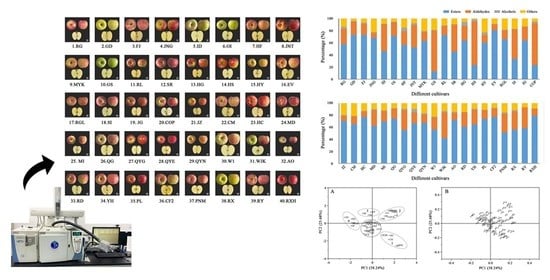
Identification, Comparison and Classification of Volatile Compounds in Peels of 40 Apple Cultivars by HS–SPME with GC–MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Physiological Characteristics Measurement
2.3. HS-SPME Procedure
2.4. GC-MS Analysis
2.5. Qualitative and Semi-Quantitative Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Identification and Determination of Volatile Compounds in Forty Apple Cultivars
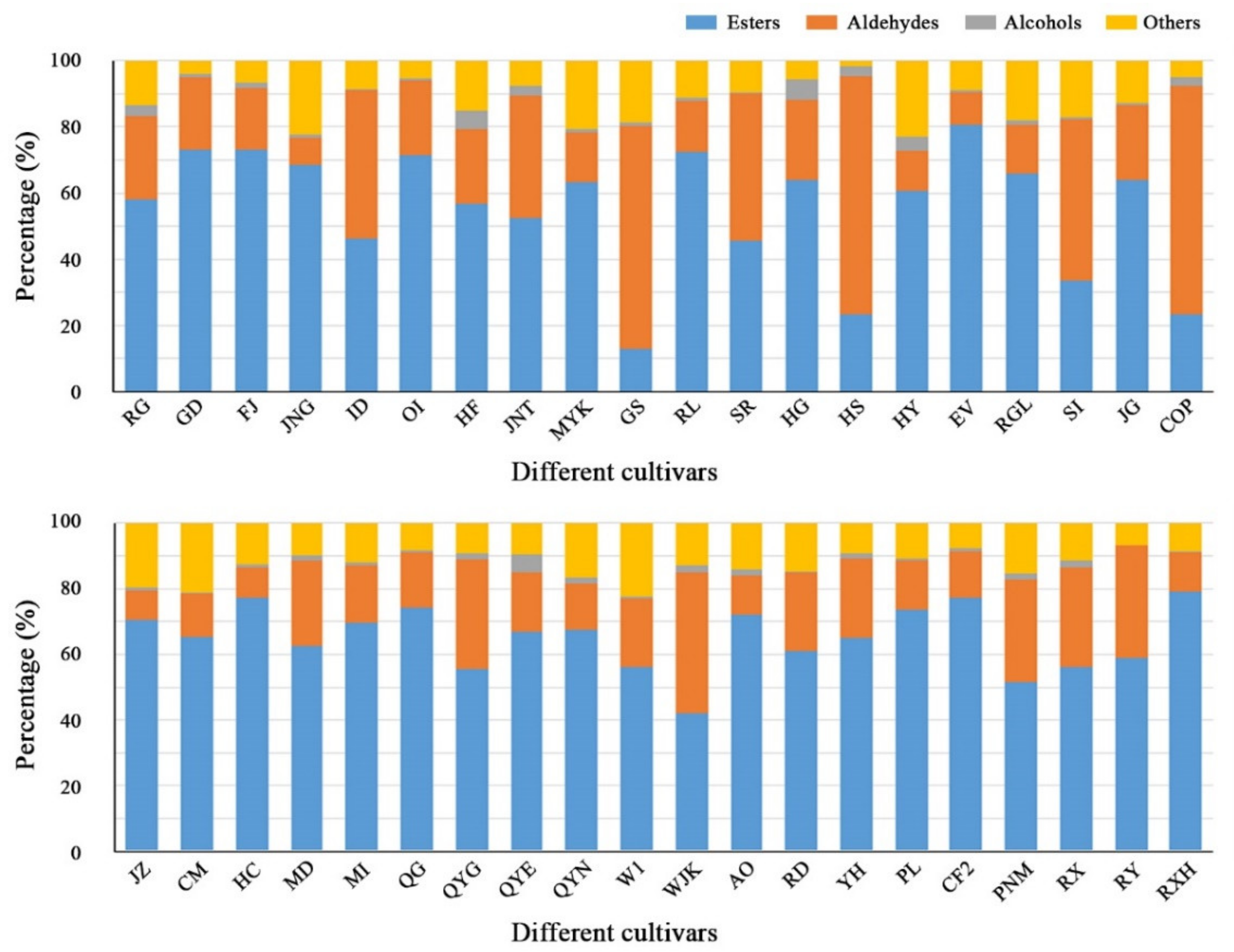
3.2. Composition and Concentration of Volatile Compounds
3.2.1. Esters
3.2.2. Aldehydes
3.2.3. Alcohols
3.2.4. Ketones, Acids and Other Compounds
3.3. Principal Component Analysis of Volatile Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harker, F.R.; Kupferman, E.M.; Marin, A.B.; Gunson, F.A.; Triggs, C.M. Eating quality standards for apples based on consumer preferences. Postharvest Biol. Technol. 2008, 50, 70–78. [Google Scholar] [CrossRef]
- Cunningham, D.G.; Acree, T.E.; Barnard, J.; Butts, R.M.; Braell, P.A. Charm analysis of apple volatiles. Food Chem. 1986, 19, 137–147. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.Y.; Zhou, L.Y.; Bi, J.F.; Wang, P.; Wu, X.Y. Influence of number of puffing times on physicochemical, color, texture, and microstructure of explosion puffing dried apple chips. Dry Technol. 2016, 34, 773–782. [Google Scholar] [CrossRef]
- Dixon, J.; Hewett, E.W. Factors affecting apple aroma/flavour volatile concentration: A review. N. Z. J. Crop. Hortic Sci. 2000, 28, 155–173. [Google Scholar] [CrossRef]
- Song, J.; Forney, C.F. Flavour volatile production and regulation in fruit. Can. J. Plant Sci. 2008, 88, 537–550. [Google Scholar] [CrossRef]
- Dunemann, F.; Ulrich, D.; Malysheva-Otto, L.; Weber, W.; Longhi, S.; Velasco, R.; Costa, F. Functional allelic diversity of the apple alcohol acyl-transferase gene MdAAT1 associated with fruit ester volatile contents in apple cultivars. Mol. Breed. 2012, 29, 609–625. [Google Scholar] [CrossRef]
- Dimick, P.S.; Hoskin, J.C.; Acree, T.E. Review of apple flavor-State of the art. CRC Crit. Rev. Food Sci. 1983, 18, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Espino-Diaz, M.; Sepulveda, D.R.; Gonzalez-Aguilar, G.; Olivas, G.I. Biochemistry of apple aroma: A review. Food Technol. Biotechnol. 2016, 54, 375–394. [Google Scholar] [CrossRef]
- Mattheis, J.P.; Fan, X.; Argenta, L.C. Interactive responses of gala apple fruit volatile production to controlled atmosphere storage and chemical inhibition of ethylene action. J. Agric. Food Chem. 2005, 53, 4510–4516. [Google Scholar] [CrossRef]
- Altisent, R.; Echeverria, G.; Grael, J.; Lopez, L.; Lara, I. Lipoxygenase activity is involved in the regeneration of volatile ester-synthesizing capacity after ultra-low oxygen storage of ‘Fuji’ apple. J. Agric. Food Chem. 2009, 57, 4305–4312. [Google Scholar] [CrossRef]
- Qin, G.; Tao, S.; Cao, Y.; Wu, J.; Zhang, H.; Huang, W. Evaluation of the volatile profile of 33 Pyrus ussuriensis cultivars by HS-SPME with GC-MS. Food Chem. 2012, 134, 2367–2382. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wu, H.; Xiong, M.; Chen, Y.; Chen, J.; Zhou, B.; Wang, H.; Li, L.; Fu, X.; Bie, Z.; et al. Comparative analysis of volatile compounds in thirty nine melon cultivars by headspace solid-phase microextraction and gas chromatography-mass spectrometry. Food Chem. 2020, 316, 126342. [Google Scholar] [CrossRef]
- Wang, Y.J.; Yang, C.X.; Li, S.H.; Yang, L.; Wang, Y.N.; Zhao, J.B.; Jiang, Q. Volatile characteristics of 50 peaches and nectarines evaluated by HP-SPME with GC-MS. Food Chem. 2009, 116, 356–364. [Google Scholar] [CrossRef]
- Moinfar, S.; Jamil, L.A.; Sami, H.Z. Determination of organophosphorus pesticides in juice and water by modified continuous sample drop flow microextraction combined with gas chromatography—Mass spectrometry. Food Anal. Methods 2020, 13, 1050–1059. [Google Scholar] [CrossRef]
- Bergler, G.; Nolleau, V.; Picou, C.; Perez, M.; Ortiz-Julien, A.; Brulfert, M.; Camarasa, C.; Bloem, A. Dispersive liquid-liquid microextraction for the quantitation of terpenes in wine. J. Agric. Food Chem. 2020, 68, 13302–13309. [Google Scholar] [CrossRef]
- Savelieva, E.I.; Gavrilova, O.P.; Gagkaeva, T.Y. Using solid-phase microextraction combined with gas chromatography-mass spectrometry for the study of the volatile products of biosynthesis released by plants and microorganisms. J. Anal. Chem. 2014, 69, 609–615. [Google Scholar] [CrossRef]
- Aprea, E.; Corollaro, M.L.; Betta, E.; Endrizzi, I.; Dematte, M.L.; Biasioli, F.; Gasperi, F. Sensory and instrumental profiling of 18 apple cultivars to investigate the relation between perceived quality and odour and flavour. Food Res. Int. 2012, 49, 677–686. [Google Scholar] [CrossRef]
- Yan, D.; Shi, J.; Ren, X.; Tao, Y.; Ma, F.; Li, R.; Liu, X.; Liu, C. Insights into the aroma profiles and characteristic aroma of ‘Honeycrisp’ apple (Malus × domestica). Food Chem. 2020, 327, 127074. [Google Scholar] [CrossRef] [PubMed]
- Villatoro, C.; Altisent, R.; Echeverria, G.; Graell, J.; Lopez, M.; Lara, I. Changes in biosynthesis of aroma volatile compounds during on-tree maturation of ‘Pink Lady’ apples. Postharvest Biol. Technol. 2008, 47, 286–295. [Google Scholar] [CrossRef]
- Qin, L.; Wei, Q.; Kang, W.; Zhang, Q.; Sun, J.; Liu, S. Comparison of volatile compounds in ‘Fuji’ apples in the different regions in China. Food Sci. Technol. Res. 2017, 23, 79–89. [Google Scholar] [CrossRef]
- Zhu, D.; Ren, X.; Wei, L.; Cao, X.; Ge, Y.; Li, J. Collaborative analysis on difference of apple fruits flavour using electronic nose and electronic tongue. Sci. Hortic. 2020, 260, 108879. [Google Scholar] [CrossRef]
- Strojnik, L.; Stopar, M.; Zlatic, E.; Kokalj, D.; Gril, M.N.; Zenko, B.; Znidarsic, M.; Bohanec, M.; Boshkovska, B.M.; Lustrek, M.; et al. Authentication of key aroma compounds in apple using stable isotope approach. Food Chem. 2019, 277, 766–773. [Google Scholar] [CrossRef]
- Zhu, Y.; Rudell, D.R.; Mattheis, J.P. Characterization of cultivar differences in alcohol acyltransferase and 1-aminocyclopropane-1-carboxylate synthase gene expression and volatile ester emission during apple fruit maturation and ripening. Postharvest Biol. Technol. 2008, 49, 330–339. [Google Scholar] [CrossRef]
- Mehinagic, E.; Royer, G.; Symoneaux, R.; Jourjon, F.; Prost, C. Characterization of odor-active volatiles in apples: Influence of cultivars and maturity stage. J. Agric. Food Chem. 2006, 54, 2678–2687. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, W.; Yu, W.; Zhao, L.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, L.; Wang, S. Study on the volatile composition of table grapes of three aroma types. LWT Food Sci. Technol. 2019, 115, 108450. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, R.; Xiao, Z.; Zhu, J.; Sun, X.; Wang, P. Characterization of ester odorants of apple juice by gas chromatography—Olfactometry, quantitative measurements, odour threshold, aroma intensity and electronic nose. Food Res. Int. 2019, 120, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yue, T.; Yuan, Y.; Sun, N.; Liu, P. Characterization of volatile and sensory profiles of apple juices to trace fruit origins and investigation of the relationship between the aroma properties and volatile constituents. LWT Food Sci. Technol. 2020, 124, 109203. [Google Scholar] [CrossRef]
- Schiller, D.; Contreras, C.; Vogt, J.; Dunemann, F.; Defilippi, B.G.; Beaudry, R.; Schwab, W. A dual positional specific lipoxygenase functions in the generation of flavor compounds during climacteric ripening of apple. Hortic. Res. 2015, 2, 15003. [Google Scholar] [CrossRef]
- Salas, N.A.; Gonzalez-Aguilar, G.A.; Jacobo-Cuellar, J.L.; Espino, M.; Sepulveda, D.; Guerrero, V.; Olivas, G.I. Volatile compounds in golden delicious apple fruit (Malus domestica) during cold storage. Rev. Fitotec Mex. 2016, 39, 159–173. [Google Scholar]
- Esmaeili, A.; Abednazari, S.; Abdollahzade, Y.M.; Abdollahzadeh, N.M.; Mahjoubian, R.; Tabatabaei-Anaraki, M. Peel volatile compounds of apple (Malus domestica) and grapefruit (Citrus Paradisi). J. Essent. Oil Bear. Plants. 2012, 15, 794–799. [Google Scholar] [CrossRef]
- Ferreira, L.; Perestrelo, R.; Caldeira, M.; Camara, J.S. Characterization of volatile substances in apples from rosaceae family by headspace solid-phase microextraction followed by GC-qMS. J. Sep. Sci. 2015, 32, 1875–1888. [Google Scholar] [CrossRef] [PubMed]
- Wandjou, J.N.; Sut, S.; Giuliani, C.; Fico, G.; Papa, F.; Ferraro, S.; Caprioli, G.; Maggi, F.; Acqua, S.D. Characterization of nutrients, polyphenols and volatile components of the ancient apple cultivar ‘mela rosa dei monti sibillini’ from marche region, central italy. Int. J. Food Sci. Nutr. 2019, 1, 1–17. [Google Scholar]
- Yang, S.; Meng, Z.; Li, Y.; Chen, R.; Yang, Y.; Zhao, Z. Evaluation of physiological characteristics, soluble sugars, organic acids and volatile compounds in ‘Orin’ apples (Malus domestica) at different ripening stages. Molecules 2021, 26, 807. [Google Scholar] [CrossRef]
- Komthong, P.; Katoh, T.; Igura, N.; Shimoda, M. Changes in the odours of apple juice during enzymatic browning. Food Qual. Prefer. 2006, 17, 497–504. [Google Scholar] [CrossRef]
- Both, V.; Brackmann, A.; Thewes, F.R.; Ferreira, D.; Wagner, R. Effect of storage under extremely low oxygen on the volatile composition of ‘Royal Gala’ apples. Food Chem. 2014, 156, 50–57. [Google Scholar] [CrossRef]
- Amaro, A.L.; Beaulieu, J.C.; Grimm, C.C.; Stein, R.E.; Almeida, D.P. Effect of oxygen on aroma volatiles and quality of fresh-cut cantaloupe and honeydew melons. Food Chem. 2012, 130, 49–57. [Google Scholar] [CrossRef]
- Gabler, F.M.; Mercier, J.; Jimenez, J.I.; Smilanick, J.L. Integration of continuous biofumigation with muscodor albus with pre-cooling fumigation with ozone or sulfur dioxide to control postharvest gray mold of table grapes. Postharvest Biol. Technol. 2010, 55, 78–84. [Google Scholar] [CrossRef]
- Gan, H.H.; Soukoulis, C.; Fisk, I. Atmospheric pressure chemical ionisation mass spectrometry analysis linked with chemometrics for food classification—A casestudy: Geographical provenance and cultivar classification of monovarietal clarified apple juices. Food Chem. 2014, 146, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Nikfardjam, M.P.; Maier, D. Development of a headspace trap HRGC/MS method for the assessment of the relevance of certain aroma compounds on the sensorial characteristics of commercial apple juice. Food Chem. 2011, 126, 1926–1933. [Google Scholar] [CrossRef]
- Bult, J.H.; Schifferstein, H.N.; Roozen, J.P.; Dalmau, B.E.; Voragen, A.G.; Kroeze, J.H. Sensory evaluation of character impact components in an apple model mixture. Chem. Senses 2002, 27, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Odeyemi, O.A.; Burke, C.M.; Bolch, C.J.; Stanley, R. Evaluation of spoilage potential and volatile metabolites production by shewanella baltica isolated from modified atmosphere packaged live mussels. Food Res. Int. 2018, 103, 415–425. [Google Scholar] [CrossRef]
- Khalil, M.N.; Fekry, M.I.; Farag, M.A. Metabolome based volatiles profling in 13 date palm fruit varieties from Egypt via SPME GC-MS and chemometrics. Food Chem. 2017, 217, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Delgado, F.J.; Gonzalez-Crespo, J.; Cava, R.; Garcia-Parra, J.; Ramirez, R. Characterisation by SPME-GC-MS of the volatile profile of a Spanish soft cheese P.D.O. Torta del Casar during ripening. Food Chem. 2010, 118, 182–189. [Google Scholar] [CrossRef]
- Echeverria, G.; Fuentes, T.; Graell, J.; Lara, I.; Lopez, M. Aroma volatile compounds of ‘Fuji’ apples in relation to harvest date and cold storage technology: A comparison of two seasons. Postharvest Biol. Technol. 2004, 32, 29–44. [Google Scholar] [CrossRef]
- Aprea, E.; Gika, H.; Carlin, S.; Theodoridis, G.; Vrhovsek, U.; Mattivi, F. Metabolite profiling on apple volatile content based on solid phase microextraction and gas chromatography time of flight mass spectrometry. J. Chromatogr. A 2011, 1218, 4517–4524. [Google Scholar] [CrossRef]


| No. | Cultivar | Code | SFW (g) | TSS (°Brix) | TA (%) |
|---|---|---|---|---|---|
| 1 | Royal Gala | RG | 175 ± 15 | 12.7 ± 0.2 | 0.42 ± 0.03 |
| 2 | Golden Delicious | GD | 262 ± 22 | 13.5 ± 0.4 | 0.44 ± 0.04 |
| 3 | Fuji | FJ | 320 ± 25 | 13.2 ± 0.2 | 0.29 ± 0.02 |
| 4 | Jonagold | JNG | 280 ± 20 | 14.3 ± 0.3 | 0.37 ± 0.03 |
| 5 | Indo | ID | 350 ± 32 | 13.8 ± 0.2 | 0.13 ± 0.01 |
| 6 | Orin | OI | 255 ± 24 | 14.5 ± 0.1 | 0.30 ± 0.02 |
| 7 | Hanfu | HF | 285 ± 18 | 13.5 ± 0.2 | 0.36 ± 0.02 |
| 8 | Jonathan | JNT | 325 ± 23 | 14.1 ± 0.2 | 0.37 ± 0.03 |
| 9 | Miyakiji | MYK | 310 ± 26 | 14.6 ± 0.4 | 0.30 ± 0.03 |
| 10 | Granny Smith | GS | 285 ± 18 | 14.4 ± 0.3 | 0.37 ± 0.01 |
| 11 | Ralls | RL | 184 ± 12 | 14.0 ± 0.2 | 0.26 ± 0.02 |
| 12 | Starkrimson | SR | 275 ± 15 | 12.3 ± 0.1 | 0.28 ± 0.02 |
| 13 | Huaguan | HG | 178 ± 12 | 13.8 ± 0.3 | 0.27 ± 0.03 |
| 14 | Huashuo | HS | 266 ± 20 | 13.6 ± 0.2 | 0.38 ± 0.01 |
| 15 | Huayu | HY | 198 ± 12 | 13.1 ± 0.2 | 0.29 ± 0.03 |
| 16 | Envy | EV | 315 ± 24 | 14.6 ± 0.3 | 0.38 ± 0.02 |
| 17 | Red General | RGL | 268 ± 17 | 15.6 ± 0.3 | 0.28 ± 0.04 |
| 18 | Starking | SI | 290 ± 22 | 12.9 ± 0.2 | 0.32 ± 0.03 |
| 19 | Jiguan | JG | 217 ± 13 | 13.7 ± 0.1 | 0.26 ± 0.03 |
| 20 | Cox Orange | COP | 256 ± 20 | 13.2 ± 0.3 | 0.36 ± 0.02 |
| 21 | Jazz | JZ | 165 ± 10 | 12.2 ± 0.2 | 0.52 ± 0.05 |
| 22 | Cameo | CM | 334 ± 26 | 13.7 ± 0.4 | 0.39 ± 0.03 |
| 23 | Honey Crips | HC | 342 ± 28 | 14.0 ± 0.3 | 0.53 ± 0.05 |
| 24 | Mollie’s Delicious | MD | 280 ± 20 | 13.5 ± 0.2 | 0.29 ± 0.04 |
| 25 | Modi | MI | 195 ± 14 | 13.8 ± 0.3 | 0.42 ± 0.02 |
| 26 | Qinguan | QG | 332 ± 22 | 13.9 ± 0.1 | 0.16 ± 0.01 |
| 27 | Qinyang | QYG | 210 ± 15 | 12.1 ± 0.1 | 0.25 ± 0.01 |
| 28 | Qinyue | QYE | 182 ± 13 | 13.2 ± 0.2 | 0.29 ± 0.02 |
| 29 | Qinyun | QYN | 190 ± 14 | 13.3 ± 0.3 | 0.26 ± 0.01 |
| 30 | World No.1 | W1 | 510 ± 35 | 14.5 ± 0.3 | 0.26 ± 0.01 |
| 31 | Weijieke | WJK | 305 ± 25 | 12.8 ± 0.2 | 0.51 ± 0.04 |
| 32 | Alps Otome | AO | 50 ± 5 | 14.0 ± 0.2 | 0.27 ± 0.03 |
| 33 | Red Delicious | RD | 295 ± 15 | 13.5 ± 0.3 | 0.32 ± 0.02 |
| 34 | Yuhuazaofu | YH | 305 ± 16 | 13.4 ± 0.2 | 0.41 ± 0.03 |
| 35 | Pink Lady | PL | 183 ± 13 | 14.8 ± 0.3 | 0.52 ± 0.04 |
| 36 | Changfu No.2 | CF2 | 330 ± 26 | 15.4 ± 0.3 | 0.15 ± 0.01 |
| 37 | Punama | PNM | 246 ± 18 | 12.0 ± 0.2 | 0.28 ± 0.02 |
| 38 | Ruixue | RX | 296 ± 21 | 14.5 ± 0.2 | 0.30 ± 0.02 |
| 39 | Ruiyang | RY | 285 ± 20 | 13.5 ± 0.1 | 0.33 ± 0.03 |
| 40 | Ruixianghong | RXH | 165 ± 15 | 14.9 ± 0.2 | 0.24 ± 0.02 |
| Code a | Compounds | CAS No b | Odour Description c | RT d | RI e/RI f | Content (μg/kg FW) |
|---|---|---|---|---|---|---|
| Esters | ||||||
| E1 | Ethyl acetate | 141-78-6 | Pineapple, balsamic | 8.74 | 894/893 | 3.01 (0–73.34) |
| E2 | Ethyl propanoate | 105-37-3 | Banana, apple | 10.20 | 964/964 | 0.24 (0–9.53) |
| E3 | Propyl acetate | 109-60-4 | Celery | 10.67 | 982/982 | 3.39 (0–52.92) |
| E4 | Ethyl butyrate | 105-54-4 | Pineapple, fruity | 12.33 | 1045/1048 | 8.52 (0–147.95) |
| E5 | Propyl propionate | 106-36-5 | Fruity, sweet | 12.57 | 1050/1045 | 2.43 (0–22.76) |
| E6 | Ethyl 2-methylbutyrate | 7452-79-1 | Fruity, berry, fresh | 12.78 | 1062/1063 | 4.85 (0–74.61) |
| E7 | Butyl acetate | 123-86-4 | Fruity, ripe banana | 13.40 | 1074/1075 | 145.31 (0–1064.54) |
| E8 | 2-Methylbutyl acetate | 624-41-9 | Fruity, banana | 14.84 | 1126/1128 | 245.02 (0–1158.08) |
| E9 | Propyl butyrate | 105-66-8 | Fruity | 14.88 | 1135/1153 | 1.37 (0–26.05) |
| E10 | Propyl 2-methylbutyrate | 37064-20-3 | Fruity, sweet | 15.29 | 1150/1150 | 13.66 (0–94.23) |
| E11 | Butyl propionate | 590-01-2 | Apple, fruity | 15.41 | 1157/1158 | 51.57 (0–343.31) |
| E12 | Amyl acetate | 628-63-7 | Pear, banana | 16.37 | 1178/1185 | 20.15 (0–89.25) |
| E13 | Amyl propionate | 624-54-4 | Fruity | 16.84 | 1195/1208 | 11.01 (0–103.27 |
| E14 | Butyl butyrate | 109-21-7 | Fruity, apple, pear | 17.69 | 1240/1240 | 103.51 (0–490.14) |
| E15 | Butyl 2-methylbutyrate | 15706-73-7 | Fruity | 18.08 | 1243/1241 | 204.79 (0–976.61) |
| E16 | 2-Methylbutyl butyrate | 51115-64-1 | Fruity | 19.06 | 1270/1270 | 11.87 (0–69.21) |
| E17 | Hexyl acetate | 142-92-7 | Sweet, flora, cherry | 19.28 | 1274/1276 | 492.44 (10.61–1649.99) |
| E18 | Pentyl valerate | 2173-56-0 | Fruity | 19.45 | 1283/1284 | 3.59 (0–143.61) |
| E19 | 2-Methylbutyl 2-methylbutyrate | 2445-78-5 | Fruity | 19.48 | 1286/1286 | 43.66 (0–268.76) |
| E20 | Pentyl butyrate | 540-18-1 | Fruity | 20.52 | 1321/1320 | 17.00 (0–81.93) |
| E21 | Propyl hexanoate | 626-77-7 | Fruity, pineapple | 20.58 | 1324/1324 | 10.08 (0–81.98) |
| E22 | Amyl 2-methylbutyrate | 68039-26-9 | Fruity, apple | 20.83 | 1330/1327 | 43.17 (0–180.78) |
| E23 | Hexyl propanoate | 2445-76-3 | Fruity, sweet | 21.14 | 1347/1344 | 180.82 (0–1404.90) |
| E24 | Hexyl isobutyrate | 2349-07-7 | Fruity, sweet | 21.18 | 1350/1353 | 14.55 (0–262.64) |
| E25 | Heptyl acetate | 112-06-1 | Fruity, orange | 22.09 | 1386/1386 | 0.30 (0–9.40) |
| E26 | Butyl caproate | 626-82-4 | Fruity, acid, rancid | 23.17 | 1410/1414 | 433.62 (7.70–1607.26) |
| E27 | Hexyl butyrate | 2639-63-6 | Fruity, green, sweet | 23.23 | 1423/1424 | 742.12 (3.62–2709.52) |
| E28 | Hexyl 2-methylbutyrate | 10032-15-2 | Fruity, green | 23.53 | 1438/1438 | 3085.20 (160.76–10087.55) |
| E29 | Ethyl octanoate | 106-32-1 | Sweet, flora, pear | 23.72 | 1445/1445 | 2.51 (0–44.50) |
| E30 | 2-Methylbutyl hexanoate | 2601-13-0 | Fruity | 24.36 | 1467/1468 | 49.51 (0–291.37) |
| E31 | trans-2-Hexenyl valerate | 56922-74-8 | Fruity | 24.94 | 1478/1478 | 5.12 (0–57.99) |
| E32 | Amyl caproate | 540-07-8 | Fruity | 25.73 | 1508/1509 | 69.62 (0–364.73) |
| E33 | Octyl hexanoate | 4887-30-3 | Fruity | 25.75 | 1512/1512 | 12.29 (0–219.39) |
| E34 | Hexyl valerate | 1117-59-5 | Fruity | 25.77 | 1516/1516 | 8.48 (0–204.26) |
| E35 | Butyl heptanoate | 5454-28-4 | Fruity | 25.78 | 1518/1518 | 34.24 (0–363.05) |
| E36 | Propyl octanoate | 624-13-5 | Fruity | 25.93 | 1525/1525 | 7.68 (0–83.56) |
| E37 | Heptyl valerate | 5451-80-9 | Fruity | 26.10 | 1529/1530 | 4.72 (0–143.09) |
| E38 | Heptyl 2-methylbutyrate | 50862-12-9 | Fruity | 26.12 | 1530/1533 | 19.23 (0–111.43) |
| E39 | 3-methylbut-2-enyl hexanoate | 76649-22-4 | Fruity | 27.53 | 1578/1575 | 0.37 (0–8.60) |
| E40 | Hexyl hexanoate | 6378-65-0 | Fruity, wine | 28.07 | 1593/1593 | 1444.76 (83.05–5688.01) |
| E41 | Butyl caprylate | 589-75-3 | Slightly fruity | 28.16 | 1603/1601 | 288.67 (0–2611.76) |
| E42 | Hexyl tiglate | 16930-96-4 | Fruity | 28.46 | 1631/1631 | 45.39 (0–233.71) |
| E43 | Butyrolactone | 96-48-0 | Fruity | 28.76 | 1638/1640 | 0.44 (0–17.42) |
| E44 | 2-Pentyl octanoate | 55193-30-1 | Fruity | 29.11 | 1647/1645 | 12.12 (0–245.97) |
| E45 | 2-Methylbutyl octanoate | 67121-39-5 | Fruity | 29.13 | 1648/1648 | 53.73 (0–320.66) |
| E46 | Hexyl caprylate | 1117-55-1 | Fruity | 31.82 | 1759/1760 | 114.96 (0–653.28) |
| E47 | Butyl caprate | 30673-36-0 | Fruity | 31.93 | 1765/1765 | 9.25 (0–163.04) |
| Aldehydes | ||||||
| A1 | Hexanal | 66-25-1 | Green, sweet | 13.76 | 1090/1089 | 242.14 (29.29–1050.38) |
| A2 | 2-Methyl-4-pentenal | 5187-71-3 | Green | 15.42 | 1156/1155 | 7.18 (0–110.33) |
| A3 | (Z)-3-Hexenal | 6789-80-6 | Grass | 15.60 | 1161/1158 | 8.11 (0–99.75) |
| A4 | (E)-2-Hexenal | 6728-26-3 | Grass, herbaceous | 17.93 | 1240/1220 | 2007.71 (569.95–4435.22) |
| A5 | Octanal | 124-13-0 | Hone, green, fatty | 19.85 | 1298/1298 | 2.13 (0–29.76) |
| A6 | (Z)-2-Heptenal | 57266-86-1 | Grass | 21.03 | 1339/1339 | 8.53 (0–53.46) |
| A7 | Nonanal | 124-19-6 | Orange, grease | 22.79 | 1401/1400 | 11.45 (0–96.12) |
| A8 | (E)-2-Octenal | 2548-87-0 | Honey, green, fatty | 23.89 | 1443/1441 | 2.61 (0–31.11) |
| A9 | (E,E)-2,4-Heptadienal | 4313-03-5 | Cucumber | 24.88 | 1497/1497 | 0.46 (0–9.32) |
| A10 | (Z)-2-Nonenal | 60784-31-8 | Wet, fat, metallic | 26.63 | 1531/1529 | 0.87 (0–23.49) |
| A11 | Benzaldehyde | 100-52-7 | Sweet, fruity | 26.66 | 1532/1532 | 5.41 (0–108.11) |
| A12 | (E)-2-Decenal | 3913-81-3 | Sour, acidic | 29.08 | 1655/1655 | 1.83 (0–38.62) |
| Alcohols | ||||||
| B1 | 1-Propanol | 71-23-8 | Alcoholic | 12.39 | 1048/1045 | 0.90 (0–35.99) |
| B2 | 1-Butanol | 71-36-3 | Sweet | 15.35 | 1156/1158 | 25.14 (0–542.07) |
| B3 | 2-Methyl-1-butanol | 137-32-6 | Acidic, sharp, spicy | 17.20 | 1210/1210 | 34.56 (0–149.4) |
| B4 | 2-Hexyn-1-ol | 764-60-3 | Green apple | 17.41 | 1225/1223 | 22.98 (0–66.21) |
| B5 | 1-Hexanol | 111-27-3 | Unpleasant, green | 21.40 | 1361/1361 | 82.66 (0–393.09) |
| Ketones | ||||||
| C1 | 1-Penten-3-one | 1629-58-9 | Mushroom | 12.07 | 1022/1020 | 1.45 (0–15.07) |
| C2 | 1-Octen-3-one | 4312-99-6 | Mushroom | 20.22 | 1305/1305 | 3.74 (0–44.64) |
| C3 | 6-Methyl-5-hepten-2-one | 110-93-0 | Earthy, strawberry | 21.26 | 1355/1348 | 3.38 (0–33.26) |
| Acids | ||||||
| D1 | 2-Methylbutanoic acid | 116-53-0 | Fatty | 29.37 | 1670/1670 | 38.34 (0–201.97) |
| Others | ||||||
| O1 | (E)-2-Pentenal | 1576-87-0 | Green | 15.23 | 1142/1140 | 0.09 (0–3.78) |
| O2 | Dodecane | 112-40-3 | Oily | 16.79 | 1187/1187 | 6.96 (0–127.3) |
| O3 | Tetradecane | 629-59-4 | Oily | 22.49 | 1398/1398 | 30.38 (0–129.62) |
| O4 | Copaene | 3856-25-5 | Woody, terpeny | 25.56 | 1503/1505 | 2.88 (0–20.71) |
| O5 | Hexadecane | 544-76-3 | Oily | 27.59 | 1581/1581 | 12.00 (0–88.67) |
| O6 | Estragole | 140-67-0 | Anise | 29.66 | 1687/1687 | 293.26 (0–2012.56) |
| O7 | α-Bergamotene | 17699-05-7 | Green | 30.37 | 1694/1695 | 343.04 (0–1291.69) |
| O8 | α-Farnesene | 502-61-4 | Green, oily, fatty | 30.75 | 1725/1754 | 850.09 (7.76–2919.09) |
| O9 | Thujopsene | 470-40-6 | Resinous | 31.48 | 1747/1760 | 19.75 (0–97.63) |
| O10 | Anethole | 25679-28-1 | Anise | 32.40 | 1780/1780 | 47.93 (0–492.16) |
| No. | Cultivars | Number of Volatile Compounds | Total Content (μg/Kg FW) |
|---|---|---|---|
| 1 | Royal Gala | 39 | 2919.26 ± 351.23 |
| 2 | Golden Delicious | 26 | 4436.74 ± 425.36 |
| 3 | Fuji | 42 | 3562.94 ± 310.02 |
| 4 | Jonagold | 38 | 23,047.24 ± 2826.62 |
| 5 | Indo | 36 | 5508.35 ± 401.23 |
| 6 | Orin | 30 | 16,863.94 ± 1806.24 |
| 7 | Hanfu | 38 | 10,988.51 ± 562.36 |
| 8 | Jonathan | 34 | 7436.91 ± 236.02 |
| 9 | Miyakiji | 38 | 12,817.30 ± 589.45 |
| 10 | Granny Smith | 27 | 3930.31 ± 328.94 |
| 11 | Ralls | 45 | 16,150.55 ± 2451.02 |
| 12 | Starkrimson | 29 | 3784.77 ± 327.05 |
| 13 | Huaguan | 34 | 12,184.76 ± 1087.69 |
| 14 | Huashuo | 21 | 2041.27 ± 120.36 |
| 15 | Huayu | 44 | 23,827.87 ± 3012.85 |
| 16 | Envy | 40 | 13,286.84 ± 1139.54 |
| 17 | Red General | 39 | 15,447.86 ± 1120.35 |
| 18 | Starking | 29 | 5411.64 ± 462.38 |
| 19 | Jiguan | 34 | 21,704.66 ± 1865.32 |
| 20 | Cox Orange | 24 | 2622.09 ± 150.74 |
| 21 | Jazz | 40 | 27,493.25 ± 3800.46 |
| 22 | Cameo | 36 | 20,118.58 ± 2010.38 |
| 23 | Honey Crips | 40 | 27,813.56 ± 2310.07 |
| 24 | Mollie’s Delicious | 29 | 5223.71 ± 362.38 |
| 25 | Modi | 44 | 12,564.23 ± 1835.44 |
| 26 | Qinguan | 32 | 26,132.20 ± 3450.20 |
| 27 | Qinyang | 33 | 5483.01 ± 280.74 |
| 28 | Qinyue | 20 | 4007.59 ± 263.58 |
| 29 | Qinyun | 27 | 10,963.94 ± 1021.56 |
| 30 | World No.1 | 34 | 10765.78 ± 1806.75 |
| 31 | Weijieke | 25 | 5878.99 ± 350.28 |
| 32 | Alps Otome | 37 | 20,460.02 ± 1805.98 |
| 33 | Red Delicious | 37 | 14,524.14 ± 1205.32 |
| 34 | Yuhuazaofu | 40 | 8650.80 ± 680.21 |
| 35 | Pink Lady | 35 | 11,086.30 ± 1008.37 |
| 36 | Changfu No.2 | 47 | 19,849.15 ± 2080.95 |
| 37 | Punama | 35 | 8480.92 ± 783.54 |
| 38 | Ruixue | 38 | 9274.25 ± 865.04 |
| 39 | Ruiyang | 25 | 4173.26 ± 280.86 |
| 40 | Ruixianghong | 43 | 27,015.38 ± 2540.92 |
| Cultivars | Esters | Aldehydes | Alcohols | Others |
|---|---|---|---|---|
| RG | 1691.11 ± 210.25 | 741.34 ± 80.43 | 94.28 ± 10.42 | 392.54 ± 32.51 |
| GD | 3240.04 ± 295.30 | 973.72 ± 85.62 | 44.02 ± 8.73 | 178.96 ± 20.98 |
| FJ | 2609.79 ± 252.76 | 662.21 ± 80.12 | 51.94 ± 4.38 | 239.01 ± 12.85 |
| JNG | 15,799.57 ± 1808.32 | 1900.45 ± 370.50 | 215.98 ± 20.84 | 5131.24 ± 486.22 |
| ID | 2542.49 ± 280.95 | 2467.84 ± 140.58 | 27.39 ± 3.85 | 470.63 ± 20.45 |
| OI | 12,073.00 ± 1500.65 | 3801.74 ± 364.02 | 94.56 ± 8.51 | 894.64 ± 107.84 |
| HF | 6242.30 ± 500.60 | 2487.06 ± 320.78 | 581.55 ± 42.89 | 1677.60 ± 137.21 |
| JNT | 3911.85 ± 410.20 | 2740.97 ± 200.36 | 215.97 ± 18.59 | 568.13 ± 46.25 |
| MYK | 8105.44 ± 742.39 | 1928.84 ± 200.45 | 143.13 ± 11.23 | 2639.89 ± 240.81 |
| GS | 504.68 ± 38.94 | 2650.55 ± 270.32 | 34.84 ± 5.20 | 740.25 ± 20.56 |
| RL | 11,716.86 ± 1520.36 | 2455.92 ± 325.60 | 150.24 ± 110.55 | 1827.52 ± 176.95 |
| SR | 1721.54 ± 160.98 | 1687.56 ± 200.85 | 18.63 ± 2.63 | 357.04 ± 40.28 |
| HG | 7797.49 ± 850.36 | 2961.00 ± 326.98 | 730.52 ± 50.46 | 695.76 ± 42.38 |
| HS | 474.23 ± 35.21 | 1475.38 ± 160.85 | 58.82 ± 5.96 | 32.84 ± 3.85 |
| HY | 14,491.84 ± 1628.32 | 2885.68 ± 203.56 | 941.15 ± 80.34 | 5509.20 ± 425.07 |
| EV | 10,691.41 ± 980.64 | 1317.54 ± 150.23 | 97.20 ± 10.55 | 1180.68 ± 140.36 |
| RGL | 10,167.00 ± 1230.52 | 2290.61 ± 180.56 | 184.35 ± 20.30 | 2805.91 ± 290.62 |
| SI | 1816.22 ± 178.21 | 2643.11 ± 250.36 | 26.48 ± 5.21 | 925.83 ± 86.33 |
| JG | 13,852.95 ± 1420.65 | 4905.73 ± 520.41 | 127.45±10.85 | 2818.53 ± 260.21 |
| COP | 608.05 ± 52.84 | 1815.17 ± 166.50 | 69.27 ± 6.21 | 129.60 ± 12.95 |
| JZ | 19,352.58 ± 1523.65 | 2509.39 ± 280.21 | 234.34 ± 19.85 | 5396.93 ± 500.42 |
| CM | 13,120.49 ± 1468.20 | 2642.90 ± 286.35 | 96.90 ± 8.55 | 4258.30 ± 480.74 |
| HC | 21,457.95 ± 2230.10 | 2609.31 ± 230.51 | 197.76 ± 15.42 | 3548.53 ± 384.19 |
| MD | 3270.42 ± 295.65 | 1359.24 ± 145.20 | 75.75 ± 8.52 | 518.30 ± 48.25 |
| MI | 8728.64 ± 865.32 | 2212.48 ± 284.50 | 103.19 ± 12.85 | 1519.92 ± 175.88 |
| QG | 19,396.06 ± 2010.57 | 4400.47 ± 385.12 | 121.38 ± 10.85 | 2214.29 ± 260.37 |
| QYG | 3036.79 ± 294.58 | 1842.62 ± 172.54 | 93.73 ± 10.25 | 509.86 ± 41.85 |
| QYE | 2684.64 ± 284.65 | 721.95 ± 85.24 | 214.52 ± 17.45 | 386.48 ± 33.06 |
| QYN | 7400.38 ± 851.54 | 1552.16 ± 160.22 | 177.79 ± 14.28 | 1833.61 ± 200.87 |
| W1 | 6042.87 ± 576.25 | 2251.65 ± 280.35 | 48.54 ± 8.46 | 2422.72 ± 284.91 |
| WJK | 2465.30 ± 294.73 | 2525.87 ± 300.14 | 121.84 ± 10.85 | 765.97 ± 80.72 |
| AO | 14,748.22 ± 1624.35 | 2432.13 ± 281.45 | 397.55 ± 40.85 | 2882.11 ± 300.95 |
| RD | 8884.26 ± 960.35 | 3429.55 ± 302.85 | 65.59 ± 5.20 | 2144.75 ± 235.48 |
| YH | 5616.93 ± 596.21 | 2105.69 ± 248.52 | 122.03 ± 10.45 | 806.15 ± 67.58 |
| PL | 8144.07 ± 756.81 | 1684.40 ± 201.35 | 53.73 ± 5.21 | 1204.09 ± 82.13 |
| CF2 | 15,358.65 ± 1742.23 | 2775.16 ± 208.95 | 175.76 ± 15.55 | 1539.57 ± 123.52 |
| PNM | 4374.62 ± 502.75 | 2657.88 ± 210.38 | 132.03 ± 15.20 | 1316.39 ± 150.70 |
| RX | 5215.59 ± 514.85 | 2815.66 ± 268.45 | 195.15 ± 20.96 | 1047.84 ± 82.09 |
| RY | 2454.10 ± 261.28 | 1434.19 ± 158.52 | 6.27 ± 1.02 | 278.70 ± 31.25 |
| RXH | 21,403.00 ± 2350.36 | 3182.62 ± 352.14 | 107.80 ± 80.56 | 2321.97 ± 213.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Hao, N.; Meng, Z.; Li, Y.; Zhao, Z. Identification, Comparison and Classification of Volatile Compounds in Peels of 40 Apple Cultivars by HS–SPME with GC–MS. Foods 2021, 10, 1051. https://doi.org/10.3390/foods10051051
Yang S, Hao N, Meng Z, Li Y, Zhao Z. Identification, Comparison and Classification of Volatile Compounds in Peels of 40 Apple Cultivars by HS–SPME with GC–MS. Foods. 2021; 10(5):1051. https://doi.org/10.3390/foods10051051
Chicago/Turabian StyleYang, Shunbo, Nini Hao, Zhipeng Meng, Yingjuan Li, and Zhengyang Zhao. 2021. "Identification, Comparison and Classification of Volatile Compounds in Peels of 40 Apple Cultivars by HS–SPME with GC–MS" Foods 10, no. 5: 1051. https://doi.org/10.3390/foods10051051
APA StyleYang, S., Hao, N., Meng, Z., Li, Y., & Zhao, Z. (2021). Identification, Comparison and Classification of Volatile Compounds in Peels of 40 Apple Cultivars by HS–SPME with GC–MS. Foods, 10(5), 1051. https://doi.org/10.3390/foods10051051