Detection of Chilling Injury in Pickling Cucumbers Using Dual-Band Chlorophyll Fluorescence Imaging
Abstract
1. Introduction
2. Materials and Methods
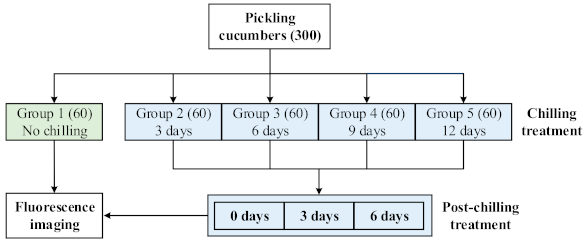
2.1. Pickling Cucumbers
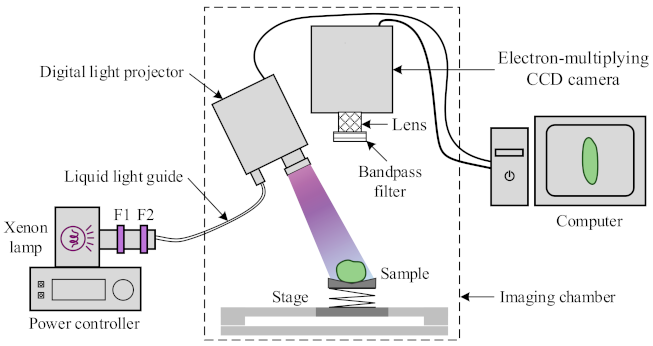
2.2. Chlorophyll Fluorescence Imaging
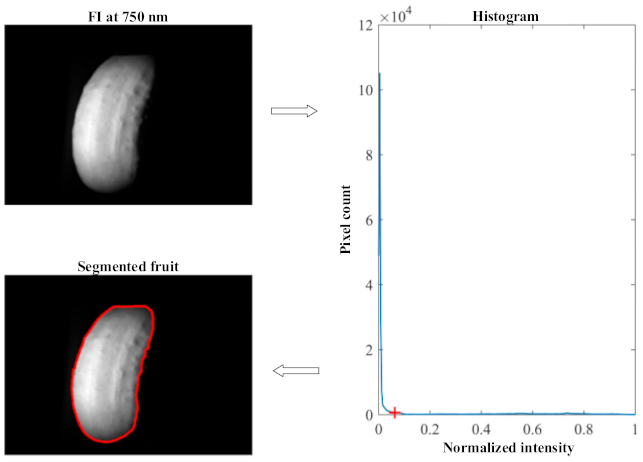
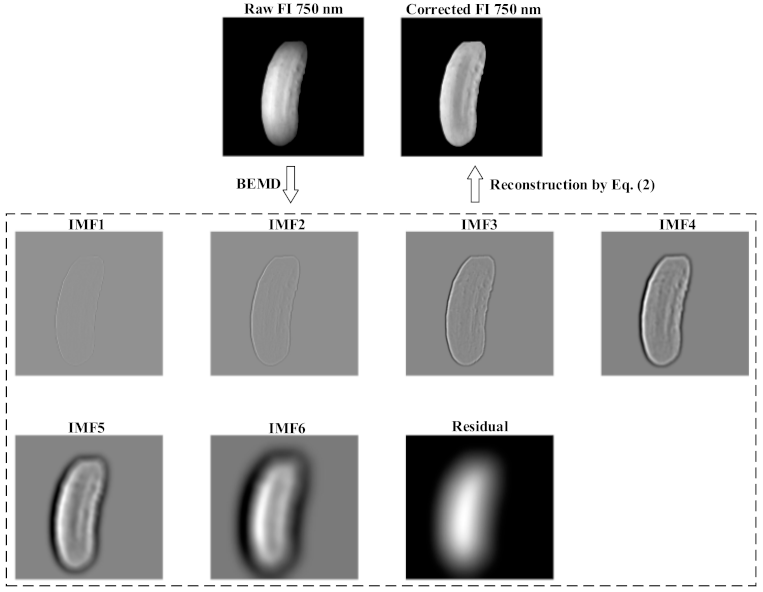
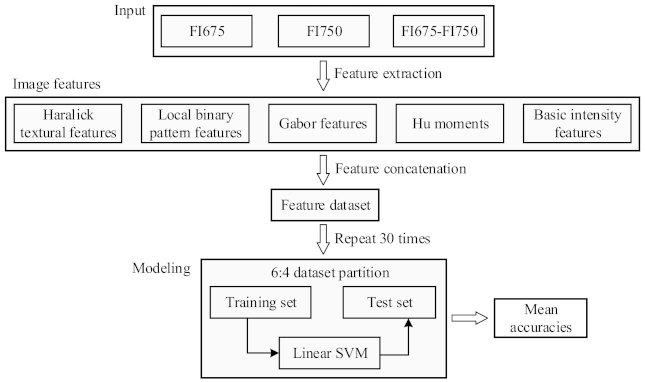
2.3. Image Processing
2.4. Machine Learning
2.5. Performance Metrics
2.6. Software
3. Results and Discussion
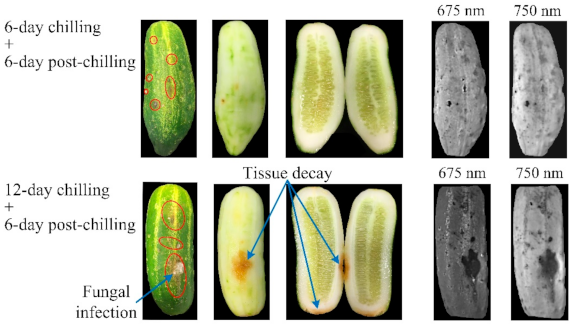
3.1. Chilling Injury Symptoms
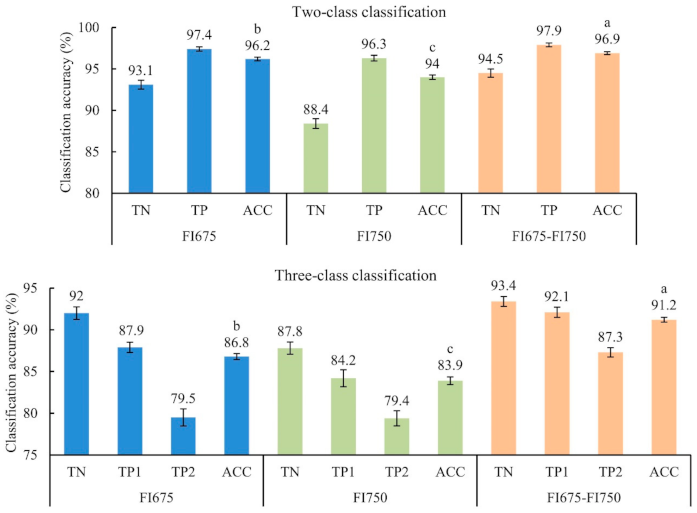
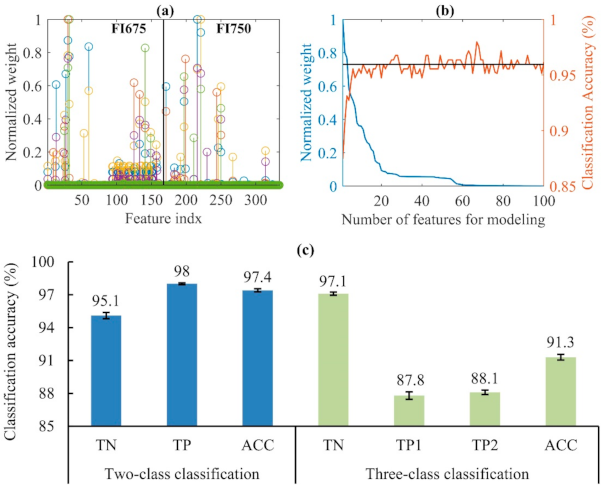
3.2. Classification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA-NASS. Vegetables 2019 Summary; U.S. Department of Agriculture, National Agricultural Statistics Service: Washington, DC, USA, 2020.
- Lower, R.L.; Edwards, M.D. Cucumber breeding. In Breeding Vegetable Crops; Bassett, M.J., Ed.; AVI Publishing: Westport, CT, USA, 1986; pp. 173–207. [Google Scholar]
- Fernandez-Trujillo, J.P.; Martinez, J.A. Ultrastructure of the oneset of chilling injury in cucumber fruit. J. Appl. Bot. Food Qual. 2006, 80, 100–110. [Google Scholar]
- Fukushima, T.; Yamazaki, M.; Tsugiyama, T. Chilling-injury in cucumber fruits. 1. effects of storage temperature on symptoms and physiological changes. Sci. Hortic. 1977, 6, 185–197. [Google Scholar] [CrossRef]
- Jackman, R.L.; Yada, R.Y.; Marangoni, A.; Pakain, K.L.; Stanley, D.W. Chilling injury: A review of quality aspects. J. Food Qual. 1988, 11, 253–278. [Google Scholar] [CrossRef]
- Cabrera, R.M.; Saltveit, M.E.; Owens, K. Cucumber cultivars differ in their response to chilling temperature. J. Am. Soc. Hortic. Sci. 1992, 117, 802–807. [Google Scholar] [CrossRef]
- Hakim, A.; Purvis, A.C.; Mullinix, B.G. Differences in chilling sensitivity of cucumber varieties depends on storage temperature and the physiological dysfunction evaluated. Postharvest Biol. Technol. 1999, 17, 97–104. [Google Scholar] [CrossRef]
- USDA-AMS. Cucumbers: Shipping Point and Market Inspection Instructions; United States Department of Agriculture Agricultural Marketing Service: Washington, DC, USA, 2017.
- USDA. United States Standards for Grades of Pickling Cucumbers; U.S. Department of Agriculture: Washington, DC, USA, 1997.
- Lu, Y.; Saeys, W.; Kim, M.; Peng, Y.; Lu, R. Hyperspectral imaging technology for quality and safety evaluation of horticultural products: A review and celebration of the past 20-year progress. Postharvest Biol. Technol. 2020, 170, 111318. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, Y.; Lu, R. Innovative Hyperspectral Imaging-based Techniques for Quality Evaluation of Fruits and Vegetables: A Review. Appl. Sci. 2017, 7, 189. [Google Scholar] [CrossRef]
- Cheng, X.; Chen, Y.R.; Tao, Y.; Wang, C.Y.; Kim, M.S.; Lefcourt, A.M. A novel integrated PCA and FLD method on hyperspectral image feature extraction for cucumber chllling damage inspection. Trans. ASAE 2004, 47, 1313–1320. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.R.; Wang, C.Y.; Chan, D.E.; Kim, M.S. Development of a simple algorithm for the detection of chilling injury in cucumbers from visible/near-infrared hyperspectralimaging. Appl. Spectrosc. 2005, 59, 78–85. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.R.; Wang, C.Y.; Chan, D.E. Development of hyperspectral imaging techniqeue for the detection of chilling injury in cucumbers: Spectral and image analysis. Appl. Eng. Agric. 2006, 22, 101–111. [Google Scholar] [CrossRef]
- Cen, H.; Lu, R.; Zhu, Q.; Mendoza, F. Nondestructive detection of chilling injury in cucumber fruit using hyperspectral imaging with feature selection and supervised classification. Postharvest Biol. Technol. 2016, 111, 352–361. [Google Scholar] [CrossRef]
- Gorbe, E.; Calatayud, A. Applications of chlorophyll flurorescence imaging technique in horticultural research: A review. Sci. Hortic. 2012, 138, 24–35. [Google Scholar] [CrossRef]
- Dell, J.R.; van Kooten, O.; Prange, P.K.; Murr, D.P. Applications of chlorophyll fluorescence techniques in postharvest physiology. Hortic. Rev. 1999, 23, 69–107. [Google Scholar]
- Martkhart III, A.H. Chilling injury: A review of possible causes. HortScience 1986, 21, 1329–1333. [Google Scholar]
- Abbott, J.A.; Massie, D.R. Delayed light emission for early detection of chilling in cucumber and bell pepper fruit. J. Am. Soc. Hortic. Sci. 1985, 110, 42–47. [Google Scholar]
- van Kooten, O.; Mensink, M.G.J.; Otma, E.C.; van Schaik, A.C.R.; Schouten, S.P. Chilled damage of dark stored cucumbers (Cucumis sativus L.) afffects the maximum quantum yield of photosystem. In Progress in Photosynthesis Research; Murata, N., Ed.; Kluwer Academic Publisher: Dordrecht, The Netherlands, 1992; pp. 161–164. [Google Scholar]
- DeEll, J.R.; Vigneault, C.; Lemerre, S. Water temperature for hydrocooling field cucumbers in relation to chilling injury during storage. Postharvest Biol. Technol. 2000, 18, 27–32. [Google Scholar] [CrossRef]
- Nedbal, L.; Whitmarsh, J. Chlorophyll fluorescence imaging of leaves and fruits. In Chlorophyll a Fluorescence. Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 389–407. [Google Scholar]
- Nedbal, L.; Soukupova, J.; Whitmarsh, J.; Trtilek, M. Postharvest imaging of chlorophyll fluorescence from lemons can be used to predict fruit quality. Photosynthetica 2000, 38, 517–579. [Google Scholar] [CrossRef]
- Obenland, D.; Neipp, P. Chlorophyll fluorescence imaging allows early detection and localization of lemon rind injury flollowing hot water treatment. HortScience 2005, 40, 1821–1823. [Google Scholar] [CrossRef]
- Slaughter, D.C.; Obenland, D.M.; Thompson, J.F.; Arpaia, M.L.; Margosan, D.A. Non-destructive freeze damage detection in oranges using machine vision and ultraviolet fluorescence. Postharvest Biol. Technol. 2008, 48, 341–346. [Google Scholar] [CrossRef]
- Kondo, N.; Kuramoto, M.; Shimizu, H.; Ogawa, Y.; Kurita, M.; Nishizu, T.; Chong, V.K.; Yamamoto, K. Identification of fluorescent substance in mandarin orange skin for machine vision system to detect rotten citrus fruits. Eng. Agric. Environ. Food 2009, 2, 54–59. [Google Scholar] [CrossRef]
- Kim, M.S.; Lef.court, A.M.; Chen, Y.R.; Tao, Y. Automated detection of fecal contamination of apples based on multispectral fluorescence image fusion. J. Food Eng. 2005, 71, 85–91. [Google Scholar] [CrossRef]
- Lohumi, S.; Cho, B.W.; Hong, S. LCTF-based multispectral fluorescence imaging: System development and potential for real-time foreign object detection in fresh-cut vegetable processing. Comput. Electron. Agric. 2021, 180, 105912. [Google Scholar] [CrossRef]
- Mo, C.; Kim, G.; Kim, M.S.; Lim, J.; Cho, H.; Barnaby, J.Y.; Cho, B.K. Fluoresence hyperspectral imaging techniques for forign substance detection on fresh-cut lettuce. J. Sci. Food Agric. 2017, 97, 3985–3993. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lu, R. Enhancing chlorophyll fluorescence imaging under structured illumination with automatic vignetting correction for detection of chilling injury in cucumbers. Comput. Electron. Agric. 2020, 68, 105145. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, R. Histogram based automatic thresholding for bruise detection of apples by structured-illumination reflectance imaging. Biosyst. Eng. 2017, 160, 30–41. [Google Scholar] [CrossRef]
- Kim, M.S.; Chen, Y.R.; Mehl, P.M. Hyperspectral reflectance and fluorescence imaging system for food quality and safety. Trans. ASAE 2001, 44, 721–729. [Google Scholar]
- Lu, Y.; Lu, R. Fast bi-dimensional empirical mode decomposition as an image enhancement technique for fruit defect detection. Comput. Electron. Agric. 2018, 152, 314–323. [Google Scholar] [CrossRef]
- Yang, J.; Yang, J.Y.; Zhang, D.; Lu, J.F. Feature fusion: Parallel strategy vs. serial strategy. Pattern Recognit. 2003, 36, 1369–1381. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, R. Detection of surface and subsurface defects of apples using structured-illumination reflectance imaging with machine learning algorithms. Trans. ASABE 2018, 61, 1831–1842. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, R. siritool: A Matlab Graphical User Interface for Image Analysis in Structured-Illumination Reflectance Imaging for Fruit Defect Detection. Trans. ASABE 2020, 3, 1037–1047. [Google Scholar] [CrossRef]
- Ben-Hur, A.; Weston, J. A user’s guide to support vector machines. In Data Mining Techniques for the Life Sciences; Carugo, O., Eisenhaber, F., Eds.; Humana Press: Totowa, NJ, USA, 2010; pp. 223–239. [Google Scholar]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Cristianini, N.; Shawe-Taylor, J. An Introduction to Support Vector Machines and Other Kernel-Based Learning Methods; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Yang, W.; Wang, K.; Zuo, W. Neighborhood component feature selection for high-dimensional data. J. Comput. 2012, 7, 61–168. [Google Scholar] [CrossRef]
- Flach, P. Machine Learning: The Art and Science of Algorithms that Make Sense of Data; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Tatsumi, Y.; Maeda, K.; Murata, T. Morphological changes in cucumber fruit surface associated with chilling injury. J. Jpn. Soc. Hort. Sci. 1987, 56, 187–192. [Google Scholar] [CrossRef]
- Buschmann, C. Variability and application of the chlorophyll fluorescence emssion ratio red/far-red of leaves. Photosynth. Res. 2007, 95, 261–271. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Lu, R. Detection of Chilling Injury in Pickling Cucumbers Using Dual-Band Chlorophyll Fluorescence Imaging. Foods 2021, 10, 1094. https://doi.org/10.3390/foods10051094
Lu Y, Lu R. Detection of Chilling Injury in Pickling Cucumbers Using Dual-Band Chlorophyll Fluorescence Imaging. Foods. 2021; 10(5):1094. https://doi.org/10.3390/foods10051094
Chicago/Turabian StyleLu, Yuzhen, and Renfu Lu. 2021. "Detection of Chilling Injury in Pickling Cucumbers Using Dual-Band Chlorophyll Fluorescence Imaging" Foods 10, no. 5: 1094. https://doi.org/10.3390/foods10051094
APA StyleLu, Y., & Lu, R. (2021). Detection of Chilling Injury in Pickling Cucumbers Using Dual-Band Chlorophyll Fluorescence Imaging. Foods, 10(5), 1094. https://doi.org/10.3390/foods10051094





