Technological, Nutritional and Sensory Properties of an Innovative Gluten-Free Double-Layered Flat Bread Enriched with Amaranth Flour
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Breadmaking
2.3. Doughs Viscometric and Textural Properties
2.4. GF Spianatas Quality
2.4.1. Proximate Chemical Composition
2.4.2. Analyses of Polyphenolic Fractions and Antioxidant Activity
2.4.3. Texture Analysis
2.4.4. Color Analysis
2.4.5. Starch Retrogradation
2.4.6. Consumer Study with CATA Test
2.5. Statistical Analysis
3. Results and Discussion
3.1. Doughs Viscometric Parameters and Textural Properties
3.2. GF Spianatas Quality
3.2.1. GF Spianatas Physico-Chemical Analyses
3.2.2. Polyphenolic Fractions and Antioxidant Activity of GF Spianatas
3.2.3. Texture Analysis of GF Spianatas during Storage
3.2.4. Starch Retrogradation
3.3. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinsek, J.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S.; Schumann, M.; et al. Spectrum of gluten-related disorders: Consensus on new nomenclature and classification. BMC Med. 2012, 10, 13. [Google Scholar] [CrossRef]
- Barada, K.; Bitar, A.; Mokadem, M.A.R.; Hashash, J.G.; Green, P. Celiac disease in middle eastern and North African countries: A new burden? World J. Gastroenterol. 2010, 16, 1449–1457. [Google Scholar] [CrossRef]
- Woomer, J.S.; Adedeji, A.A. Current applications of gluten-free grains—A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Anton, A.A.; Artfield, S.D. Hydrocolloids in gluten-free breads: A review. Int. J. Food Sci. Nutr. 2008, 59, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Sparks, B.; Hill, I.; Ediger, T. Going Beyond Gluten-Free: A Review of Potential Future Therapies for Celiac Disease. Curr. Treat. Options Pediatr. 2021, 7, 17–31. [Google Scholar] [CrossRef]
- Kulai, T.; Rashid, M. Assessment of nutritional adequacy of packaged gluten-free food products. Can. J. Diet. Pract. Res. 2014, 75, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Conte, P.; Del Caro, A.; Balestra, F.; Piga, A.; Fadda, C. Bee pollen as a functional ingredient in gluten-free bread: A physical-chemical, technological and sensory approach. LWT Food Sci. Technol. 2018, 90, 1–7. [Google Scholar] [CrossRef]
- Pilco-Quesada, S.; Tian, Y.; Yang, B.; Repo-Carrasco-Valencia, R.; Suomela, J.P. Effects of germination and kilning on the phenolic compounds and nutritional properties of quinoa (Chenopodium quinoa) and kiwicha (Amaranthus caudatus). J. Cereal Sci. 2020, 94, 102996. [Google Scholar] [CrossRef]
- Banerji, A.; Ananthanarayan, L.; Lele, S. Rheological and nutritional studies of amaranth enriched wheat chapatti (Indian flat bread). J. Food Process. Preserv. 2018, 42, e13361. [Google Scholar] [CrossRef]
- Liu, S.; Chen, D.; Xu, J. Characterization of amaranth and bean flour blends and the impact on quality of gluten-free breads. J. Food Meas. Charact. 2019, 13, 1440–1450. [Google Scholar] [CrossRef]
- Qarooni, J. Flat Bread Technology; Chapman & Hall: New York, NY, USA, 1996; pp. 121–140. [Google Scholar]
- Fadda, C.; Santos, E.M.; Piga, A.; Collar, C. Innovative traditional Italian durum wheat breads: Influence of yeast and gluten on performance of sourdough Moddizzosu breads. Cereal Chem. 2010, 87, 204–213. [Google Scholar] [CrossRef]
- Catzeddu, P. Sourdough Breads. In Flour and Breads and their Fortification. In Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press, Elsevier: London, UK, 2011; pp. 37–46. [Google Scholar]
- Al-Dmoor, H.M. Flat bread: Ingredients and fortification. Qual. Assur. Saf. Crop. Foods 2012, 4, 2–8. [Google Scholar] [CrossRef]
- Kim, M.; Yun, Y.; Jeong, Y. Effects of corn, potato, and tapioca starches on the quality of gluten-free rice bread. Food Sci. Biotechnol. 2015, 24, 913–919. [Google Scholar] [CrossRef]
- Collar, C.; Conte, P.; Fadda, C.; Piga, A. Gluten-free dough-making of specialty breads: Significance of blended starches, flours and additives on dough behaviour. Food Sci. Technol. Int. 2015, 21, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Sadeghnia, N.; Azizi, M.H.; Neyestani, T.R.; Mortazavian, A.M. Development of gluten-free flat bread using hydrocolloids: Xanthan and CMC. J. Ind. Eng. Chem. 2014, 20, 1812–1818. [Google Scholar] [CrossRef]
- Padalino, L.; Conte, A.; Del Nobile, M. Overview on the General Approaches to Improve Gluten-Free Pasta and Bread. Foods 2016, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Houben, A.; Höchstötter, A.; Becker, T. Possibilities to increase the quality in gluten-free bread production: An overview. Eur. Food Res. Technol. 2012, 235, 195–208. [Google Scholar] [CrossRef]
- Nunes, M.H.B.; Ryan, L.A.M.; Arendt, E.K. Effect of low lactose dairy powder addition on the properties of gluten-free batters and bread quality. Eur. Food Res. Technol. 2009, 229, 31–41. [Google Scholar] [CrossRef]
- Renzetti, S.; Rosell, C.M. Role of enzymes in improving the functionality of proteins in non-wheat dough systems. J. Cereal Sci. 2016, 67, 35–45. [Google Scholar] [CrossRef]
- Matos Segura, M.E.; Rosell, C.M. Chemical Composition and Starch Digestibility of Different Gluten-free Breads. Plant Foods Hum. Nutr. 2011, 66, 224–230. [Google Scholar] [CrossRef]
- Witczak, M.; Ziobro, R.; Juszczak, L.; Korus, J. Starch and starch derivatives in gluten-free systems—A review. J. Cereal Sci. 2016, 67, 46–57. [Google Scholar] [CrossRef]
- Matos, M.E.; Rosell, C.M. Understanding gluten-free dough for reaching breads with physical quality and nutritional balance. J. Sci. Food Agric. 2015, 95, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, R.M.; Yousif, E.I.; Gadallah, M.G.E.; Alawneh, A.R. Formulations and quality characterization of gluten-free Egyptian balady flat bread. Ann. Agric. Sci. 2013, 58, 19–25. [Google Scholar] [CrossRef][Green Version]
- Ministero Della Salute. Relazione Annuale al Parlamento Sulla Celiachia Anno 2019. 2021; pp. 40–49. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_3025_allegato.pdf (accessed on 10 April 2021).
- ICC, International Association for Cereal Science and Technology. Method n.162, Rapid Pasting Method using Newport Rapid Visco Analyser. ICC Stand. 1996. Available online: https://www.perten.com/Global/Application%20notes/RVA/General%20Pasting%20Method%20-%20RVA%2001.05.pdf (accessed on 16 December 2020).
- Fois, S.; Fadda, C.; Tonelli, R.; Sanna, M.; Urgeghe, P.P.; Roggio, T.; Catzeddu, P. Effects of the fermentation process on gas-cell size two-dimensional distribution and rheological characteristics of durum-wheat-based doughs. Food Res. Int. 2012, 49, 193–200. [Google Scholar] [CrossRef]
- AACC Approved Methods of the AACC, Method 74–09. Am. Assoc. Cereal Chem. 2005. Available online: https://img67.chem17.com/1/20170312/636249351441623177119.pdf (accessed on 8 January 2021).
- Singh, H.; Rockall, A.; Martin, C.R.; Chung, O.K.; Lookhart, G.L. The analysis of stress relaxation data of some viscoelastic foods using a texture analyzer. J. Texture Stud. 2006, 37, 383–392. [Google Scholar] [CrossRef]
- Sciarini, L.S.; Ribotta, P.D.; León, A.E.; Pérez, G.T. Incorporation of several additives into gluten free breads: Effect on dough properties and bread quality. J. Food Eng. 2012, 111, 590–597. [Google Scholar] [CrossRef]
- Conte, P.; Del Caro, A.; Urgeghe, P.P.; Petretto, G.L.; Montanari, L.; Piga, A.; Fadda, C. Nutritional and aroma improvement of gluten-free bread: Is bee pollen effective? LWT 2020, 118, 108711. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Angioloni, A.; Collar, C. Polyphenol composition and “in vitro” antiradical activity of single and multigrain breads. J. Cereal Sci. 2011, 53, 90–96. [Google Scholar] [CrossRef]
- Giannone, V.; Giarnetti, M.; Spina, A.; Todaro, A.; Pecorino, B.; Summo, C.; Caponio, F.; Paradiso, V.M.; Pasqualone, A. Physico-chemical properties and sensory profile of durum wheat Dittaino PDO (Protected Designation of Origin) bread and quality of re-milled semolina used for its production. Food Chem. 2018, 241, 242–249. [Google Scholar] [CrossRef]
- Piccinini, M.; Fois, S.; Secchi, N.; Sanna, M.; Roggio, T.; Catzeddu, P. The Application of NIR FT-Raman Spectroscopy to Monitor Starch Retrogradation and Crumb Firmness in Semolina Bread. Food Anal. Methods 2012, 5, 1145–1149. [Google Scholar] [CrossRef]
- Ares, G.; Reis, F.; Oliveira, D.; Antúnez, L.; Vidal, L.; Giménez, A.; Chheang, S.L.; Hunter, D.C.; Kam, K.; Roigard, C.M.; et al. Recommendations for use of balanced presentation order of terms in CATA questions. Food Qual. Prefer. 2015, 46, 137–141. [Google Scholar] [CrossRef]
- Mishra, S.; Rai, T. Morphology and functional properties of corn, potato and tapioca starches. Food Hydrocoll. 2006, 20, 557–566. [Google Scholar] [CrossRef]
- Bahnassey, Y.A.; Breene, W.M. Rapid Visco-Analyzer (RVA) Pasting Profiles of Wheat, Corn, Waxy Corn, Tapioca and Amaranth Starches (A. hypochondriacus and A. cruentus) in the Presence of Konjac Flour, Gellan, Guar, Xanthan and Locust Bean Gums. Starch Stärke 1994, 46, 134–141. [Google Scholar] [CrossRef]
- Waterschoot, J.; Gomand, S.V.; Fierens, E.; Delcour, J.A. Starch blends and their physicochemical properties. Starch Staerke 2015, 67, 1–13. [Google Scholar] [CrossRef]
- Gunaratne, A.; Corke, H. Gelatinizing, pasting, and gelling properties of potato and amaranth starch mixtures. Cereal Chem. 2007, 84, 22–29. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Z.; Hong, Y.; Li, Z.; Cheng, L. Pasting and rheologic properties of potato starch and maize starch mixtures. Starch Staerke 2011, 63, 11–16. [Google Scholar] [CrossRef]
- Sanz-Penella, J.M.; Wronkowska, M.; Soral-Smietana, M.; Haros, M. Effect of whole amaranth flour on bread properties and nutritive value. LWT Food Sci. Technol. 2013, 50, 679–685. [Google Scholar] [CrossRef]
- Nasir, S.; Allai, F.M.; Gani, M.; Ganaie, S.; Gul, K.; Jabeen, A.; Majeed, D. Physical, Textural, Rheological, and Sensory Characteristics of Amaranth-Based Wheat Flour Bread. Int. J. Food Sci. 2020, 2020, 8874872. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Serrano, J.; Goñi, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007, 101, 492–501. [Google Scholar] [CrossRef]
- Bhatt, S.; Kumari, N.; Abhishek, V.; Gupta, M. Elucidating the role of amaranth flour in formulation of gluten free black rice muffins and its premix: Nutritional, physico-chemical and textural characteristics. J. Food Meas. Charact. 2021, 15, 675–685. [Google Scholar] [CrossRef]
- Viereck, N.; Salomonsen, T.; Van Den Berg, F.; Engelsen, S.B. Raman Applications in Food Analysis. In Raman Spectroscopy for Soft Matter Applications; Amer, M.S., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 199–223. ISBN 9780470453834. [Google Scholar]
- Ayo, J.A. The effect of amaranth grain flour on the quality of bread. Int. J. Food Prop. 2001, 4, 341–351. [Google Scholar] [CrossRef]
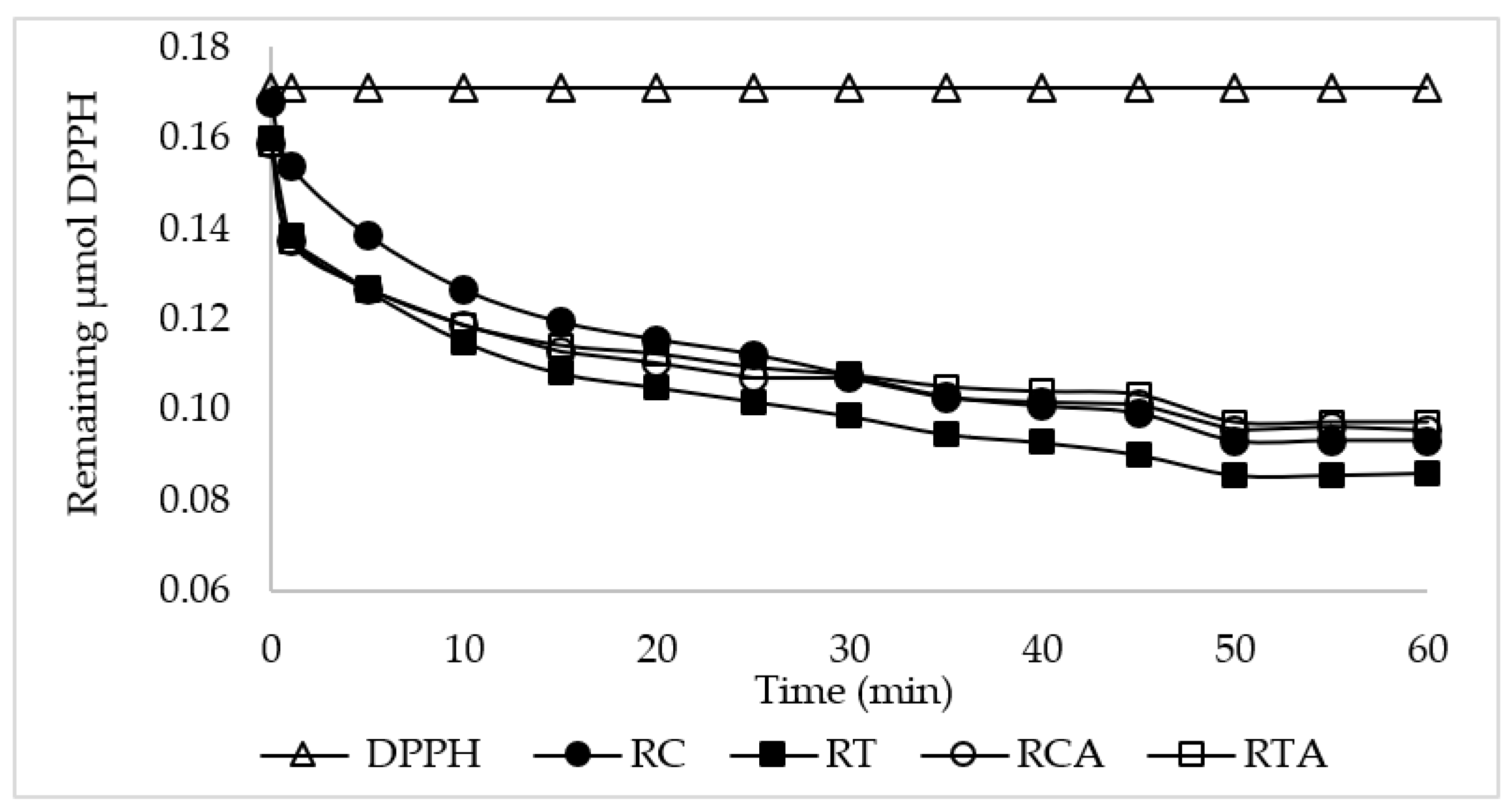
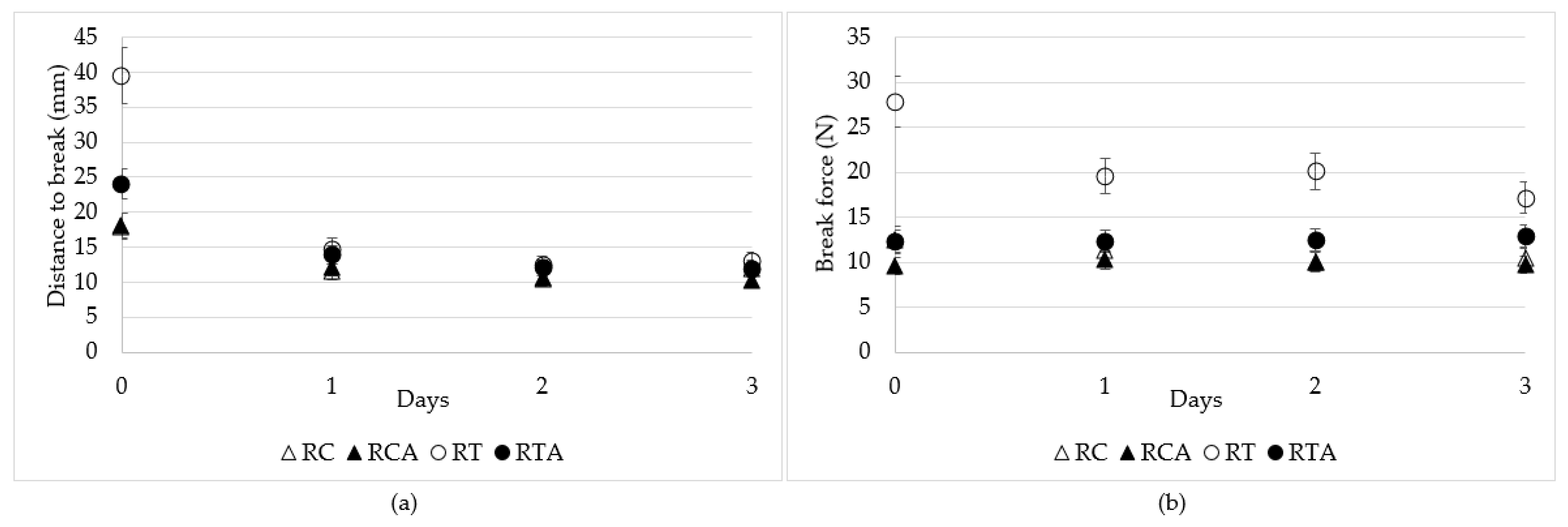
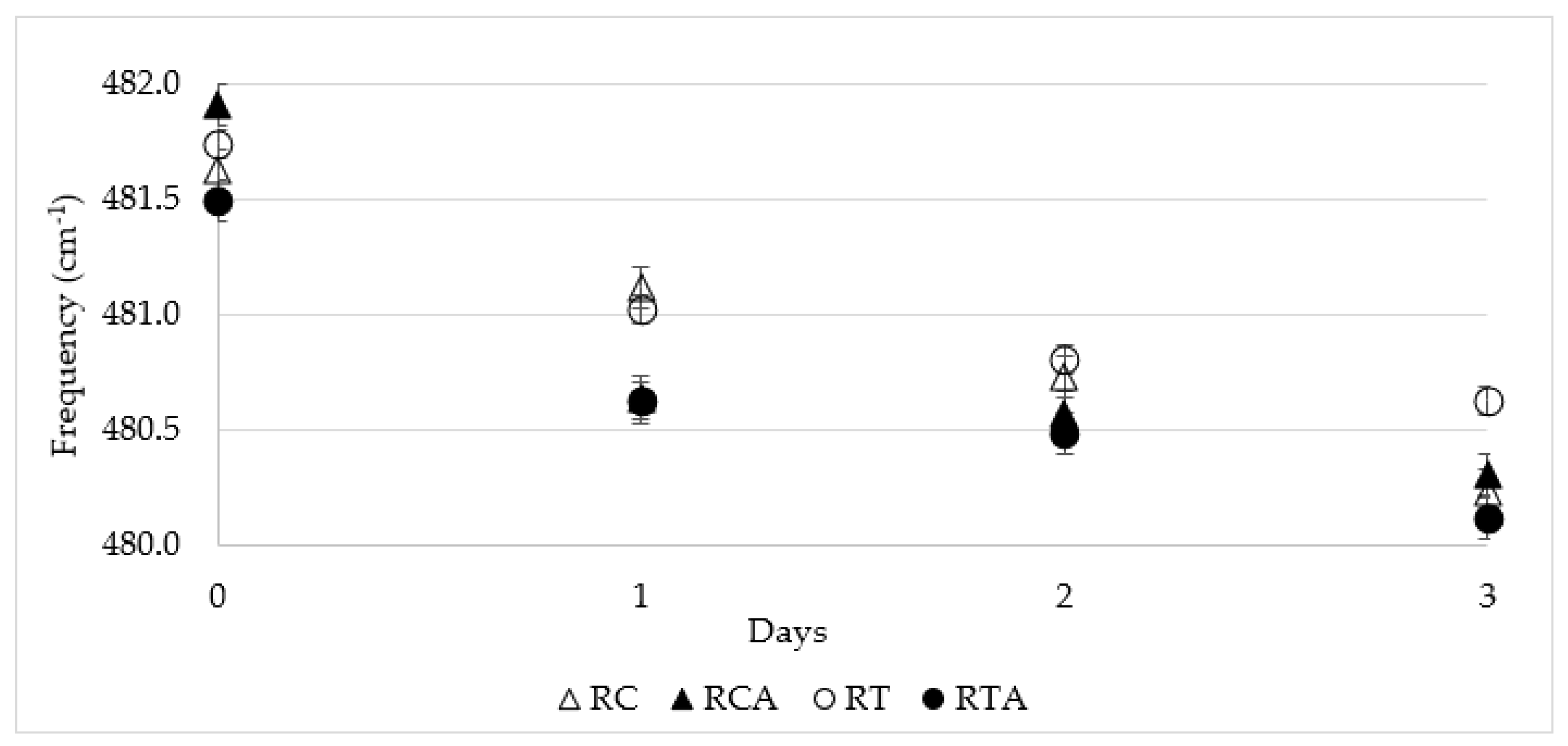
| Ingredients | R | C | T | A | P |
|---|---|---|---|---|---|
| Protein | 7.1 | 0.3 | 0.5 | 14.5 | 2.5 |
| Carbohydrate | 76.5 | 88 | 86 | 51 | 4 |
| Lipid | 1.3 | 0 | 0.5 | 6.5 | 0.5 |
| Fiber | 0.2 | 0.0 | 0.5 | 15 | 81 * |
| Moisture | 14 | 12 | 12.6 | 14.5 | 10 |
| Ash | 0.8 | 0 | 0.2 | 2.4 | 2 |
| Samples | Parameters | |||||
|---|---|---|---|---|---|---|
| Peak Viscosity (mPa·s) | Breakdown (mPa·s) | Setback (mPa·s) | Final Viscosity (mPa·s) | Peak Time (min) | Pasting Temperature (°C) | |
| RC | 4100 ± 163a * | 1026 ± 66c | 2317 ± 172a | 5391 ± 261a | 5.5 ± 0.2b | 72 ± 7 ns |
| RT | 4170 ± 71a | 1752 ± 21a | 1086 ± 96d | 3504 ± 47c | 5.1 ± 0.1c | 67 ± 10 |
| RCA | 3701 ± 70b | 634 ± 5d | 2038 ± 74b | 5105 ± 31a | 6.0 ± 0.0a | 77 ± 0 |
| RTA | 3838 ± 45b | 1196 ± 145b | 1788 ± 41c | 4430 ± 206b | 5.4 ± 0.0b | 73 ± 0 |
| Dough Samples | Stress Relaxation (%) | Resistance to Penetration (N) |
|---|---|---|
| RC | 45.39 ± 0.62a * | 0.599 ± 0.002b |
| RT | 43.35 ± 0.33a | 0.725 ± 0.022a |
| RCA | 37.75 ± 0.35b | 0.462 ± 0.018c |
| RTA | 37.10 ± 0.77b | 0.608 ± 0.049b |
| FB Samples | Moisture | Lipid | Ash | Protein | Total Carbohydrate |
|---|---|---|---|---|---|
| RC | 32.3 ± 0.0c * | 0.06 ± 0.02c | 1.85 ± 0.02d | 4.23 ± 0.21b | 61.6 ± 0.1a |
| RT | 33.7 ± 0.1b | 0.08 ± 0.01bc | 1.96 ± 0.06c | 4.03 ± 0.01b | 60.2 ± 0.0b |
| RCA | 35.0 ± 0.1a | 0.18 ± 0.03a | 2.22 ± 0.03b | 5.86 ± 0.07a | 56.8 ± 0.1c |
| RTA | 31.9 ± 0.1d | 0.14 ± 0.01ab | 2.33 ± 0.00a | 5.69 ± 0.03a | 60.0 ± 0.0b |
| FB Samples | L | a | b | Browning Index |
|---|---|---|---|---|
| RC | 75.04 ± 2.05a * | −0.19 ± 0.14b | 5.77 ± 0.12c | 24.96 ± 2.05a * |
| RT | 73.78 ± 1.47a | −0.05 ± 0.07b | 5.84 ± 0.07c | 26.22 ± 1.47a |
| RCA | 72.71 ± 2.47a | 0.96 ± 0.17a | 9.66 ± 0.29a | 27.29 ± 2.47a |
| RTA | 74.24 ± 0.91a | −0.05 ± 0.43b | 8.30 ± 0.24b | 25.76 ± 0.91a |
| FB Samples | Polyphenol Fractions (mg GAE */100 g Fresh Bread) | Antioxidant Activity % ** | |||
|---|---|---|---|---|---|
| Soluble | Insoluble | Total | Bioaccessible | ||
| RC | 23.3 ± 1.1d | 80.6 ± 6.1b | 104.0 ± 7.2b | 62.1 ± 2c | 44.5a |
| RT | 30.9 ± 4.8c | 60.3 ± 3.0c | 91.2 ± 2.0c | 71.6 ± 1b | 46.3a |
| RCA | 49.8 ± 1.2a | 88.3 ± 5.1ab | 138.1 ± 4.5a | 80.8 ± 1a | 39.9b |
| RTA | 38.3 ± 0.6b | 91.6 ± 4.7a | 129.8 ± 4.2a | 79.3 ± 1a | 37.2b |
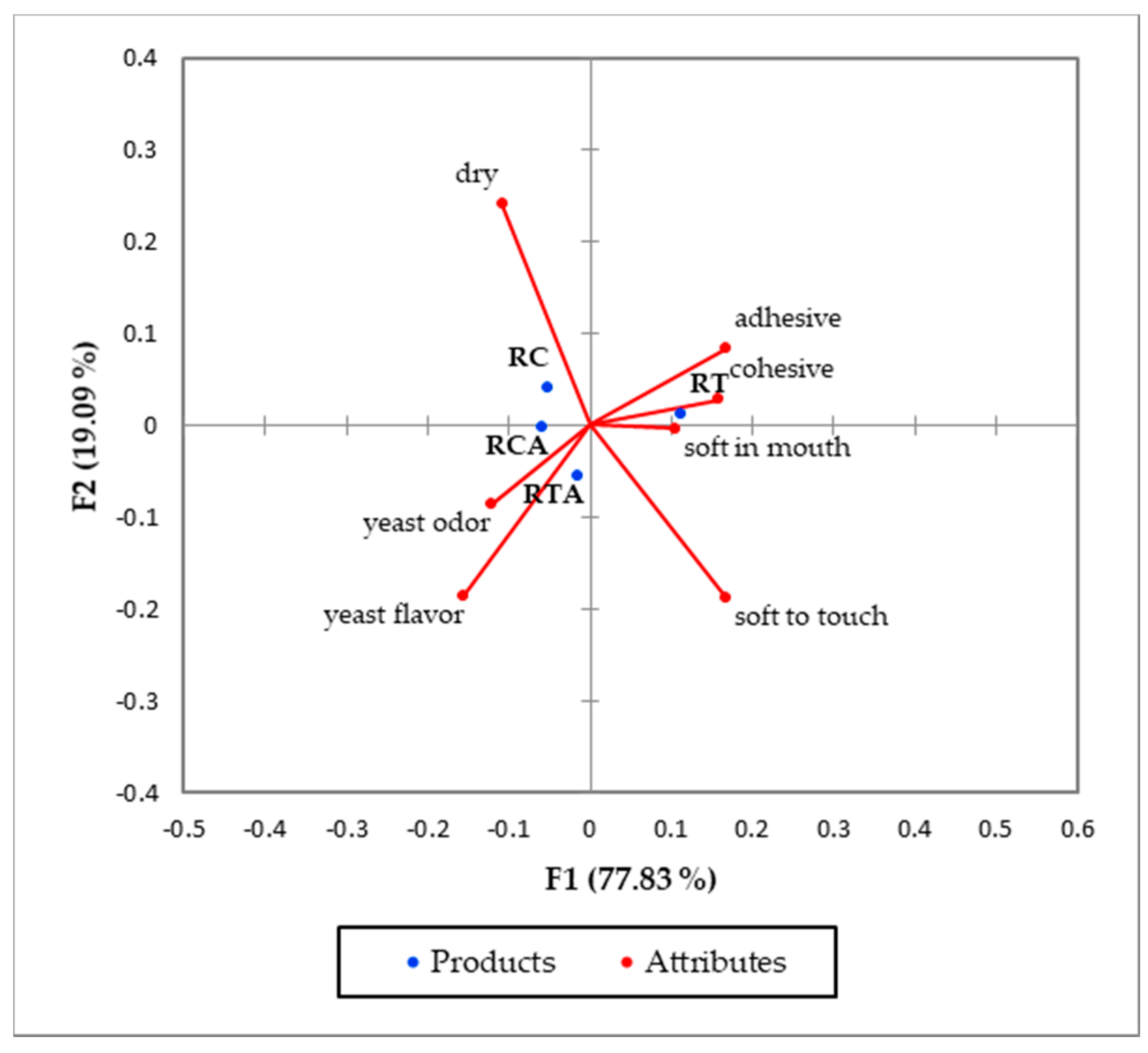
| CATA Attributes | p-Values | RC | RT | RCA | RTA |
|---|---|---|---|---|---|
| Soft to touch | 0.000 | 0.054b 1 | 0.339a | 0.089b | 0.214ab |
| Yeast odor | 0.023 | 0.589ab | 0.339b | 0.482ab | 0.607a |
| yeast flavor | 0.008 | 0.304ab | 0.143b | 0.393a | 0.492a |
| adhesive | 0.000 | 0.107ab | 0.304a | 0.036b | 0.089ab |
| cohesive | 0.000 | 0.107b | 0.357a | 0.089b | 0.125ab |
| Soft in mouth | 0.029 | 0.036b | 0.161a | 0.054b | 0.054b |
| dry | 0.000 | 0.768a | 0.446b | 0.589ab | 0.357b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piga, A.; Conte, P.; Fois, S.; Catzeddu, P.; Del Caro, A.; Sanguinetti, A.M.; Fadda, C. Technological, Nutritional and Sensory Properties of an Innovative Gluten-Free Double-Layered Flat Bread Enriched with Amaranth Flour. Foods 2021, 10, 920. https://doi.org/10.3390/foods10050920
Piga A, Conte P, Fois S, Catzeddu P, Del Caro A, Sanguinetti AM, Fadda C. Technological, Nutritional and Sensory Properties of an Innovative Gluten-Free Double-Layered Flat Bread Enriched with Amaranth Flour. Foods. 2021; 10(5):920. https://doi.org/10.3390/foods10050920
Chicago/Turabian StylePiga, Antonio, Paola Conte, Simonetta Fois, Pasquale Catzeddu, Alessandra Del Caro, Anna Maria Sanguinetti, and Costantino Fadda. 2021. "Technological, Nutritional and Sensory Properties of an Innovative Gluten-Free Double-Layered Flat Bread Enriched with Amaranth Flour" Foods 10, no. 5: 920. https://doi.org/10.3390/foods10050920
APA StylePiga, A., Conte, P., Fois, S., Catzeddu, P., Del Caro, A., Sanguinetti, A. M., & Fadda, C. (2021). Technological, Nutritional and Sensory Properties of an Innovative Gluten-Free Double-Layered Flat Bread Enriched with Amaranth Flour. Foods, 10(5), 920. https://doi.org/10.3390/foods10050920








