Pulsed Electric Field (PEF) Processing of Chilled and Frozen-Thawed Lamb Meat Cuts: Relationships between Sensory Characteristics and Chemical Composition of Meat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Meat Samples
2.2. PEF Processing
2.3. Sample Preparation and Temporal Dominance of Sensations (TDS) Procedure
2.4. Determination of Volatile Profile Using Headspace/HS-SPME Analysis
2.5. Fatty Acid and Amino Acid Analyses
2.6. Data Analysis
3. Results and Discussion
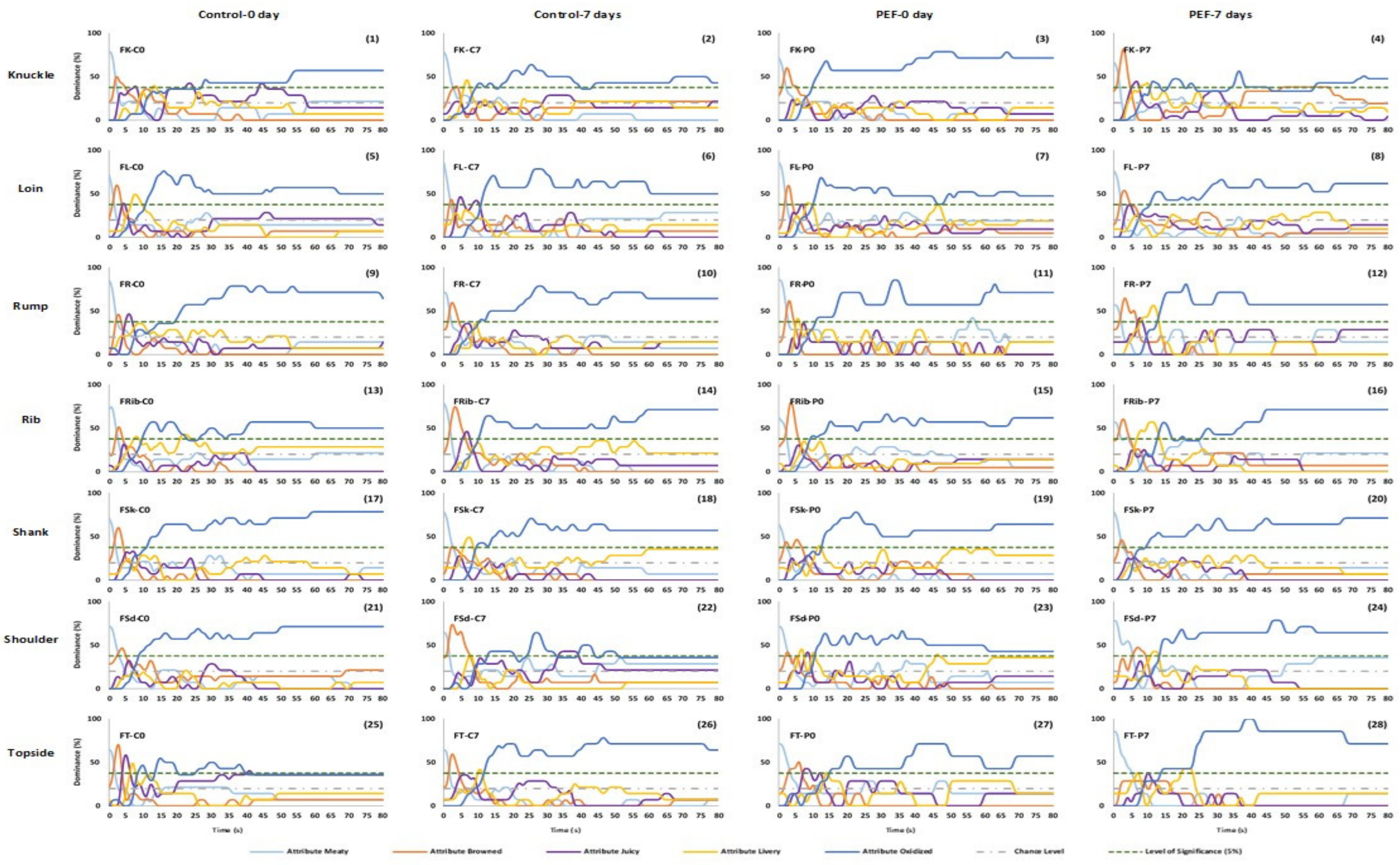
3.1. Sensory Evaluation
3.1.1. Panel dominance curves
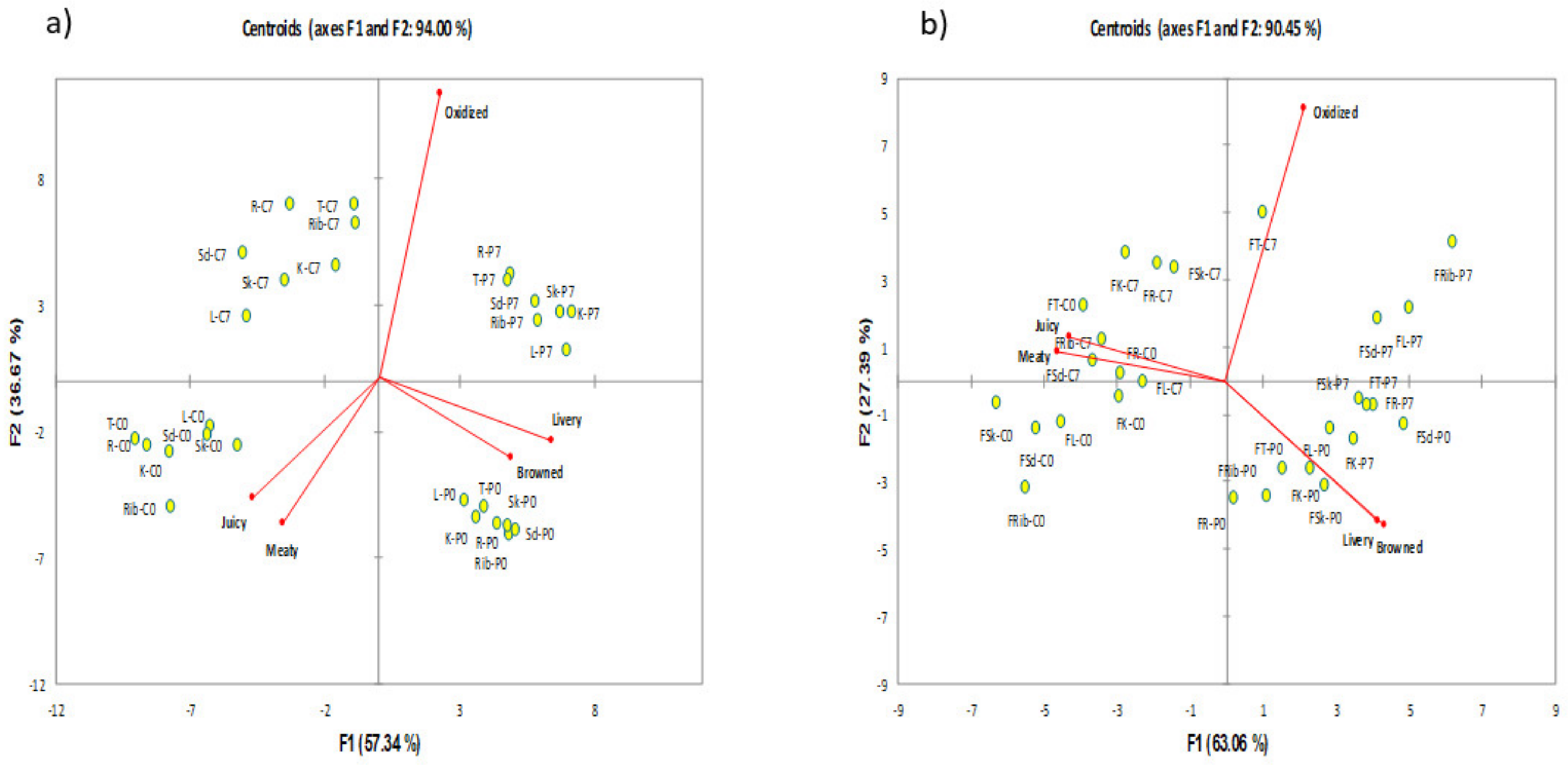
3.1.2. Canonical Variate Analysis
3.2. Volatile Analysis
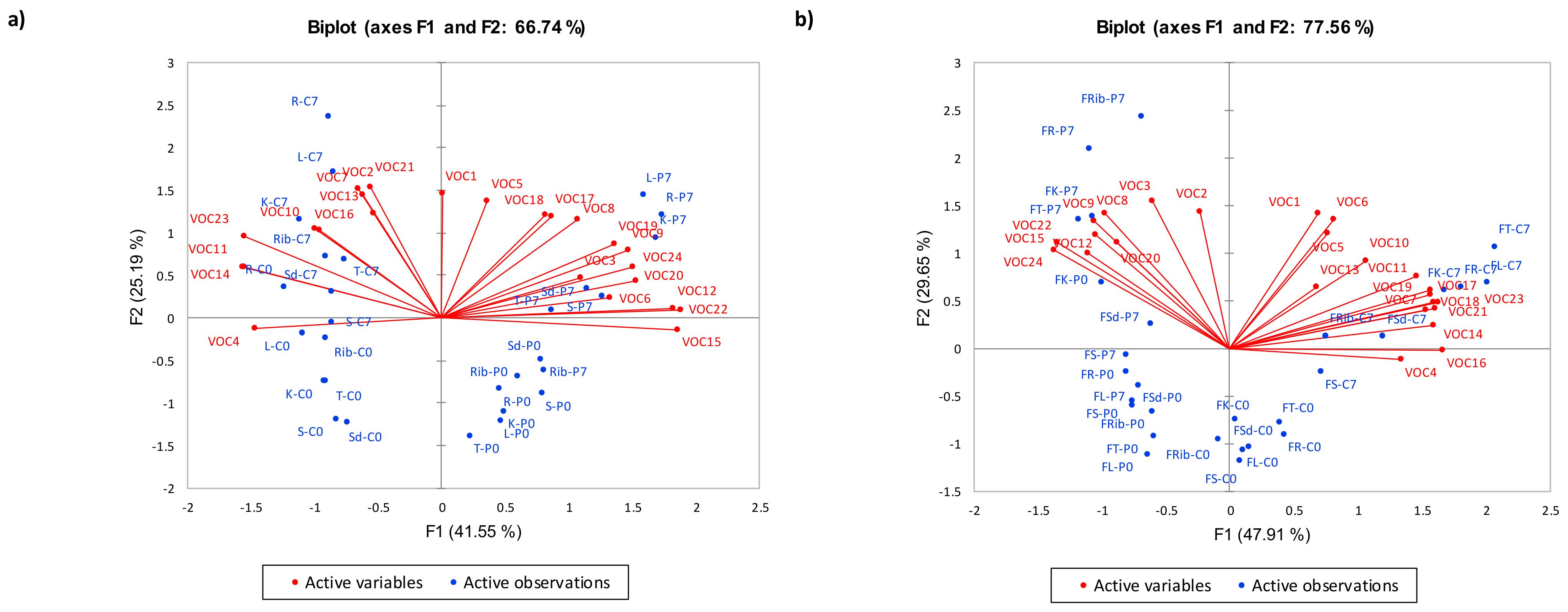
3.2.1. Effects of Storage, PEF Processing, and Cuts on Volatile Composition
PEF-Treated Chilled Samples
PEF-Treated Frozen Samples
3.3. Partial Least Squares Regression Analysis to Determine the Relationship between Sensory and Chemical Measures
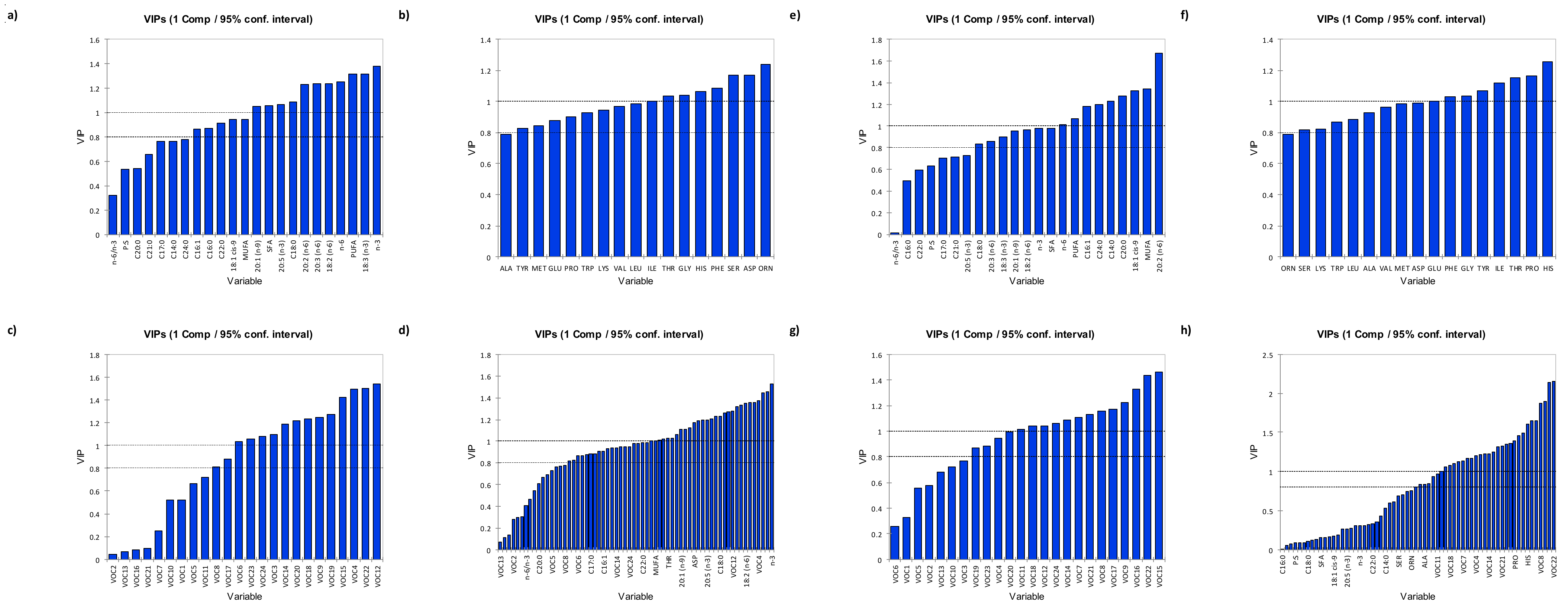
3.3.1. Variable Importance in Projection (VIP)
3.3.2. Chilled Samples
3.3.3. Frozen Samples
3.4. PLSR Correlations between Chemical Composition and Sensory Perception
3.4.1. Chilled Samples
3.4.2. Frozen Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Arroyo, C.; Eslami, S.; Brunton, N.P.; Arimi, J.M.; Noci, F.; Lyng, J.G. An assessment of the impact of pulsed electric fields processing factors on oxidation, color, texture, and sensory attributes of turkey breast meat. Poult. Sci. 2015, 94, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Bekhit Ael, D.; Suwandy, V.; Carne, A.; van de Ven, R.; Hopkins, D.L. Effect of repeated pulsed electric field treatment on the quality of hot-boned beef loins and topsides. Meat Sci. 2016, 111, 139–146. [Google Scholar] [CrossRef]
- Faridnia, F.; Ma, Q.L.; Bremer, P.J.; Burritt, D.J.; Hamid, N.; Oey, I. Effect of freezing as pre-treatment prior to pulsed electric field processing on quality traits of beef muscles. Innov. Food Sci. Emerg. Technol. 2015, 29, 31–40. [Google Scholar] [CrossRef]
- O’Dowd, L.P.; Arimi, J.M.; Noci, F.; Cronin, D.A.; Lyng, J.G. An assessment of the effect of pulsed electrical fields on tenderness and selected quality attributes of post rigour beef muscle. Meat Sci. 2013, 93, 303–309. [Google Scholar] [CrossRef]
- Ma, Q.; Hamid, N.; Oey, I.; Kantono, K.; Faridnia, F.; Yoo, M.; Farouk, M. Effect of chilled and freezing pre-treatments prior to pulsed electric field processing on volatile profile and sensory attributes of cooked lamb meats. Innov. Food Sci. Emerg. Technol. 2016, 37, 359–374. [Google Scholar] [CrossRef]
- Kantono, K.; Hamid, N.; Oey, I.; Wang, S.; Xu, Y.; Ma, Q.; Faridnia, F.; Farouk, M. Physicochemical and sensory properties of beef muscles after Pulsed Electric Field processing. Food Res. Int. 2019, 121, 1–11. [Google Scholar] [CrossRef]
- Luo, Q.; Hamid, N.; Oey, I.; Leong, S.Y.; Kantono, K.; Alfaro, A.; Lu, J. Physicochemical changes in New Zealand abalone (Haliotis iris) with pulsed electric field (PEF) processing and heat treatments. LWT 2019, 115, 108438. [Google Scholar] [CrossRef]
- Kantono, K.; Hamid, N.; Ma, Q.; Oey, I.; Farouk, M. Changes in the physicochemical properties of chilled and frozen-thawed lamb cuts subjected to pulsed electric field processing. Food Res. Int. 2021, 141, 110092. [Google Scholar] [CrossRef]
- Xu, Y.; Hamid, N.; Shepherd, D.; Kantono, K.; Reay, S.; Martinez, G.; Spence, C. Background soundscapes influence the perception of ice-cream as indexed by electrophysiological measures. Food Res. Int. 2019, 125, 108564. [Google Scholar] [CrossRef] [PubMed]
- Monrozier, R.; Danzart, M. A quality measurement for sensory profile analysis The contribution of extended cross-validation and resampling techniques. Food Qual. Prefer. 2001, 12, 393–406. [Google Scholar] [CrossRef]
- Reineccius, G. Flavor chemistry and technology; CRC press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Machiels, D. Gas chromatography-olfactometry analysis of beef meat originating from differently fed Belgian Blue, Limousin and Aberdeen Angus bulls. Food Chem. 2004, 86, 377–383. [Google Scholar] [CrossRef]
- Yang, H.S.; Lee, E.J.; Moon, S.H.; Paik, H.D.; Ahn, D.U. Addition of garlic or onion before irradiation on lipid oxidation, volatiles and sensory characteristics of cooked ground beef. Meat Sci. 2011, 88, 286–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madruga, M.S.; Stephen Elmore, J.; Dodson, A.T.; Mottram, D.S. Volatile flavour profile of goat meat extracted by three widely used techniques. Food Chem. 2009, 115, 1081–1087. [Google Scholar] [CrossRef]
- Ruiz, J.; Ventanas, J.; Cava, R.; Andrés, A.; Garcıía, C. Volatile compounds of dry-cured Iberian ham as affected by the length of the curing process. Meat Sci. 1999, 52, 19–27. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavour formation in meat and meat products: A review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Mottram, D.S.; Szauman-Szumski, C.; Dodson, A. Interaction of thiol and disulfide flavor compounds with food components. J. Agric. Food Chem. 1996, 44, 2349–2351. [Google Scholar] [CrossRef]
- Stetzer, A.J.; Tucker, E.; McKeith, F.K.; Brewer, M.S. Quality changes in beef complexus, serratus ventralis, vastus lateralis, vastus medialis, and longissimus dorsi muscles enhanced prior to aging. J. Food Sci. 2008, 73, S6–S10. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.; Richardson, R.; Nute, G.; Fisher, A.; Campo, M.; Kasapidou, E.; Sheard, P.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef]
- Calkins, C.R.; Hodgen, J.M. A fresh look at meat flavor. Meat Sci. 2007, 77, 63–80. [Google Scholar] [CrossRef]
- Koutsidis, G.; Elmore, J.S.; Oruna-Concha, M.J.; Campo, M.M.; Wood, J.D.; Mottram, D.S. Water-soluble precursors of beef flavour. Part II: Effect of post-mortem conditioning. Meat Sci. 2008, 79, 270–277. [Google Scholar] [CrossRef]
- Mottram, D.S.; Nobrega, I.C. Formation of sulfur aroma compounds in reaction mixtures containing cysteine and three different forms of ribose. J. Agric. Food Chem. 2002, 50, 4080–4086. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, M.; Biancolillo, A.; Marini, F. Chemometric Methods for Classification and Feature Selection. Compr. Anal. Chem. 2018, 265–299. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Kantono, K.; Hamid, N.; Oey, I.; Wu, Y.C.; Ma, Q.; Farouk, M.; Chadha, D. Effect of High Hydrostatic Pressure Processing on the Chemical Characteristics of Different Lamb Cuts. Foods 2020, 9, 1444. [Google Scholar] [CrossRef]
- You, L.; Luo, R. Effect of Chilled Ageing Conditioning at 4 °C in Lamb Longissimus Dorsi Muscles on Water-Soluble Flavour Precursors as Revealed by a Metabolomic Approach. J. Food Qual. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Bueno, M.; Resconi, V.C.; Campo, M.M.; Cacho, J.; Ferreira, V.; Escudero, A. Gas chromatographic–olfactometric characterisation of headspace and mouthspace key aroma compounds in fresh and frozen lamb meat. Food Chem. 2011, 129, 1909–1918. [Google Scholar] [CrossRef]
- Gkarane, V.; Brunton, N.P.; Harrison, S.M.; Gravador, R.S.; Allen, P.; Claffey, N.A.; Diskin, M.G.; Fahey, A.G.; Farmer, L.J.; Moloney, A.P.; et al. Volatile Profile of Grilled Lamb as Affected by Castration and Age at Slaughter in Two Breeds. J. Food Sci. 2018, 83, 2466–2477. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.B.; Oliveira, R.L.; Silva, T.M.; Barbosa, A.M.; Borja, M.S.; de Pellegrini, C.B.; Oliveira, V.D.S.; Ribeiro, R.D.X.; Bezerra, L.R. Fatty acid, physicochemical composition and sensory attributes of meat from lambs fed diets containing licuri cake. PLoS ONE 2018, 13, e0206863. [Google Scholar] [CrossRef]
- Nute, G.R.; Richardson, R.I.; Wood, J.D.; Hughes, S.I.; Wilkinson, R.G.; Cooper, S.L.; Sinclair, L.A. Effect of dietary oil source on the flavour and the colour and lipid stability of lamb meat. Meat Sci. 2007, 77, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.V.; Enser, M.; Richardson, R.I.; Wood, J.D.; Nute, G.R.; Kurt, E.; Sinclair, L.A.; Wilkinson, R.G. Fatty acid composition and eating quality of lamb types derived from four diverse breed x production systems. Meat Sci. 2000, 55, 141–147. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kemp, R.; Samuelsson, L.M. Effects of dry-aging on meat quality attributes and metabolite profiles of beef loins. Meat Sci. 2016, 111, 168–176. [Google Scholar] [CrossRef]
- Al-Sabagh, E.S.; El-Far, A.H.; Sadek, K.M.; Taha, N.M.; Saleh, E.A. Effect of Freezing and Frozen Storage on Amino Acid Profile and Fatty Acid Pattern in Imported and Local Meat. Alex. J. Vet. Sci. 2016, 49. [Google Scholar]
- Jensen, M.T.; Hansen, L.L.; Andersen, H.J. Transfer of the Meat Aroma Precursors (Dimethyl Sulfide, Dimethyl Disulfide and Dimethyl Trisulfide) from Feed to Cooked Pork. LWT Food Sci. Technol. 2002, 35, 485–489. [Google Scholar] [CrossRef]
- Raes, K.; Balcaen, A.; Dirinck, P.; De Winne, A.; Claeys, E.; Demeyer, D.; De Smet, S. Meat quality, fatty acid composition and flavour analysis in Belgian retail beef. Meat Sci. 2003, 65, 1237–1246. [Google Scholar] [CrossRef]
- Caporaso, F.; Sink, J.D.; Dimick, P.S.; Mussinan, C.J.; Sanderson, A. Volatile flavor constituents of ovine adipose tissue. J. Agric. Food Chem. 2002, 25, 1230–1234. [Google Scholar] [CrossRef]
- Resconi, V.C.; Escudero, A.; Campo, M.M. The development of aromas in ruminant meat. Molecules 2013, 18, 6748–6781. [Google Scholar] [CrossRef] [Green Version]
- Yancey, E.J.; Grobbel, J.P.; Dikeman, M.E.; Smith, J.S.; Hachmeister, K.A.; Chambers, E.C.t.; Gadgil, P.; Milliken, G.A.; Dressler, E.A. Effects of total iron, myoglobin, hemoglobin, and lipid oxidation of uncooked muscles on livery flavor development and volatiles of cooked beef steaks. Meat Sci. 2006, 73, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Joo, S.T. Fatty Acid Profiles, Meat Quality, and Sensory Palatability of Grain-fed and Grass-fed Beef from Hanwoo, American, and Australian Crossbred Cattle. Korean J. Food Sci. Anim. Resour. 2017, 37, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Hwang, Y.H.; Joo, S.T. Fatty Acid Profiles of Ten Muscles from High and Low Marbled (Quality Grade 1(++) and 2) Hanwoo Steers. Korean J. Food Sci. Anim. Resour. 2016, 36, 679–688. [Google Scholar] [CrossRef] [Green Version]
- Smit, B.A.; Engels, W.J.; Smit, G. Branched chain aldehydes: Production and breakdown pathways and relevance for flavour in foods. Appl. Microbiol. Biotechnol. 2009, 81, 987–999. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.; Liu, Q.; Jia, H.; Jiang, Y.; Nie, S.; Xie, J.; Li, C.; Xie, M. Simultaneous determination of furan and 2-alkylfurans in heat-processed foods by automated static headspace gas chromatography-mass spectrometry. LWT Food Sci. Technol. 2016, 72, 44–54. [Google Scholar] [CrossRef]
- Mussinan, C.J.; Walradt, J.P. Volatile constituents of pressure cooked pork liver. J. Agric. Food Chem. 2002, 22, 827–831. [Google Scholar] [CrossRef]
- Wang, S.; Adhikari, K.; Hung, Y.C. Acceptability and Preference Drivers of Freshly Roasted Peanuts. J. Food Sci. 2017, 82, 174–184. [Google Scholar] [CrossRef] [Green Version]
- Coleman, E.C.; Ho, C.-T. Chemistry of baked potato flavor. 1. Pyrazines and thiazoles indentified in the volatile flavor of baked potato. J. Agric. Food Chem. 2002, 28, 66–68. [Google Scholar] [CrossRef]
- Hornstein, I.; Wasserman, A. Chemistry of meat flavour. In The science of meat and meat products, 3rd ed.; Price, J.F., Schweigert, B.S., Eds.; Food and Nutrition Press Inc.: Westport, CT, USA, 1987; pp. 329–347. [Google Scholar]
- Legako, J.F. 114 Effect of altering fatty acid profile on fresh meat palatability. J. Anim. Sci. 2019, 97, 108. [Google Scholar] [CrossRef]
- Smith, G.; Carpenter, Z. Eating quality of animal products and their fat content. In Proceedings of the Proceedings of the symposium on changing the fat content and composition of animal products, Washingtong, DC, USA, 12–13 December 1974; pp. 1–63. [Google Scholar]
- Insausti, K.; Goni, V.; Petri, E.; Gorraiz, C.; Beriain, M.J. Effect of weight at slaughter on the volatile compounds of cooked beef from Spanish cattle breeds. Meat Sci. 2005, 70, 83–90. [Google Scholar] [CrossRef]
- Cho, S.H.; Park, B.Y.; Kim, J.H.; Hwang, I.H.; Kim, J.H.; Lee, J.M. Fatty Acid Profiles and Sensory Properties of Longissimus dorsi, Triceps brachii, and Semimembranosus Muscles from Korean Hanwoo and Australian Angus Beef. Asian-Australas. J. Anim. Sci. 2005, 18, 1786–1793. [Google Scholar] [CrossRef]
- Morrissey, P.A.; Sheehy, P.J.; Galvin, K.; Kerry, J.P.; Buckley, D.J. Lipid stability in meat and meat products. Meat Sci. 1998, 49S1, S73–S86. [Google Scholar] [CrossRef]
- Campo, M.M.; Nute, G.R.; Hughes, S.I.; Enser, M.; Wood, J.D.; Richardson, R.I. Flavour perception of oxidation in beef. Meat Sci. 2006, 72, 303–311. [Google Scholar] [CrossRef]
- Hou, L.; Xie, J.; Zhao, J.; Zhao, M.; Fan, M.; Xiao, Q.; Liang, J.; Chen, F. Roles of different initial Maillard intermediates and pathways in meat flavor formation for cysteine-xylose-glycine model reaction systems. Food Chem. 2017, 232, 135–144. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Toldra, F. Proteolysis and lipolysis in flavour development of dry-cured meat products. Meat Sci. 1998, 49S1, S101–S110. [Google Scholar] [CrossRef]
- Shahidi, F. Flavor of meat and meat products—an overview. Flavor Meat Meat Prod. 1994, 1–3. [Google Scholar] [CrossRef]
- Huang, L.; Xiong, Y.L.; Kong, B.; Huang, X.; Li, J. Influence of storage temperature and duration on lipid and protein oxidation and flavour changes in frozen pork dumpling filler. Meat Sci. 2013, 95, 295–301. [Google Scholar] [CrossRef]
- Adams, A.; Bouckaert, C.; Van Lancker, F.; De Meulenaer, B.; De Kimpe, N. Amino acid catalysis of 2-alkylfuran formation from lipid oxidation-derived alpha, beta-unsaturated aldehydes. J. Agric. Food Chem. 2011, 59, 11058–11062. [Google Scholar] [CrossRef]
- Schutte, L.; Teranishi, R. Precursors of sulfur-containing flavor compounds. CRC Crit. Rev. Food Technol. 1974, 4, 457–505. [Google Scholar] [CrossRef]
- Macleod, G. The flavour of beef. Flavor Meat Meat Prod. 1994, 4–37. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Toldra, F. Evolution of oxidised peptides during the processing of 9months Spanish dry-cured ham. Food Chem. 2018, 239, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, S.; Ahn, D.U. Protein oxidation: Basic principles and implications for meat quality. Crit. Rev. Food Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kantono, K.; Hamid, N.; Chadha, D.; Ma, Q.; Oey, I.; Farouk, M.M. Pulsed Electric Field (PEF) Processing of Chilled and Frozen-Thawed Lamb Meat Cuts: Relationships between Sensory Characteristics and Chemical Composition of Meat. Foods 2021, 10, 1148. https://doi.org/10.3390/foods10051148
Kantono K, Hamid N, Chadha D, Ma Q, Oey I, Farouk MM. Pulsed Electric Field (PEF) Processing of Chilled and Frozen-Thawed Lamb Meat Cuts: Relationships between Sensory Characteristics and Chemical Composition of Meat. Foods. 2021; 10(5):1148. https://doi.org/10.3390/foods10051148
Chicago/Turabian StyleKantono, Kevin, Nazimah Hamid, Diksha Chadha, Qianli Ma, Indrawati Oey, and Mustafa M. Farouk. 2021. "Pulsed Electric Field (PEF) Processing of Chilled and Frozen-Thawed Lamb Meat Cuts: Relationships between Sensory Characteristics and Chemical Composition of Meat" Foods 10, no. 5: 1148. https://doi.org/10.3390/foods10051148
APA StyleKantono, K., Hamid, N., Chadha, D., Ma, Q., Oey, I., & Farouk, M. M. (2021). Pulsed Electric Field (PEF) Processing of Chilled and Frozen-Thawed Lamb Meat Cuts: Relationships between Sensory Characteristics and Chemical Composition of Meat. Foods, 10(5), 1148. https://doi.org/10.3390/foods10051148








