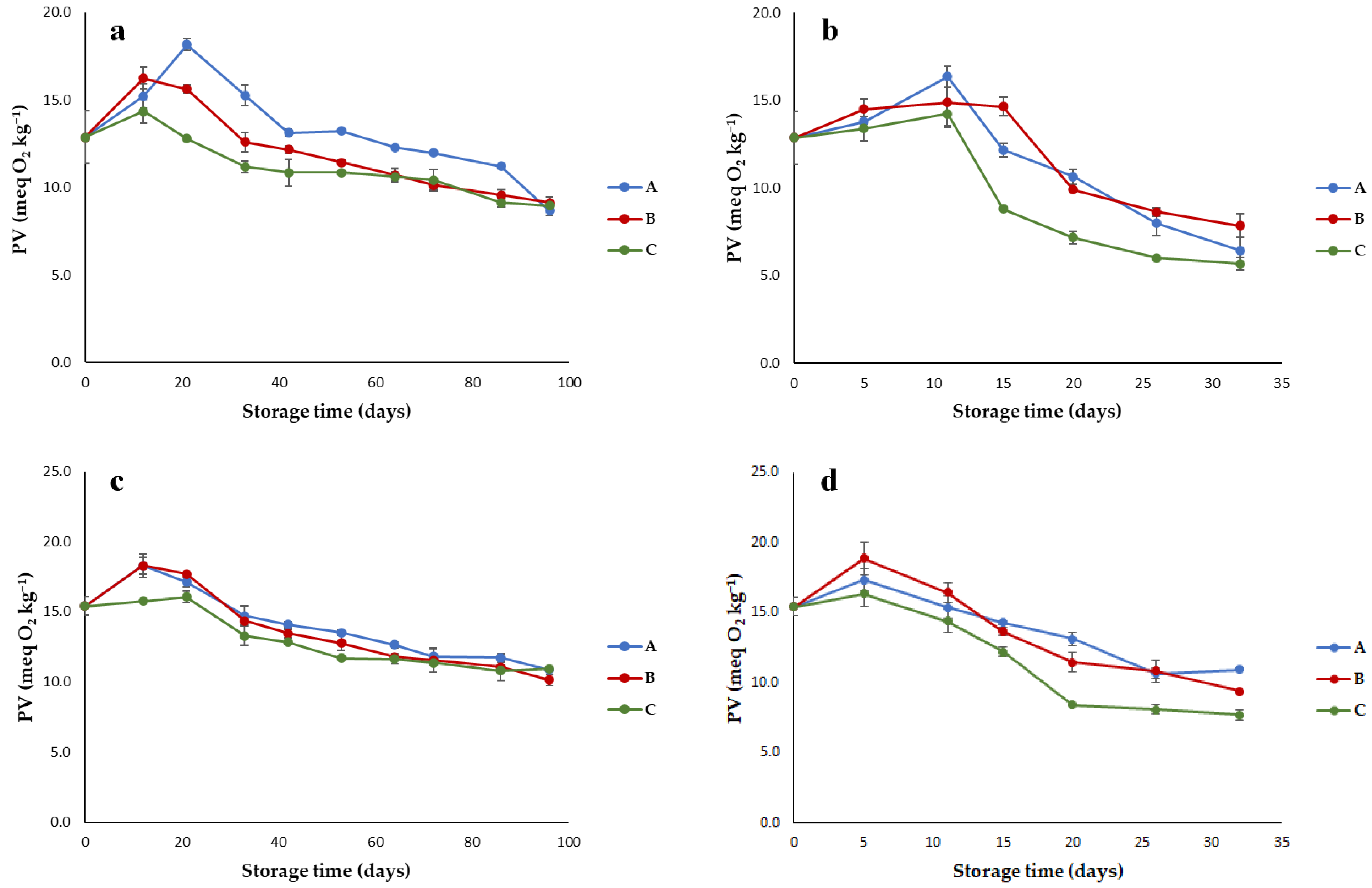
- Peroxide value (PV) corresponding to the amount of hydroperoxides (meq O2kg−1);
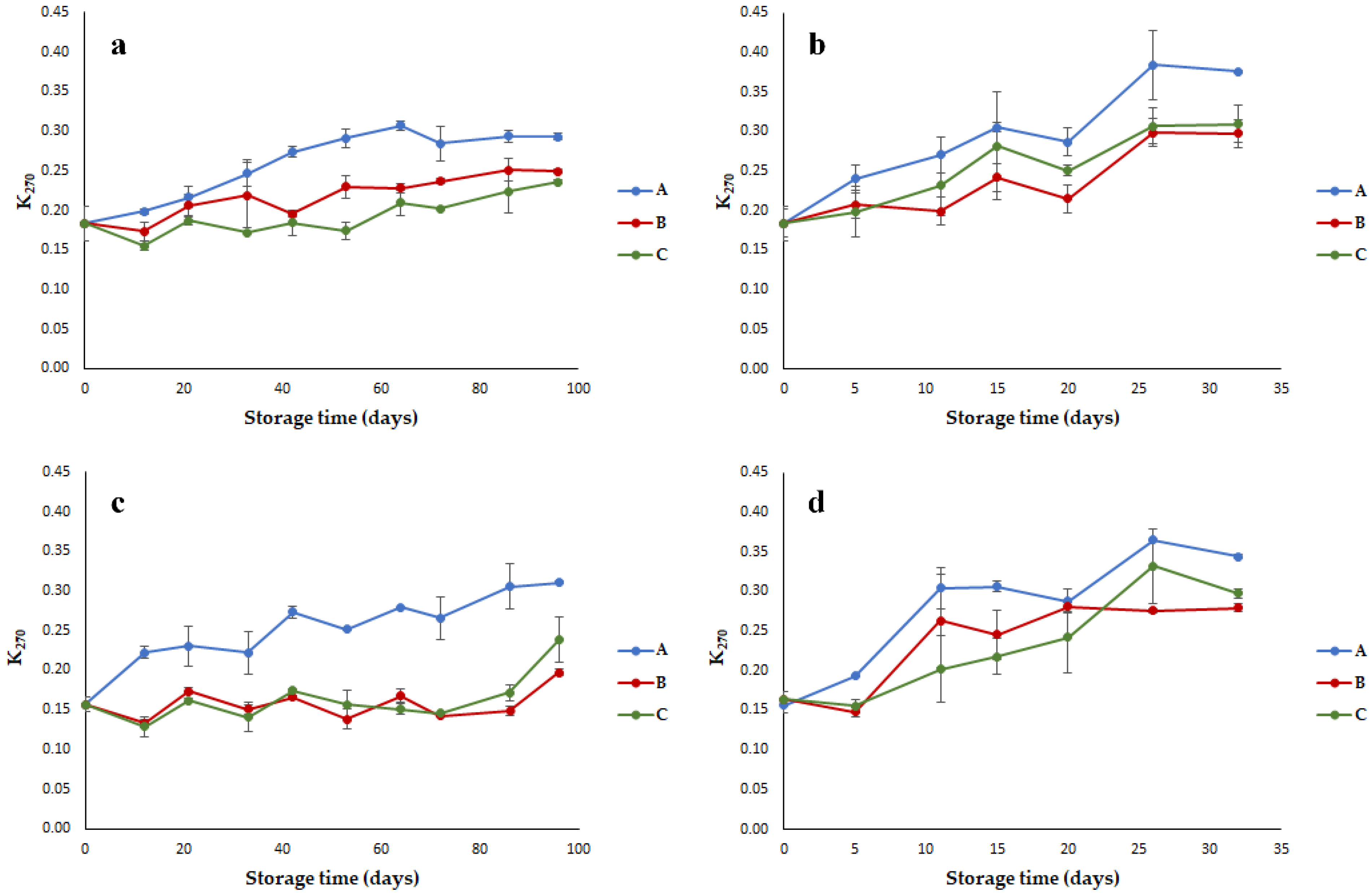
- Specific extinction at 270 nm (K270) providing a measurement of the secondary oxidation products.
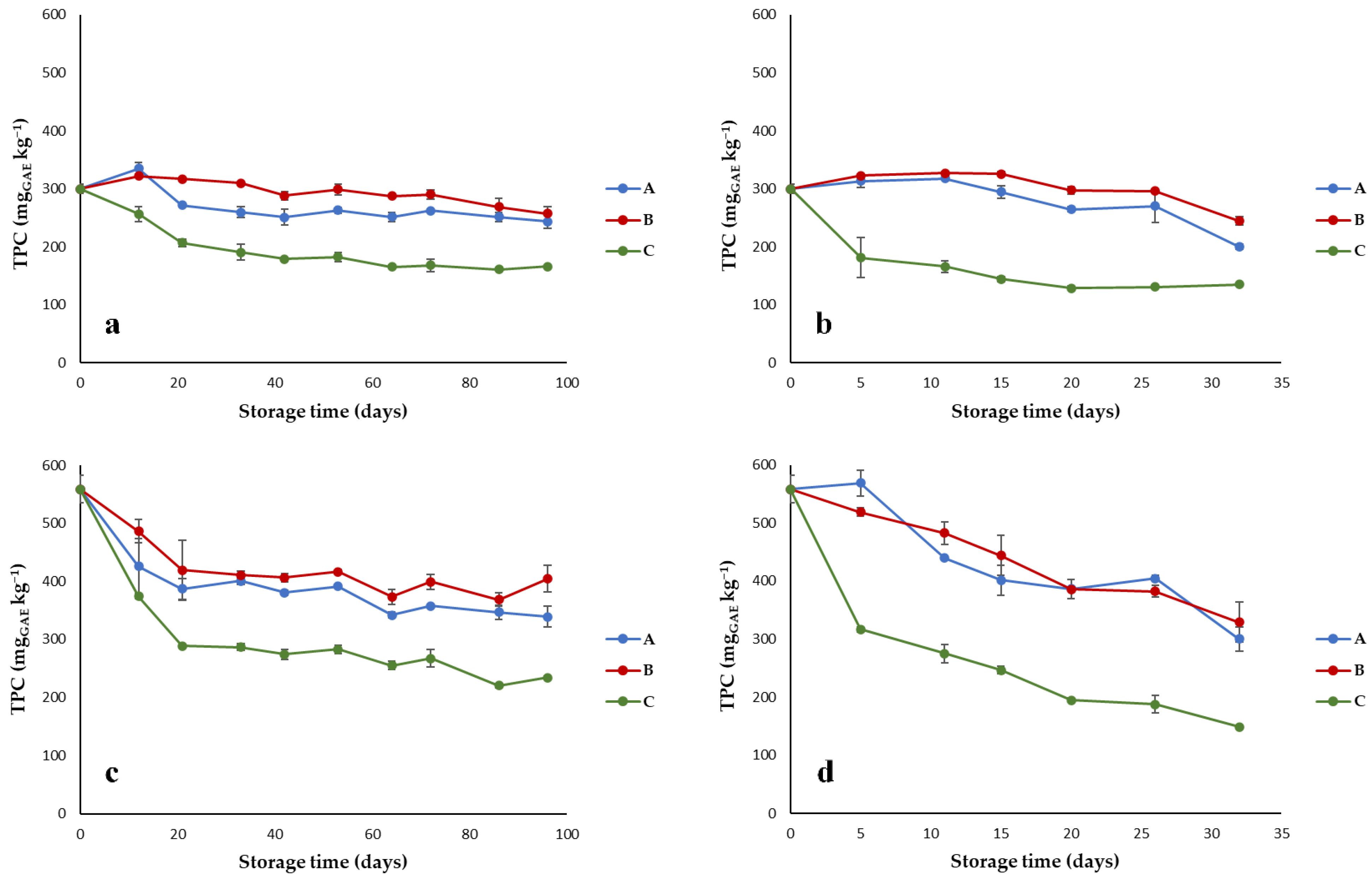
- Total phenolic content (TPC): oil samples were extracted with pure methanol as follows: 2 g oil was added to 5 mL methanol in a centrifuge tube, and the mixture was sonicated for 15 min. After sonication, the tube was centrifuged at 3500 rpm for 15 min at 15 °C, and the methanolic phase (extract) was separated; each sample was extracted in duplicate. TPC were determined by the Folin–Ciocalteu method [13], modified as follow: 0.5 mL of extract was added with 2.5 mL distilled water, 0.5 mL Folin–Ciocalteu reagent, and 2 mL Na2CO3 10%, and the mixture was taken to 10 mL with distilled water. After 90 min rest in the dark, the mixture was filtered with 0.2 mm Whatman filter, and the absorbance was read at 750 nm (Spectrophotometer V-650, Jasco, Japan). Results were expressed as gallic acid equivalents (mgGAE kg−1). Each extract was analyzed in duplicate.
2.5. Electronic Nose Analysis
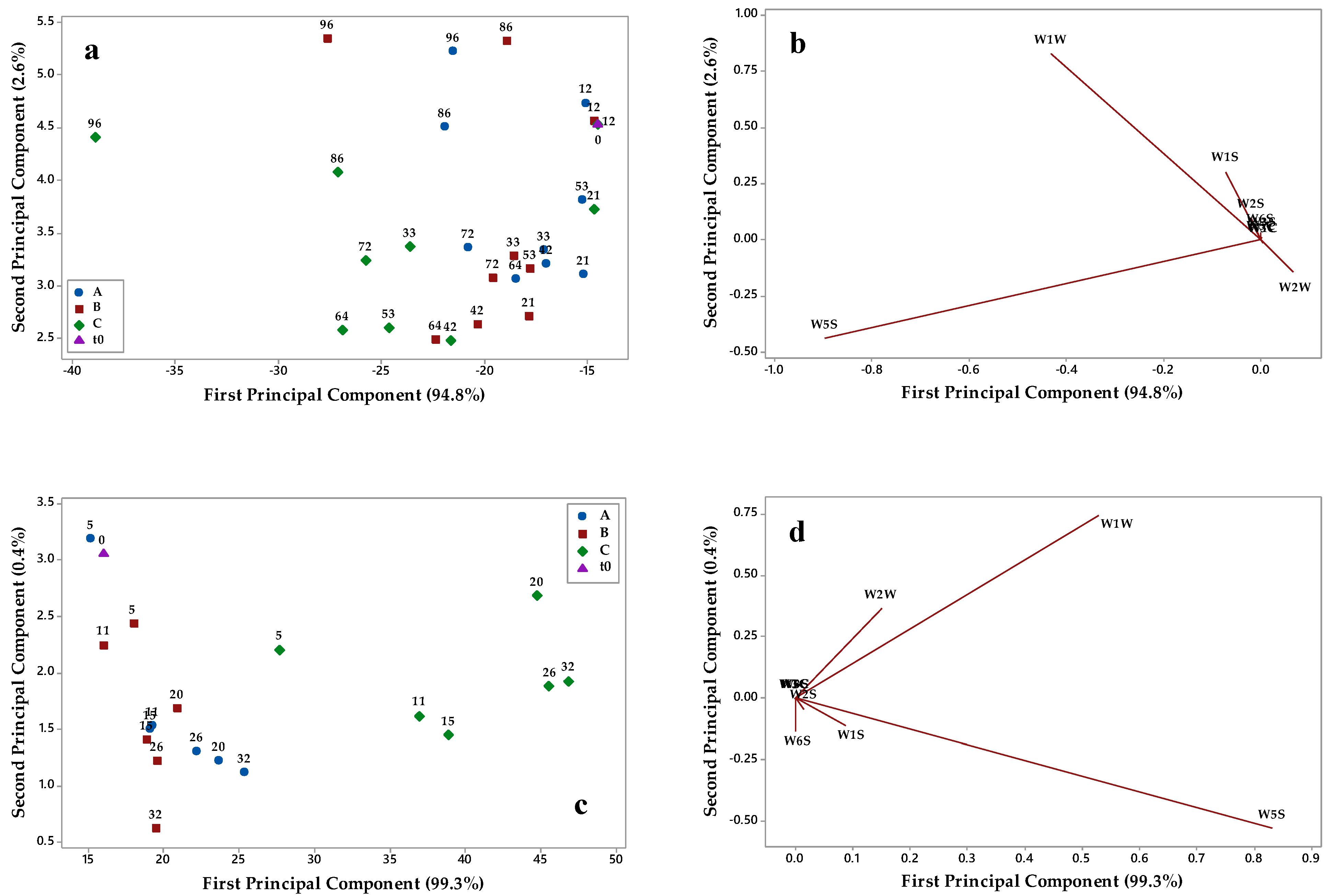
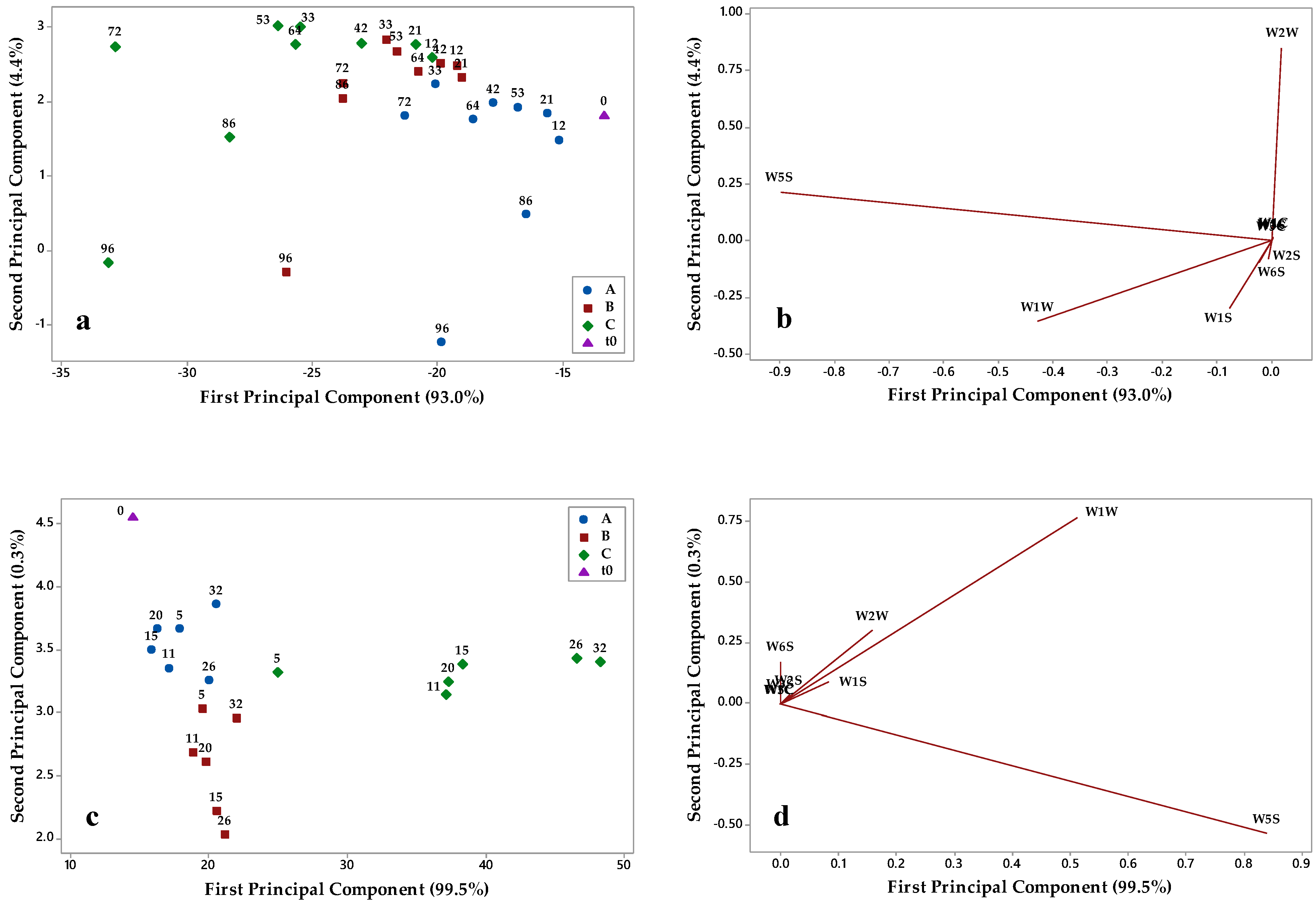
2.6. Data Analysis
3. Results and Discussion
3.1. Quality Parameters of Olive Oils Stored under Different Conditions
3.2. E-Nose Aromatic Profile of Olive Oils Stored under Different Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The next generation of sustainable food packaging to preserve our environment in a circular economy context. Front. Nutr. 2018, 5, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Boesen, S.; Bey, N.; Niero, M. Environmental sustainability of liquid food packaging: Is there a gap between Danish consumers’ perception and learnings from life cycle assessment? J. Clean. Prod. 2019, 210, 1193–1206. [Google Scholar] [CrossRef]
- Giovenzana, V.; Casson, A.; Beghi, R.; Tugnolo, A.; Grassi, S.; Alamprese, C.; Casiraghi, E.; Farris, S.; Fiorindo, I.; Guidetti, R. Environmental Benefits: Traditional vs. Innovative Packaging for Olive Oil. Chem. Eng. Trans. 2019, 75, 193–198. [Google Scholar]
- Gomes, T.S.; Visconte, L.L.Y.; Pacheco, E. Life cycle assessment of polyethylene terephthalate packaging: An overview. J. Polym. Environ. 2019, 27, 533–548. [Google Scholar] [CrossRef]
- Ferrara, C.; De Feo, G. Comparative life cycle assessment of alternative systems for wine packaging in Italy. J. Clean. Prod. 2020, 259, 120888–120900. [Google Scholar] [CrossRef]
- Flexible Packaging Association. Available online: https://www.flexpack.org/sustainable-packaging (accessed on 1 January 2021).
- Pristouri, G.; Badeka, A.; Kontominas, M.G. Effect of packaging material headspace, oxygen and light transmission, temperature and storage time on quality characteristics of extra virgin olive oil. Food Control 2010, 21, 412–418. [Google Scholar] [CrossRef]
- Gargouri, B.; Zribi, A.; Bouaziz, M. Effect of containers on the quality of Chemlali olive oil during storage. J. Food Sci. Technol. 2015, 52, 1948–1959. [Google Scholar] [CrossRef]
- Lolis, A.; Badeka, A.V.; Kontominas, M.G. Effect of bag-in-box packaging material on quality characteristics of extra virgin olive oil stored under household and abuse temperature conditions. Food Packag. Shelf Life 2019, 21, 100368–100377. [Google Scholar] [CrossRef]
- Lolis, A.; Badeka, A.V.; Kontominas, M.G. Quality retention of extra virgin olive oil, Koroneiki cv. packaged in bag-inbox containers under long term storage: A comparison to packaging in dark glass bottles. Food Packag. Shelf Life 2020, 26, 100549–100559. [Google Scholar] [CrossRef]
- Tan, J.; Xu, J. Applications of electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties determination: A review. Artif. Intell. Agric. 2020, 4, 104–115. [Google Scholar] [CrossRef]
- Commission Regulation (EEC) No 2568/91 of 11 July 1991 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Eur. Union 1991, L248, 1–128.
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Xu, L.; Yu, X.; Liu, L.; Zhang, R. A novel method for qualitative analysis of edible oil oxidation using an electronic nose. Food Chem. 2016, 202, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Borgognone, M.G.; Bussi, J.; Hough, G. Principal component analysis in sensory analysis: Covariance or correlation matrix? Food Qual. Prefer. 2001, 12, 323–326. [Google Scholar] [CrossRef]
- Ben-Hassine, K.; Taamalli, A.; Ferchichi, S.; Mlaouah, A.; Benincasa, C.; Romano, E.; Flamini, G.; Lazzez, A.; Grati-kamoun, N.; Perri, E.; et al. Physico-chemical and sensory characteristics of virgin olive oils in relation to cultivar, extraction system and storage conditions. Food Res. Int. 2013, 54, 1915–1925. [Google Scholar] [CrossRef]
- Vacca, V.; Del Caro, A.; Poiana, M.; Piga, A. Effect of storage period and exposure conditions on the quality of Bosana extra-virgin olive oil. J. Food Qual. 2006, 29, 139–150. [Google Scholar] [CrossRef]
- Ciafardini, G.; Zullo, B.A.; Iride, A. Lipase production by yeasts from extra virgin olive oil. Food Microbiol. 2006, 23, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Bustan, A.; Kerem, Z.; Yermiyahu, U.; Ben-Gal, A.; Lichter, A.; Droby, S.; Zchori-Fein, E.; Orbach, D.; Zipori, I.; Dag, A. Preharvest circumstances leading to elevated oil acidity in ‘Barnea’ olives. Sci. Hortic. 2014, 176, 11–21. [Google Scholar] [CrossRef]
- Ciafardini, G.; Zullo, B.A. Effect of lipolytic activity of Candida adriatica, Candida diddensiae and Yamadazyma terventina on the acidity of extra-virgin olive oil with a different polyphenol and water content. Food Microbiol. 2015, 47, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Commission Delegated Regulation (EU) 2016/2095 of 26 September 2016 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Eur. Union 2016, L326, 1–6.
- Conte, L.; Milani, A.; Calligaris, S.; Rovellini, P.; Lucci, P.; Nicoli, M.C. Temperature dependence of oxidation kinetics of extra virgin olive oil (EVOO) and shelf-life prediction. Foods 2020, 9, 295. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Gertz, C.; Klostermann, S. A new analytical procedure to differentiate virgin or non-refined from refined vegetable fats and oils. Eur. J. Lipid Sci. Technol. 2000, 102, 329–336. [Google Scholar] [CrossRef]
- Krichene, D.; Salvador, M.D.; Fregapane, G. Stability of virgin olive oil phenolic compounds during long-term storage (18 months) at temperatures of 5–50 °C. J. Agric. Food Chem. 2015, 63, 6779–6786. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak, T.; Wojnowski, W.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Electronic noses in classification and quality control of edible oils: A review. Food Chem. 2018, 246, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Pedan, V.; Popp, M.; Rohn, S.; Nyfeler, M.; Bongartz, A. Characterization of phenolic compounds and their contribution to sensory properties of olive oil. Molecules 2019, 24, 2041. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Caporaso, N.; Villani, V.; Paduano, A.; Sacchi, R. Olive oil phenolic compounds affect the release of aroma compounds. Food Chem. 2015, 181, 284–294. [Google Scholar] [CrossRef]
- Giovenzana, V.; Beghi, R.; Buratti, S.; Civelli, R.; Guidetti, R. Monitoring of fresh-cut Valerianella locusta Laterr. shelf life by electronic nose and VIS–NIR spectroscopy. Talanta 2014, 120, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, S.; Buratti, S.; Spinardi, A.; Mannino, S.; Mignani, I. Electronic nose as a non-destructive tool to characterise peach cultivars and to monitor their ripening stage during shelf-life. Postharvest Biol. Technol. 2008, 47, 181–188. [Google Scholar] [CrossRef]
| Source | Acidity | PV | K270 | TPC (Squared) |
|---|---|---|---|---|
| Covariate | ||||
| Storage time | 476.41 *** | 206.32 *** | 93.88 *** | 129.81 *** |
| Main effects | ||||
| Packaging | 10.13 *** | 16.47 *** | 51.02 *** | 354.02 *** |
| Oil cultivar | 4000.51 *** | 36.97 *** | 15.72 *** | 486.69 *** |
| Storage temperature | 2.91 n.s. | 124.73 *** | 176.04 *** | 60.93 *** |
| Interactions | ||||
| Packaging × Cultivar | 8.57 *** | 0.14 n.s. | 1.68 n.s. | 2.53 n.s. |
| Packaging × Temperature | 10.11 *** | 2.55 n.s. | 5.15 ** | 22.34 *** |
| Cultivar × Temperature | 4.77 * | 1.79 n.s. | 3.06 n.s. | 0.74 n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farris, S.; Buratti, S.; Benedetti, S.; Rovera, C.; Casiraghi, E.; Alamprese, C. Influence of Two Innovative Packaging Materials on Quality Parameters and Aromatic Fingerprint of Extra-Virgin Olive Oils. Foods 2021, 10, 929. https://doi.org/10.3390/foods10050929
Farris S, Buratti S, Benedetti S, Rovera C, Casiraghi E, Alamprese C. Influence of Two Innovative Packaging Materials on Quality Parameters and Aromatic Fingerprint of Extra-Virgin Olive Oils. Foods. 2021; 10(5):929. https://doi.org/10.3390/foods10050929
Chicago/Turabian StyleFarris, Stefano, Susanna Buratti, Simona Benedetti, Cesare Rovera, Ernestina Casiraghi, and Cristina Alamprese. 2021. "Influence of Two Innovative Packaging Materials on Quality Parameters and Aromatic Fingerprint of Extra-Virgin Olive Oils" Foods 10, no. 5: 929. https://doi.org/10.3390/foods10050929
APA StyleFarris, S., Buratti, S., Benedetti, S., Rovera, C., Casiraghi, E., & Alamprese, C. (2021). Influence of Two Innovative Packaging Materials on Quality Parameters and Aromatic Fingerprint of Extra-Virgin Olive Oils. Foods, 10(5), 929. https://doi.org/10.3390/foods10050929








