An Event-Specific Real-Time PCR Method for Measuring Transgenic Lysozyme Goat Content in Trace Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. DNA Extraction
2.3. Primers and Probes
2.4. Conventional PCR and Quantitative Real-Time PCR
2.5. Evaluation of the Performance of the Event-Specific Real-Time PCR Assay
2.6. Application of the Developed Real-Time PCR Assay in Practical Sample Analysis
3. Results and Discussion
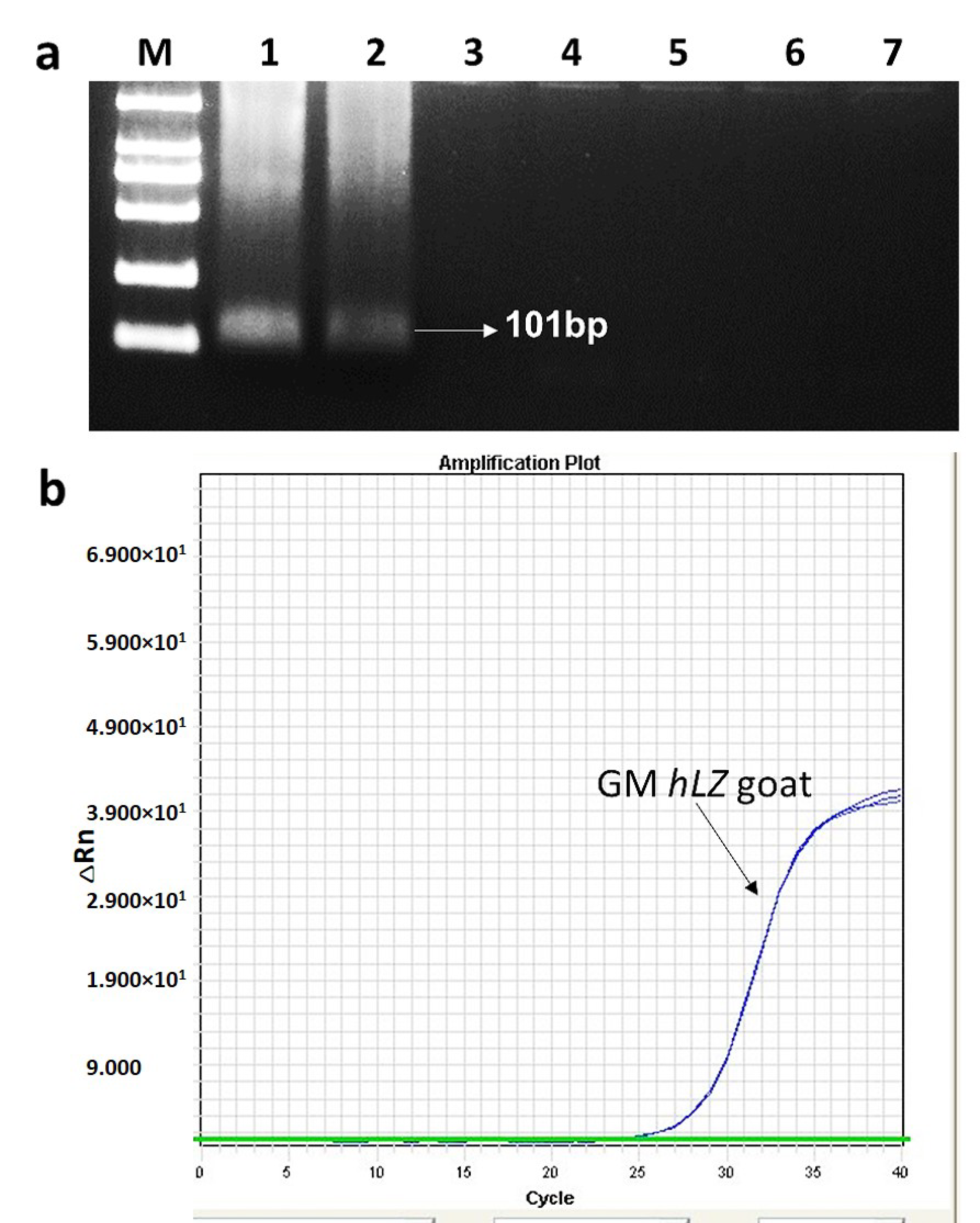
3.1. Specificity of the Event-Specific Real-Time PCR Assay for Transgenic hLZ Goats
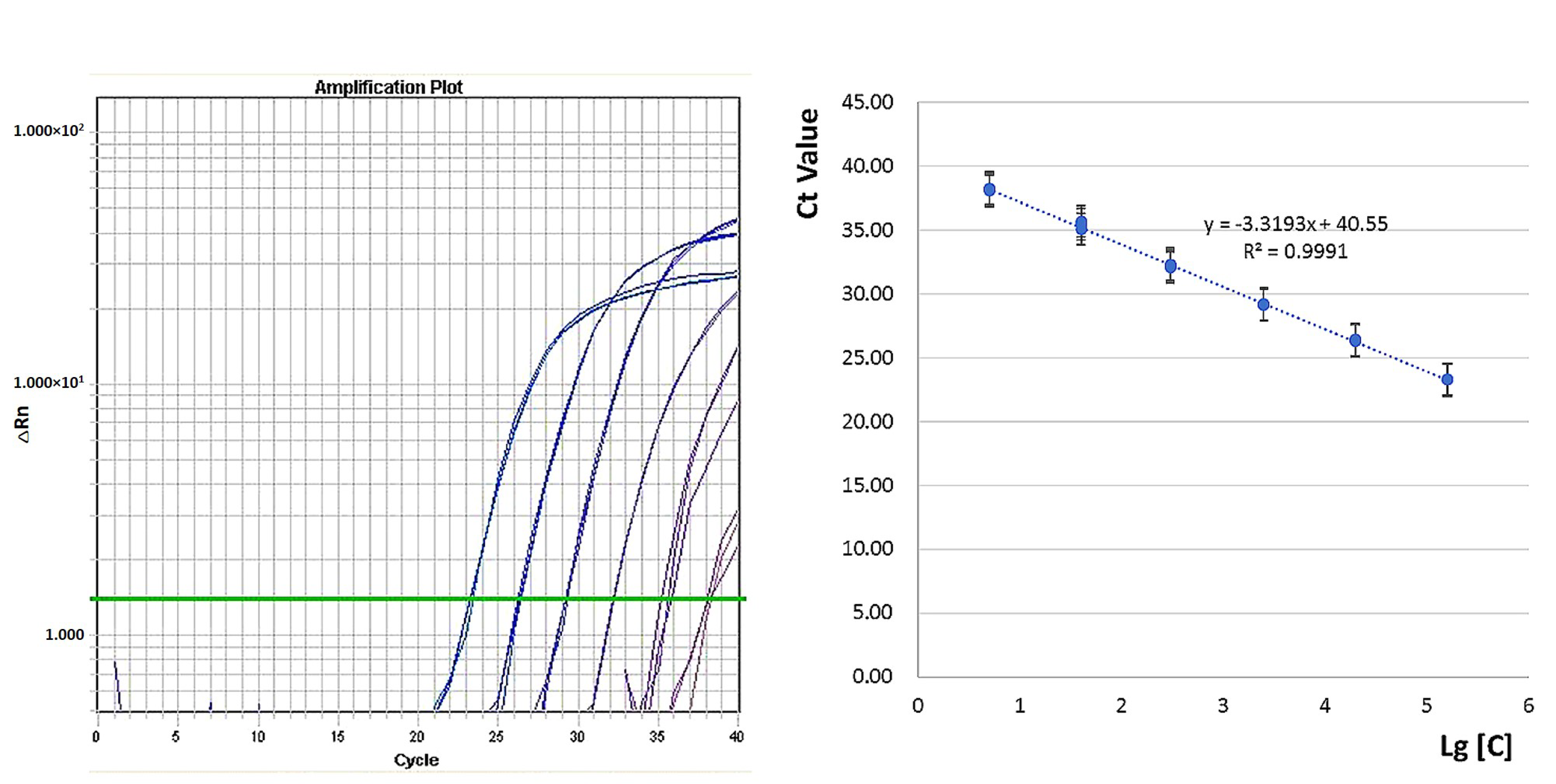
3.2. Standard Curve Construction and Dynamic Range Determination
3.3. LOD and LOQ Determination
3.4. Repeatability of the qPCR Assay
3.5. Quantitative Analysis of Simulated Blood Samples
3.6. Determination of Transgenic hLZ Goat Content in Practical Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miao, X. Recent advances in the development of new transgenic animal technology. Cell Mol. Life Sci. 2013, 70, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Van Veen, H.A.; Koiter, J.; Vogelezang, C.J.; Van Wessel, N.; Van Dam, T.; Velterop, I.; Van Houdt, K.; Kupers, L.; Horbach, D.; Salaheddine, M.; et al. Characterization of recombinant human C1 inhibitor secreted in milk of transgenic rabbits. J. Biotechnol. 2012, 162, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Ledford, H. Salmon approval heralds rethink of transgenic animals. Nature 2015, 527, 417–418. [Google Scholar] [CrossRef] [PubMed]
- CAC. Guideline for the Conduct of Food Safety Assessment of Foods Derived from Recombinant-DNA Animals; FAO/WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Moghissi, A.A.; Jaeger, L.M.; Shafei, D.; Bloom, L.L. Regulatory science requirements of labeling of genetically modified food. Crit. Rev. Biotechnol. 2018, 38, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, C.M.; Shan, G.M.; Layton, D.T.; Bell, T.A.; Whipkey, S.; Shillito, R.D. Application of DNA and Protein Based Detection Methods in Agricultural Biotechnology. J. Agric. Food Chem. 2019, 67, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Salisu, I.B.; Shahid, A.A.; Yaqoob, A.; Ali, Q.; Bajwa, K.S.; Rao, A.Q.; Husnain, T. Molecular Approaches for High Throughput Detection and Quantification of Genetically Modified Crops: A Review. Front. Plant. Sci. 2017, 8, 1670. [Google Scholar] [CrossRef] [PubMed]
- Kamle, S.; Ali, S. Genetically modified crops: Detection strategies and biosafety issues. Gene 2013, 522, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Guo, J. The development and standardization of testing methods for genetically modified organisms and their derived products. J. Integr. Plant. Biol. 2011, 53, 539–551. [Google Scholar] [CrossRef]
- Huang, J.; Wang, A.; Huang, C.; Sun, Y.; Song, B.; Zhou, R.; Li, L. Generation of marker-free pbd-2 knock-in pigs using the CRISPR/Cas9 and Cre/loxP systems. J. Genes. 2020, 11, 951. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhu, Z.W.; Yu, F.X.; Huang, J.; Hu, X.R.; Pan, J.Z. Production of germline transgenic pigs co-expressing double fluorescent proteins by lentiviral vector. J. Anim. Reprod. Sci. 2016, 174, 11–19. [Google Scholar] [CrossRef]
- Li, X.L.; Wang, Q.; Zhu, Z.; Wu, S.Q.; Qiu, S.Y.; Liu, X.F.; Sun, M.; Pan, D.K.; Lin, X.M. Establishment of multiplex PCR for detection of myostatin knockout pigs. J. Nanjing Agric. Univ. 2015, 38, 993–997. [Google Scholar]
- Yang, X.; Cheng, Y.T.; Tan, M.F.; Zhang, H.W.; Liu, W.Q.; Zou, G.; Zhang, L.S.; Zhang, C.Y.; Deng, S.M.; Yu, L.; et al. Over expression of porcine beta-defensin 2 enhances resistance to actinobacillus pleuropneumoniae infection in pigs. J. Infect. Immun. 2015, 83, 2836–2843. [Google Scholar] [CrossRef]
- Liu, X.F.; Qiu, S.Y.; Li, X.L.; Liu, D.D.; Jing, H.L.; Wang, Q.; Lin, X.M.; Pan, D.K.; Shi, N.N. Establishment of a Decaplex PCR-Capillary Gel Electrophoresis Method for the Simultaneous Detection of Six Kinds of Genetically Modified Animals. J. AOAC Int. 2018, 2, 2. [Google Scholar] [CrossRef]
- Hafsa, A.B.; Nabi, N.; Zellama, S.M.; Said, K.; Chaouachi, M. A new specific reference gene based on growth hormone gene (GH1) used for detection and relative quantification of Aquadvantage GM salmon (Salmo salar L.) in food products. Food Chem. 2016, 190, 1040–1045. [Google Scholar] [CrossRef]
- Nakajima, O.; Nakamura, K.; Kondo, K.; Akiyama, H.; Teshima, R. Method of detecting genetically modified chicken containing human erythropoietin gene. Biol. Pharm. Bull. 2013, 36, 1454–1459. [Google Scholar] [CrossRef]
- Ragland, S.A.; Criss, A.K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS. Pathog. 2017, 13, 9. [Google Scholar] [CrossRef]
- Gui, T.; Zhang, M.; Chen, J.W.; Zhang, Y.L.; Zhou, N.; Zhang, Y.; Tao, J.; Sui, L.C.; Li, Y.S.; Liu, Y.; et al. In vitro evaluation of a mammary gland specific expression vector encoding recombinant human lysozyme for development of transgenic dairy goat embryos. Biotechnol. Lett. 2012, 34, 1445–1452. [Google Scholar] [CrossRef]
- Maga, E.A.; Shoemaker, C.F.; Rowe, J.D.; Bondurant, R.H.; Anderson, G.B.; Murray, J.D. Production and processing of milk from transgenic goats expressing human lysozyme in the mammary gland. J. Dairy Sci. 2006, 89, 518–524. [Google Scholar] [CrossRef]
- Yu, H.; Chen, J.Q.; Liu, S.; Zhang, A.; Xu, X.J.; Wang, X.B.; Lu, P.; Cheng, G.X. Large-scale production of functional human lysozyme in transgenic cloned goats. J. Biotechnol. 2013, 168, 676–683. [Google Scholar] [CrossRef]
- Wang, Q.; Cai, Y.; He, Y.; Yang, L.; Pan, L. Droplet digital PCR (ddPCR) method for the detection and quantification of goat and sheep derivatives in commercial meat products. Eur. Food Res. Technol. 2018, 244, 767–774. [Google Scholar] [CrossRef]
- Marchiesi, U.; Mazzara, M.; Broll, H.; Giacomo, D.M.; European Network of GMO Laboratories. Definition of minimum performance requirements for analytical methods of GMO testing. 2015. Available online: http://gmo-crl.jrc.eceuropa.eu/doc/Mm_Perf Requirements_Analytical methods.pdf (accessed on 27 December 2020).
- Waigmann, E.; Paoletti, C.; Davies, H.; Perry, J.; Kärenlampi, S.; Kuiper, H. Special issue: Risk assessment of Genetically Modified Organisms (GMOs). EFSA J. 2012, 10, 1008. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Mesnage, R.; Tsatsakis, A.M.; Golokhvast, K.S.; Yang, S.H.; Antoniou, M.N.; Chung, G. Addressing concerns over the fate of DNA derived from genetically modified food in the human body: A review. Food Chem. Toxicol. 2019, 124, 423–430. [Google Scholar] [CrossRef]
- Sharma, R.; Damgaard, D.; Alexander, T.W.; Dugan, M.E.; Aalhus, J.L.; Stanford, K.; McAllister, T.A. Detection of transgenic and endogenous plant DNA in digesta and tissues of sheep and pigs fed Roundup Ready canola meal. J. Agric Food Chem. 2006, 54, 1699–1709. [Google Scholar] [CrossRef]
- Guo, L.; Qian, J.-P.; Guo, Y.-S.; Guo, X.H.; Liu, -Q.; Luo, J.X.; Ya, M. Simultaneous identification of bovine and equine DNA in milks and dairy products inferred from triplex TaqMan real-time PCR technique. J. Dairy Sci 2018, 101, 6776–6786. [Google Scholar] [CrossRef]
- Yu, Y.J.; Majumdar, A.P.; Nechvatal, J.M.; Ram, J.L.; Basson, M.D.; Heilbrun, L.K.; Kato, I. Exfoliated cells in stool: A source for reverse transcription-PCR-based analysis of biomarkers of gastrointestinal cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 455–458. [Google Scholar] [CrossRef]
- Anderson, N.; Suliman, I.; Bandaletova, T.; Obichere, A.; Lywood, R.; Loktionov, A. Protein biomarkers in exfoliated cells collected from the human rectal mucosa: Implications for colorectal disease detection and monitoring. Int. J. Colorectal. Dis. 2011, 26, 1287–1297. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Guo, H.; Huang, J.; Li, X.; Sun, Q.; Wang, B.; Xie, E.; Jiang, L.; Xia, Q. Potential of transferring transgenic DNA from silkworm to chicken. Int. J. Biol. Macromol. 2020, 142, 311–319. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, J.; Wang, J.; Zhao, Y.; Zhang, L.; Chu, M.; Li, N. Molecular-based environmental risk assessment of three varieties of genetically engineered cows. Transgenic Res. 2011, 20, 1043–1054. [Google Scholar] [CrossRef]
- Murray, D.; Meidinger, R.G.; Golovan, S.P.; Phillips, J.P.; O’Halloran, I.P.; Fan, M.Z.; Hacker, R.R.; Forsberg, C.W. Transgene and mitochondrial DNA are indicators of efficient composting of transgenic pig carcasses. Bioresour Technol. 2007, 98, 1795–1804. [Google Scholar] [CrossRef]
- Bao, Z.; Gao, X.; Zhang, Q.; Lin, J.; Hu, W.; Yu, H.; Chen, J.; Yang, Q.; Yu, Q. The Effects of GH Transgenic Goats on the Microflora of the Intestine, Feces and Surrounding Soil. PLoS ONE 2015, 10, e0139822. [Google Scholar]

| Assays | Primer Name | Sequence (5′—3′) | Amplicon (bp) |
|---|---|---|---|
| Event-specific | GM-LYZ-F | TCTTGTCATACAGTGTACTGATAAAGC | 101 |
| GM-LYZ-R | CTCTTCAGACCTAATAACTTCGTATAGC | ||
| GM-LYZ-P | FAM TGCTCCTGATAAATTAGTTCCCTTCCCACCTTTCG-BHQ1 | ||
| Goat | Goat-F | CCAACATGCCTTTAAACCCTCAA | 88 |
| Goat-R | GGAACTGTAGCCTTCTGACTCG | ||
| Goat-P | FAM-TGCCTTTCCTTCCCCGCCAGTCTC-BHQ1 |
| Copy Number | Ct Values | SD | RSD (%) | Quantified Copy Number | Bias (%) | |||
|---|---|---|---|---|---|---|---|---|
| Rep1 | Rep2 | Rep3 | Mean | |||||
| 2000 | 30.19 | 29.24 | 29.26 | 29.56 | 0.54 | 1.83 | 2041.08 | 2.05 |
| 200 | 32.73 | 33.11 | 33.20 | 33.02 | 0.25 | 0.76 | 186.20 | −6.90 |
| 100 | 33.95 | 33.83 | 34.35 | 34.04 | 0.27 | 0.80 | 91.36 | −8.64 |
| 50 | 34.72 | 34.92 | 34.76 | 34.80 | 0.11 | 0.30 | 53.87 | 7.74 |
| 10 | 36.51 | 37.35 | 36.96 | 36.94 | 0.42 | 1.13 | 12.22 | 22.25 |
| 5 | 38.37 | 37.33 | 37.52 | 37.74 | 0.55 | 1.47 | 7.04 | 40.78 |
| Sample Name | PCR Assay | Ct Values | Mean | SD | RSD (%) | Quantified Copy Number | GM Content (%) | Bias (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| S1 (5.0%) | Event-specific | 30.87 | 31.31 | 30.79 | 30.99 | 0.28 | 0.90 | 758.80 | 5.22 | 4.40 |
| Goat species | 24.62 | 25.00 | 24.57 | 24.73 | 0.24 | 0.95 | 14,526.36 | |||
| S2 (2.0%) | Event-specific | 32.45 | 32.62 | 31.82 | 32.3 | 0.42 | 1.30 | 306.53 | 1.91 | −4.50 |
| Goat species | 24.42 | 24.56 | 24.77 | 24.59 | 0.18 | 0.72 | 16,036.77 | |||
| S3 (0.9%) | Event-specific | 33.59 | 32.86 | 33.45 | 33.3 | 0.39 | 1.16 | 152.83 | 0.95 | 5.56 |
| Goat species | 24.42 | 24.56 | 24.77 | 24.59 | 0.18 | 0.72 | 16,036.77 | |||
| S4 (0.5%) | Event-specific | 34.87 | 34.31 | 34.79 | 34.66 | 0.30 | 0.87 | 59.63 | 0.39 | −22.00 |
| Goat species | 24.69 | 24.57 | 24.76 | 24.67 | 0.10 | 0.39 | 15,116.24 | |||
| Sample Type | Sample Name | Animal Type | hLZ Event-Specific Assay | Goat Species Assay | ||||
|---|---|---|---|---|---|---|---|---|
| Mean Ct | SD | RSD (%) | Mean Ct | SD | RSD (%) | |||
| Blood | B106 | GM | 24.21 | 0.28 | 1.16 | 22.68 | 0.17 | 0.75 |
| B176 | GM | 25.62 | 0.19 | 0.73 | 22.39 | 0.28 | 1.25 | |
| B254 | GM | 24.45 | 0.18 | 0.72 | 22.81 | 0.27 | 1.18 | |
| B350 | GM | 26.47 | 0.21 | 0.79 | 24.66 | 0.23 | 0.93 | |
| B418 | GM | 25.37 | 0.22 | 0.88 | 23.26 | 0.29 | 1.25 | |
| B476 | GM | 25.34 | 0.31 | 1.22 | 23.58 | 0.19 | 0.81 | |
| B494 | GM | 26.59 | 0.22 | 0.84 | 24.32 | 0.18 | 0.74 | |
| B496 | GM | 24.76 | 0.15 | 0.62 | 22.47 | 0.16 | 0.71 | |
| B498 | GM | 24.34 | 0.34 | 1.41 | 22.59 | 0.24 | 1.06 | |
| B502 | GM | 25.68 | 0.15 | 0.58 | 23.34 | 0.12 | 0.51 | |
| B518 | GM | 25.47 | 0.29 | 1.14 | 23.37 | 0.19 | 0.81 | |
| B522 | GM | 26.79 | 0.31 | 1.17 | 24.92 | 0.16 | 0.64 | |
| B540 | GM | 25.42 | 0.36 | 1.42 | 23.05 | 0.11 | 0.48 | |
| B18006 | GM | 26.36 | 0.26 | 0.99 | 24.97 | 0.12 | 0.48 | |
| B18014 | GM | 25.88 | 0.35 | 1.35 | 23.24 | 0.16 | 0.69 | |
| B18046 | GM | 25.02 | 0.49 | 1.96 | 23.16 | 0.09 | 0.39 | |
| Milk | M418 | GM | 27.67 | 0.35 | 1.26 | 25.14 | 0.29 | 1.15 |
| M494 | GM | 26.32 | 0.29 | 1.10 | 24.26 | 0.24 | 0.99 | |
| M502 | GM | 27.04 | 0.41 | 1.52 | 25.19 | 0.19 | 0.75 | |
| M522 | GM | 26.94 | 0.31 | 1.15 | 25.03 | 0.27 | 1.08 | |
| M-N1 | Non-GM | neg | / | / | 24.96 | 0.18 | 0.72 | |
| Faeces | F106 | GM | neg | / | / | 32.55 | 1.175 | 3.61 |
| F176 | GM | neg | / | / | neg | / | / | |
| F254 | GM | 33.31 | 0.60 | 1.79 | 29.40 | 0.19 | 0.63 | |
| F350 | GM | neg | / | / | neg | / | / | |
| F418 | GM | neg | / | / | neg | / | / | |
| F494 | GM | 32.27 | 0.67 | 2.09 | 28.56 | 0.65 | 2.27 | |
| F476 | GM | neg | / | / | neg | / | / | |
| F496 | GM | neg | / | / | neg | / | / | |
| F498 | GM | neg | / | / | neg | / | / | |
| F502 | GM | 31.47 | 0.20 | 0.62 | 28.02 | 0.75 | 2.69 | |
| F518 | GM | neg | / | / | neg | / | / | |
| F522 | GM | neg | / | / | neg | / | / | |
| F540 | GM | 33.15 | 0.52 | 1.57 | 29.57 | 0.56 | 1.91 | |
| F18006 | GM | neg | / | / | neg | / | / | |
| F18014 | GM | 31.48 | 0.38 | 1.21 | 27.81 | 0.20 | 0.71 | |
| F18046 | GM | neg | / | / | neg | / | / | |
| F-N1 | Non-GM | neg | / | / | neg | / | / | |
| F-N2 | Non-GM | neg | / | / | 26.49 | 0.49 | 1.84 | |
| Soil | S1 | GM | neg | / | / | neg | / | / |
| S2 | GM | neg | / | / | neg | / | / | |
| S3 | GM | neg | / | / | neg | / | / | |
| S4 | GM | neg | / | / | neg | / | / | |
| S5 | GM | neg | / | / | neg | / | / | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Cui, J.; Liu, B.; Yang, L. An Event-Specific Real-Time PCR Method for Measuring Transgenic Lysozyme Goat Content in Trace Samples. Foods 2021, 10, 925. https://doi.org/10.3390/foods10050925
Xu W, Cui J, Liu B, Yang L. An Event-Specific Real-Time PCR Method for Measuring Transgenic Lysozyme Goat Content in Trace Samples. Foods. 2021; 10(5):925. https://doi.org/10.3390/foods10050925
Chicago/Turabian StyleXu, Wenting, Jinjie Cui, Biao Liu, and Litao Yang. 2021. "An Event-Specific Real-Time PCR Method for Measuring Transgenic Lysozyme Goat Content in Trace Samples" Foods 10, no. 5: 925. https://doi.org/10.3390/foods10050925
APA StyleXu, W., Cui, J., Liu, B., & Yang, L. (2021). An Event-Specific Real-Time PCR Method for Measuring Transgenic Lysozyme Goat Content in Trace Samples. Foods, 10(5), 925. https://doi.org/10.3390/foods10050925





