Process Simulation and Techno-Economic Analysis of Large-Scale Bioproduction of Sweet Protein Thaumatin II
Abstract
:1. Introduction
2. Materials and Methods
2.1. Process Simulation and Economics
2.2. Base Case Process Assumptions
2.3. Indoor Vertical Farming
2.4. Transient Production in Spinach
3. Results
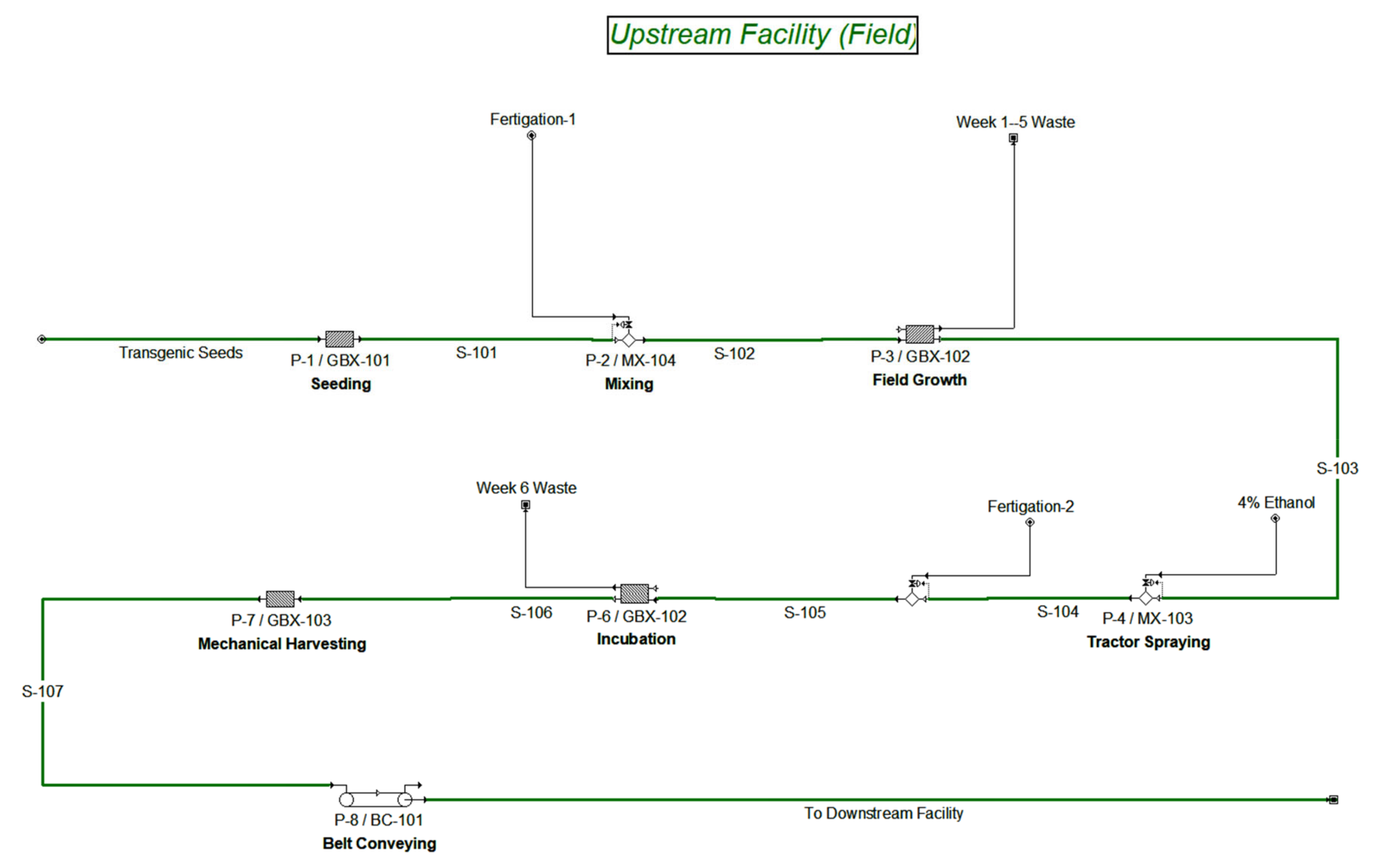
3.1. Field Upstream Transgenic Production Facility
3.2. Indoor Upstream Transgenic Production Facility
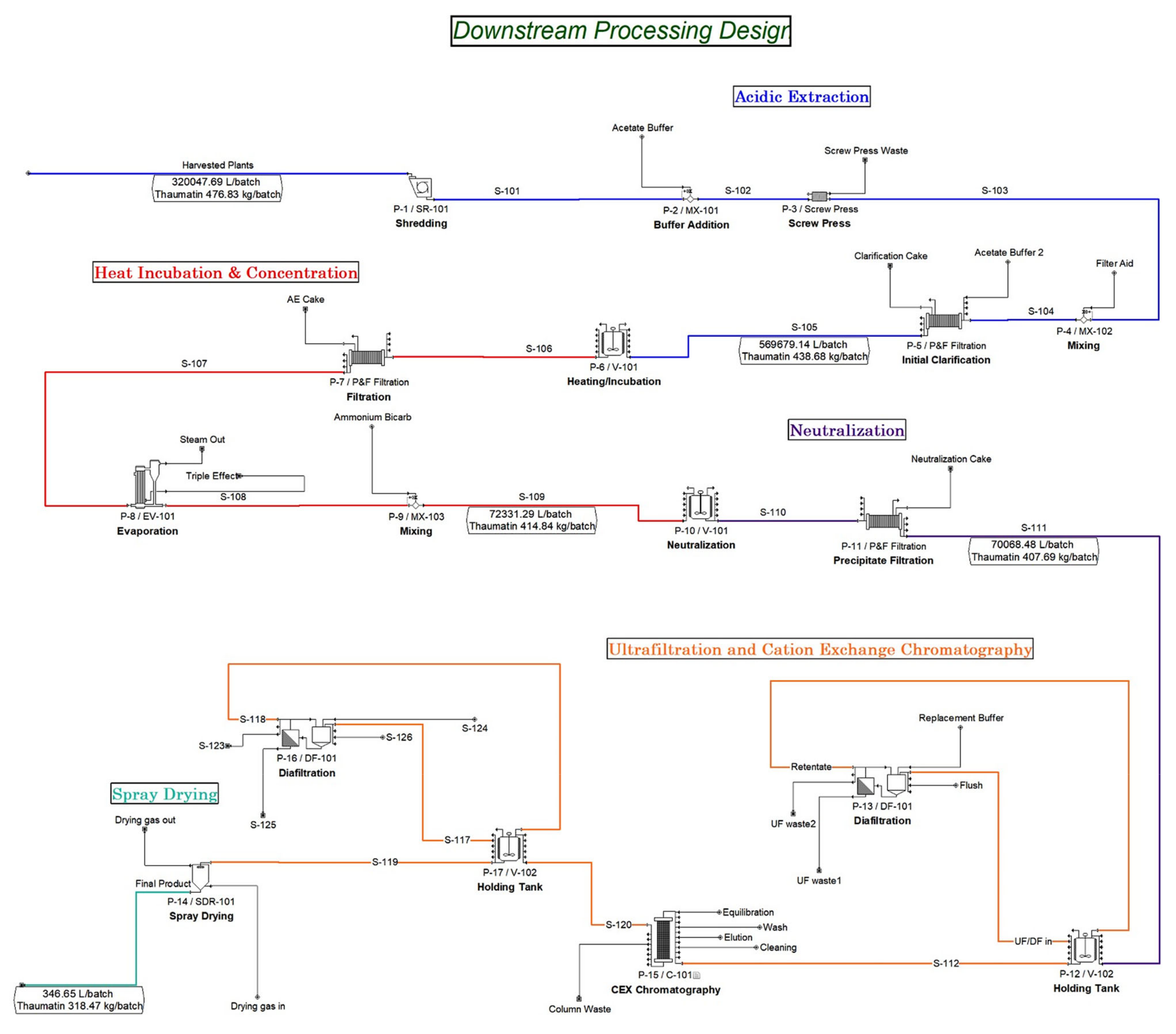
3.3. Transgenic Production Downstream Processing Facility
3.4. Spinach Transient Production Facility
3.5. Economic Analysis of Transgenic Production Facilities
3.6. Expression Level and Production Capacity Scenario Analysis
3.7. Economic Analysis of Transient Production Facility
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lichtenstein, A.H.; Karpyn, A. History and Development of the 2015–2020 Dietary Guidelines for Americans. In Food and Public Health; Oxford University Press: New York, NY, USA, 2018; ISBN 9780190626686. [Google Scholar]
- Joseph, J.A.; Akkermans, S.; Nimmegeers, P.; van Impe, J.F.M. Bioproduction of the recombinant sweet protein thaumatin: Current state of the art and perspectives. Front. Microbiol. 2019, 10, 1–19. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Nutrition for Health and Development Guideline: Sugars Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Brisbois, T.D.; Marsden, S.L.; Anderson, G.H.; Sievenpiper, J.L. Estimated Intakes and Sources of Total and Added Sugars in the Canadian Diet. Nutrients 2014, 6, 1899–1912. [Google Scholar] [CrossRef] [Green Version]
- Bleich, S.N.; Wolfson, J.A. U.S. adults and child snacking patterns among sugar-sweetened beverage drinkers and non-drinkers. Prev. Med. 2015, 72, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- HHS and USDA. Dietary Guidelines for Americans 2015–2020, 8th ed.; HHS and USDA: Washington, DC, USA, 2015. [Google Scholar]
- Brownell, K.D.; Farley, T.; Willett, W.C.; Popkin, B.M.; Chaloupka, F.J.; Thompson, J.W.; Ludwig, D.S. The public health and economic benefits of taxing sugar-sweetened beverages. N. Engl. J. Med. 2009, 361, 1599–1605. [Google Scholar] [CrossRef]
- Falbe, J.; Thompson, H.R.; Becker, C.M.; Rojas, N.; McCulloch, C.E.; Madsen, K.A. Impact of the Berkeley Excise Tax on Sugar-Sweetened Beverage Consumption. Am. J. Public Health 2016, 106, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Changes to the Nutrition Facts Label. Available online: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/ucm385663.htm (accessed on 2 April 2020).
- Agboola, D.A.; Fawibe, O.O.; Ogunyale, O.G.; Ajiboye, A.A. Botanical and Protein Sweeteners. J. Adv. Lab. Res. Biol. 2014, 5, 169–187. [Google Scholar]
- Pearlman, M.; Obert, J.; Casey, L. The Association between Artificial Sweeteners and Obesity. Curr. Gastroenterol. Rep. 2017, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, C.R.; Boullata, J.; McCauley, L.A. The Potential Toxicity of Artificial Sweeteners. AAOHN J. 2008, 56, 251–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokotou, M.G.; Asimakopoulos, A.G.; Thomaidis, N.S. Artificial sweeteners as emerging pollutants in the environment: Analytical methodologies and environmental impact. Anal. Methods 2012, 4, 3057–3070. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Rother, K.I. Trends in the consumption of low-calorie sweeteners. Physiol. Behav. 2016, 164, 446–450. [Google Scholar] [CrossRef] [Green Version]
- Anton, S.D.; Martin, C.K.; Han, H.; Coulon, S.; Cefalu, W.T.; Geiselman, P.; Williamson, D.A. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite 2010, 55, 37–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciriminna, R.; Meneguzzo, F.; Pecoraino, M.; Pagliaro, M. A bioeconomy perspective for natural sweetener Stevia. Biofuels Bioprod. Biorefining 2019, 13, 445–452. [Google Scholar] [CrossRef] [Green Version]
- von Rymon Lipinski, G.-W. Sweeteners. In Ullmann’s Encyclopedia of Industrial Chemistry; American Cancer Society: Atlanta, GA, USA, 2015; pp. 1–25. ISBN 9783527306732. [Google Scholar]
- Tao, R.; Cho, S. Consumer-Based Sensory Characterization of Steviol Glycosides (Rebaudioside A, D, and M). Foods 2020, 9, 1026. [Google Scholar] [CrossRef]
- Nelson, G.; Chandrashekar, J.; Hoon, M.A.; Feng, L.; Zhao, G.; Ryba, N.J.P.; Zuker, C.S. An amino-acid taste receptor. Nature 2002, 416, 199–202. [Google Scholar] [CrossRef]
- Vigues, S.; Dotson, C.D.; Munger, S.D. The Receptor Basis of Sweet Taste in Mammals. In Chemosensory Systems in Mammals, Fishes, and Insects; Korsching, S., Meyerhof, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 20–23. ISBN 978-3-540-69919-4. [Google Scholar]
- Fernstrom, J.D.; Munger, S.D.; Sclafani, A.; de Araujo, I.E.; Roberts, A.; Molinary, S. Mechanisms for Sweetness. J. Nutr. 2012, 142, 1134S–1141S. [Google Scholar] [CrossRef]
- Masuda, T. Sweet-Tasting Protein Thaumatin: Physical and Chemical Properties. In Sweeteners: Pharmacology, Biotechnology, and Applications; Merillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–31. ISBN 978-3-319-26478-3. [Google Scholar]
- Glenday, C. Guinness World Records 2008; Bantam Books: New York, NY, USA, 2008; ISBN 0553589954. [Google Scholar]
- Healey, R.D.; Lebhar, H.; Hornung, S.; Thordarson, P.; Marquis, C.P. An improved process for the production of highly purified recombinant thaumatin tagged-variants. Food Chem. 2017, 237, 825–832. [Google Scholar] [CrossRef]
- Cohen, S.M.; Eisenbrand, G.; Fukushima, S.; Gooderham, N.J.; Guengerich, F.P.; Hecht, S.S.; Rietjens, I.M.C.M.; Harman, C.; Taylor, S.V. GRAS 28 Flavoring Substances. Food Technol. 2018, 72, 62–77. [Google Scholar]
- Lee, T.D. Sweeteners. In Kirk-Othmer Encyclopedia of Chemical Technology; American Cancer Society: Atlanta, GA, USA, 2000; ISBN 9780471238966. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives; World Health Organization; Food and Agriculture Organization of the United Nations. Evaluation of Certain Food Additives and Contaminants: Twenty-Second Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization Technical Report Series; World Health Organization: Geneva, Switzerland, 1978; p. 39. [Google Scholar]
- iCrowdNewswire Thaumatin Industry 2019-Market Analysis, Size, Growth, Share, Global Statistics and Forecast Analysis 2025. Available online: https://advance.lexis.com/api/document?collection=news&id=urn:contentItem:5VDT-43X1-F08D-528N-00000-00&context=1516831 (accessed on 5 July 2019).
- Puetz, J.; Wurm, F.M. Recombinant Proteins for Industrial versus Pharmaceutical Purposes: A Review of Process and Pricing. Processes 2019, 7, 476. [Google Scholar] [CrossRef] [Green Version]
- Masuda, T.; Tamaki, S.; Kaneko, R.; Wada, R.; Fujita, Y.; Mehta, A.; Kitabatake, N. Cloning, expression and characterization of recombinant sweet-protein thaumatin II using the methylotrophic yeast Pichia pastoris. Biotechnol. Bioeng. 2004, 85, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Illingworth, C.; Larson, G.; Hellekant, G. Secretion of the sweet-tasting plant protein thaumatin byStreptomyces lividans. J. Ind. Microbiol. 1989, 4, 37–42. [Google Scholar] [CrossRef]
- Faus, I.; del Moral, C.; Adroer, N.; del Río, J.L.; Patiño, C.; Sisniega, H.; Casas, C.; Bladé, J.; Rubio, V. Secretion of the sweet-tasting protein thaumatin by recombinant strains of Aspergillus niger var. awamori. Appl. Microbiol. Biotechnol. 1998, 49, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Bartoszewski, G.; Niedziela, A.; Szwacka, M.; Niemirowicz-Szczytt, K. Modification of tomato taste in transgenic plants carrying a thaumatin gene from Thaumatococcus daniellii Benth. Plant Breed. 2003, 122, 347–351. [Google Scholar] [CrossRef]
- Mir-Artigues, P.; Twyman, R.M.; Alvarez, D.; Cerda, P.; Balcells, M.; Christou, P.; Capell, T. A simplified techno-economic model for the molecular pharming of antibodies. Biotechnol. Bioeng. 2019, 116, 2526–2539. [Google Scholar] [CrossRef]
- Fischer, R.; Buyel, J.F. Molecular farming—The slope of enlightenment. Biotechnol. Adv. 2020, 40, 107519. [Google Scholar] [CrossRef] [PubMed]
- Petrides, D.; Cooney, C.L.; Evans, L.B.; Field, R.P.; Snoswell, M. Bioprocess simulation: An integrated approach to process development. Comput. Chem. Eng. 1989, 13, 553–561. [Google Scholar] [CrossRef]
- Petrides, D.P.; Koulouris, A.; Lagonikos, P.T. The role of process simulation in pharmaceutical process development and product commercialization. Pharm. Eng. 2002, 22, 56–65. [Google Scholar]
- Nandi, S.; Kwong, A.T.; Holtz, B.R.; Erwin, R.L.; Marcel, S.; McDonald, K.A. Techno-economic analysis of a transient plant-based platform for monoclonal antibody production. mAbs 2016, 8, 1456–1466. [Google Scholar] [CrossRef]
- Alam, A.; Jiang, L.; Kittleson, G.A.; Steadman, K.D.; Nandi, S.; Fuqua, J.L.; Palmer, K.E.; Tusé, D.; McDonald, K.A. Technoeconomic Modeling of Plant-Based Griffithsin Manufacturing. Front. Bioeng. Biotechnol. 2018, 6, 102. [Google Scholar] [CrossRef] [Green Version]
- Tusé, D.; Tu, T.; McDonald, K.A. Manufacturing economics of plant-made biologics: Case studies in therapeutic and industrial enzymes. BioMed Res. Int. 2014, 2014, 256135. [Google Scholar] [CrossRef] [Green Version]
- Nandi, S.; Yalda, D.; Lu, S.; Nikolov, Z.; Misaki, R.; Fujiyama, K.; Huang, N. Process development and economic evaluation of recombinant human lactoferrin expressed in rice grain. Transgenic Res. 2005, 14, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Corbin, J.M.; McNulty, M.J.; Macharoen, K.; McDonald, K.A.; Nandi, S. Technoeconomic analysis of semicontinuous bioreactor production of biopharmaceuticals in transgenic rice cell suspension cultures. Biotechnol. Bioeng. 2020, 117, 3053–3065. [Google Scholar] [CrossRef]
- Walwyn, D.R.; Huddy, S.M.; Rybicki, E.P. Techno-Economic Analysis of Horseradish Peroxidase Production Using a Transient Expression System in Nicotiana benthamiana. Appl. Biochem. Biotechnol. 2015, 175, 841–854. [Google Scholar] [CrossRef] [PubMed]
- McNulty, M.J.; Gleba, Y.; Tusé, D.; Hahn-Löbmann, S.; Giritch, A.; Nandi, S.; McDonald, K.A. Techno-economic analysis of a plant-based platform for manufacturing antimicrobial proteins for food safety. Biotechnol. Prog. 2019, 36, e2896. [Google Scholar] [CrossRef] [PubMed]
- Marillonnet, S.; Thoeringer, C.; Kandzia, R.; Klimyuk, V.; Gleba, Y. Systemic Agrobacterium tumefaciens—Mediated transfection of viral replicons for efficient transient expression in plants. Nat. Biotechnol. 2005, 23, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Breus, O.; Symonenko, Y.; Marillonnet, S.; Gleba, Y. High-level recombinant protein expression in transgenic plants by using a double-inducible viral vector. Proc. Natl. Acad. Sci. USA 2011, 108, 14061–14066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparrow, P.A.C.; Irwin, J.A.; Dale, P.J.; Twyman, R.M.; Ma, J.K.C. Pharma-Planta: Road testing the developing regulatory guidelines for plant-made pharmaceuticals. Transgenic Res. 2007, 16, 147–161. [Google Scholar] [CrossRef]
- Buyel, J.F. Plant Molecular Farming—Integration and Exploitation of Side Streams to Achieve Sustainable Biomanufacturing. Front. Plant Sci. 2019, 9, 1893. [Google Scholar] [CrossRef]
- Lico, C.; Chen, Q.; Santi, L. Viral vectors for production of recombinant proteins in plants. J. Cell. Physiol. 2008, 216, 366–377. [Google Scholar] [CrossRef]
- Chen, Q.; Dent, M.; Hurtado, J.; Stahnke, J.; McNulty, A.; Leuzinger, K.; Lai, H. Transient Protein Expression by Agroinfiltration in Lettuce. In Recombinant Proteins from Plants: Methods and Protocols; MacDonald, J., Kolotilin, I., Menassa, R., Eds.; Springer: New York, NY, USA, 2016; pp. 55–67. ISBN 978-1-4939-3289-4. [Google Scholar]
- Fischer, R.; Vaquero-Martin, C.; Sack, M.; Drossard, J.; Emans, N.; Commandeur, U. Towards molecular farming in the future: Transient protein expression in plants. Biotechnol. Appl. Biochem. 1999, 30, 113–116. [Google Scholar] [CrossRef]
- Pogue, G.P.; Lindbo, J.A.; Garger, S.J.; Fitzmaurice, W.P. Making an Ally from an Enemy: Plant Virology and the New Agriculture. Annu. Rev. Phytopathol. 2002, 40, 45–74. [Google Scholar] [CrossRef]
- Hahn, S.; Giritch, A.; Bartels, D.; Bortesi, L.; Gleba, Y. A novel and fully scalable Agrobacterium spray-based process for manufacturing cellulases and other cost-sensitive proteins in plants. Plant Biotechnol. J. 2015, 13, 708–716. [Google Scholar] [CrossRef]
- Torti, S.; Schlesier, R.; Thümmler, A.; Bartels, D.; Römer, P.; Koch, B.; Werner, S.; Panwar, V.; Kanyuka, K.; von Wirén, N.; et al. Transient reprogramming of crop plants for agronomic performance. Nat. Plants 2021, 7, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Schachtsiek, J.; Stehle, F. Nicotine-free, nontransgenic tobacco (Nicotiana tabacum L.) edited by CRISPR-Cas9. Plant Biotechnol. J. 2019, 17, 2228–2230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buyel, J.F.; Twyman, R.M.; Fischer, R. Extraction and downstream processing of plant-derived recombinant proteins. Biotechnol. Adv. 2015, 33, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Buyel, J.F.; Gruchow, H.M.; Boes, A.; Fischer, R. Rational design of a host cell protein heat precipitation step simplifies the subsequent purification of recombinant proteins from tobacco. Biochem. Eng. J. 2014, 88, 162–170. [Google Scholar] [CrossRef]
- Gergerich, R.C.; Dolja, V.V. Introduction to plant viruses, the invisible foe. Plant Health Instr. Available online: https://www.apsnet.org/edcenter/disandpath/viral/introduction/Pages/PlantViruses.aspx (accessed on 29 May 2020).
- Koike, S.T.; Cahn, M.; Cantwell, M.; Fennimore, S.; Lestrange, M.; Natwick, E.; Smith, R.F.; Takele, E. Spinach Production in California; University of California Agriculture and Natural Resources: Oakland, CA, USA, 2011. [Google Scholar] [CrossRef] [Green Version]
- Ben, P.; Ron, G. Using Linear Bed Feet for Calculating Fertigation and Banded Fertilizer Rates. Available online: https://www.canr.msu.edu/news/using_linear_bed_feet_for_calculating_fertigation_and_banded_fertilizer_rat (accessed on 17 April 2020).
- Smith, R.F.; Tumber, K.P. Sample Costs to Produce and Harvest Organic Spinach; University of California Agriculture and Natural Resources: Oakland, CA, USA, 2015. [Google Scholar]
- Ramsay Highlander, I. Spinach/Spring Mix Mechanical Harvesters. Available online: http://www.ramsayhighlander.com/products/spinach-spring/spinach.htm (accessed on 18 April 2020).
- Barbera, E.; Zorzetto, L.; Sharif, A.O.; Bertucco, A. Simultaneous Clean Water and Power Production from Seawater Using Osmosis: Process Simulation and Techno-economic Analysis. In Renewable Energy and Sustainable Buildings; Springer: Berlin/Heidelberg, Germany, 2020; pp. 121–137. [Google Scholar]
- Petrides, D. Bioprocess Design and Economics, 2nd ed.; Intelligen, Inc.: Scotch Plains, NJ, USA, 2015. [Google Scholar]
- Ahmad, S.Y.; Azad, M.B.; Friel, J.; MacKay, D. Recent evidence for the effects of nonnutritive sweeteners on glycaemic control. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Alexander Bentley, R.; Ruck, D.J.; Fouts, H.N. U.S. obesity as delayed effect of excess sugar. Econ. Hum. Biol. 2020, 36, 100818. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.-P.; Hu, F.B. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 2010, 121, 1356–1364. [Google Scholar] [CrossRef]
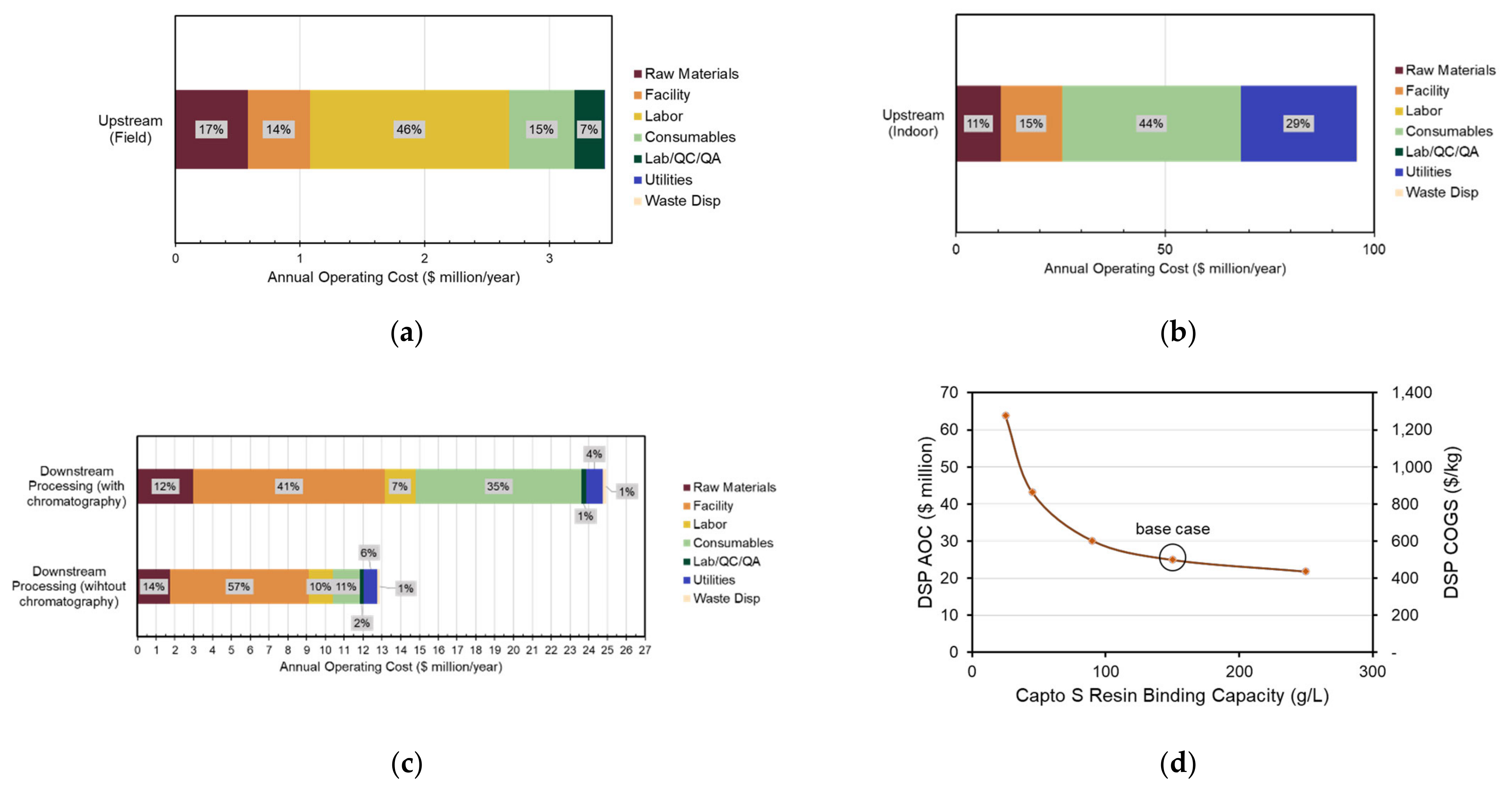
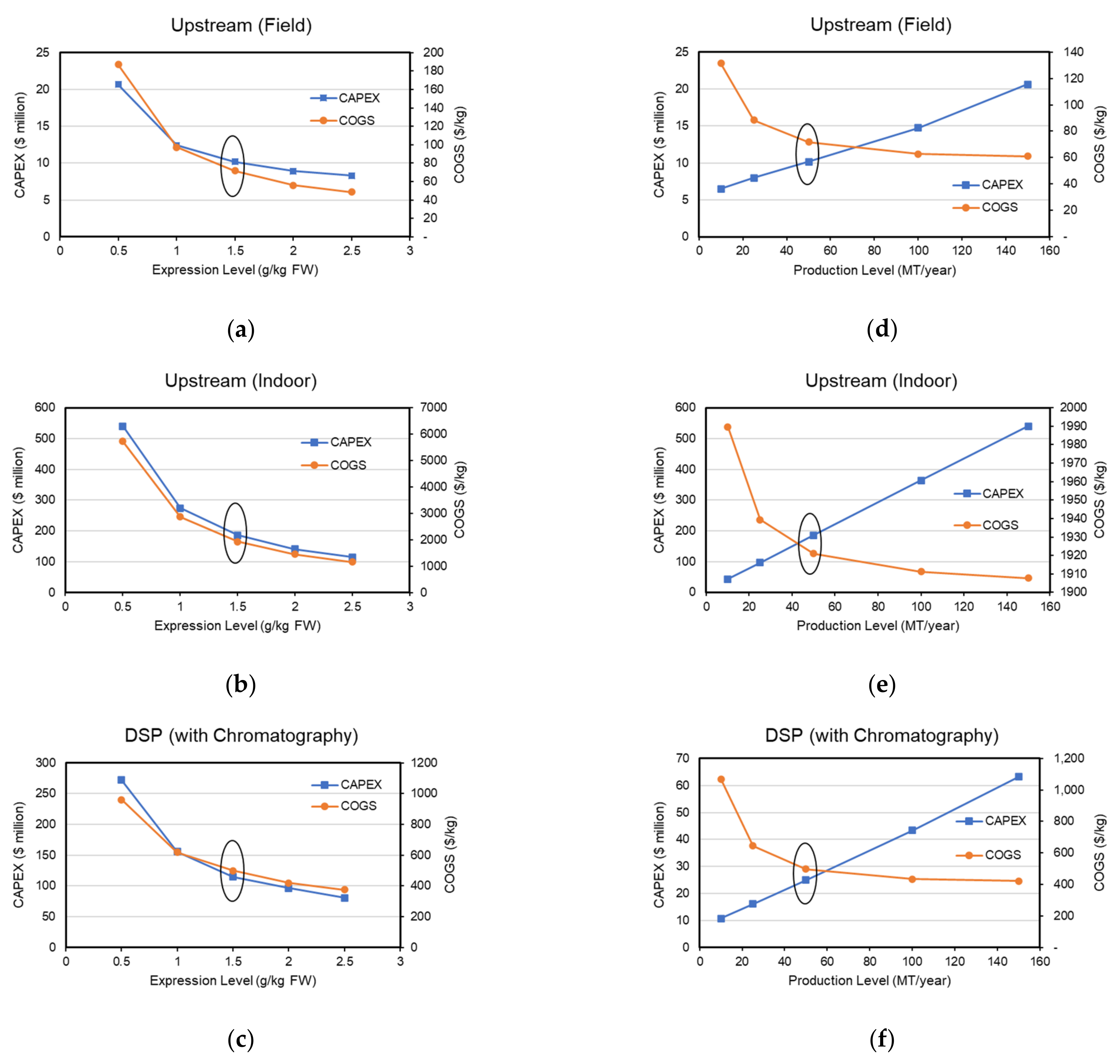
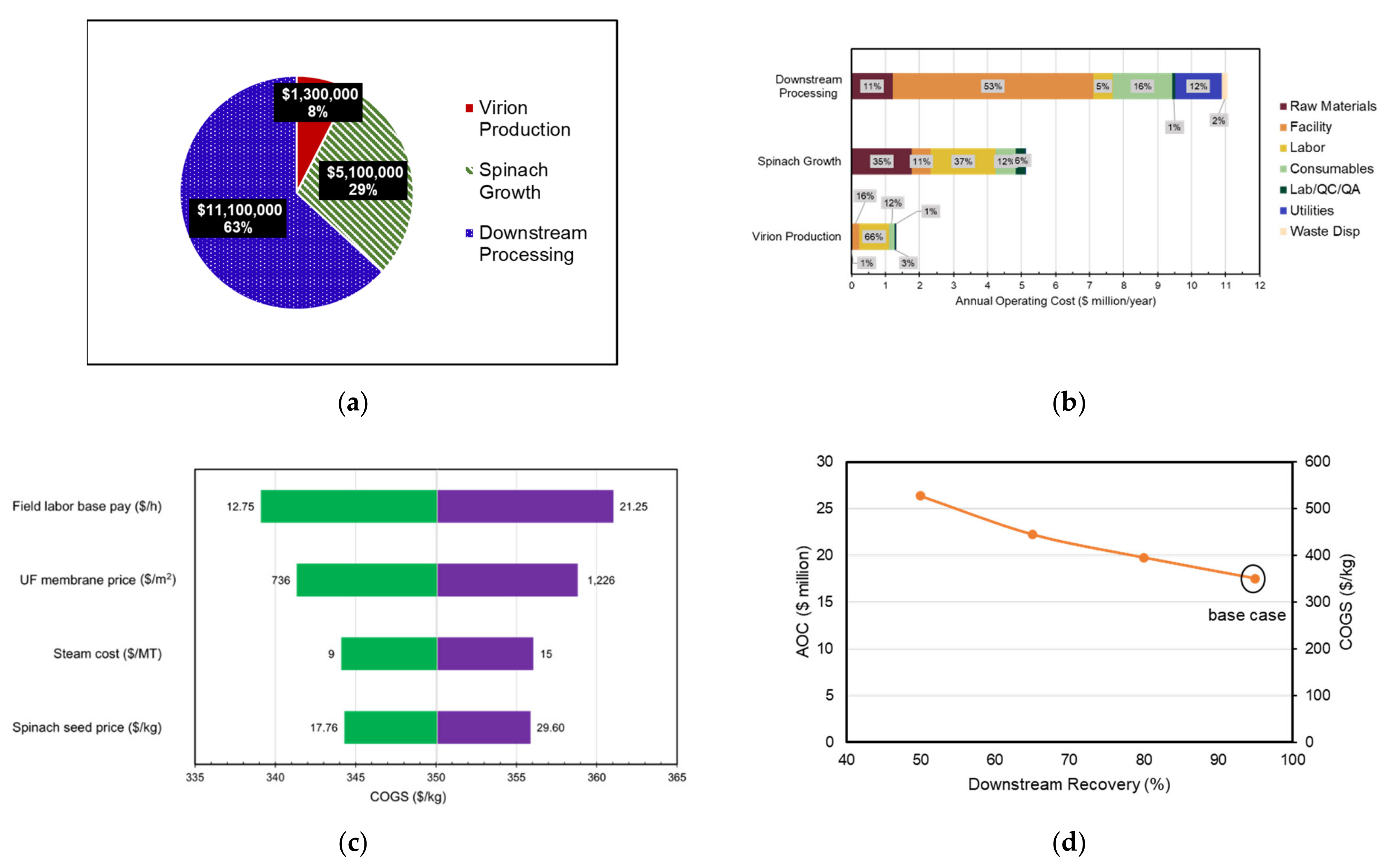
| Facility | Upstream (Field) | Upstream (Indoor) | Downstream (With Chromatography) |
|---|---|---|---|
| CAPEX ($ million) | 10.2 | 186 | 115 |
| AOC without depreciation ($ million) | 3.59 | 96.1 | 25.0 |
| COGS without depreciation ($/kg) | 71.7 | 1920 | 499 |
| AOC with depreciation ($ million) | 4.22 | 110 | 35.3 |
| COGS with depreciation ($/kg) | 84.5 | 2200 | 706 |
| Upstream (Field) | Upstream (Indoor) | Downstream (Without Chromatography) | |
| CAPEX ($ million) | 9.22 | 157 | 83.7 |
| AOC without depreciation ($ million) | 3.00 | 80.3 | 12.8 |
| COGS without depreciation ($/kg) | 60.0 | 1600 | 258 |
| AOC with depreciation ($ million) | 3.56 | 94.3 | 20.4 |
| COGS with depreciation ($/kg) | 71.2 | 1890 | 408 |
| Viral Particles Production | Spinach Growth | Downstream Processing | Working Capital, Start-Up, and Validation Costs | Total | |
|---|---|---|---|---|---|
| DFC(section)/CAPEX(total) | 2.53 | 9.74 | 64.4 | 4.49 | 81.2 |
| ($ million) | 1.32 | 5.12 | 11.1 | - | 17.5 |
| AOC without depreciation ($ million) | 26.4 | 103 | 221 | - | 350 |
| COGS without depreciation ($/kg) | 1.56 | 5.38 | 17.2 | - | 24.1 |
| AOC with depreciation | 31.2 | 108 | 344 | - | 482 |
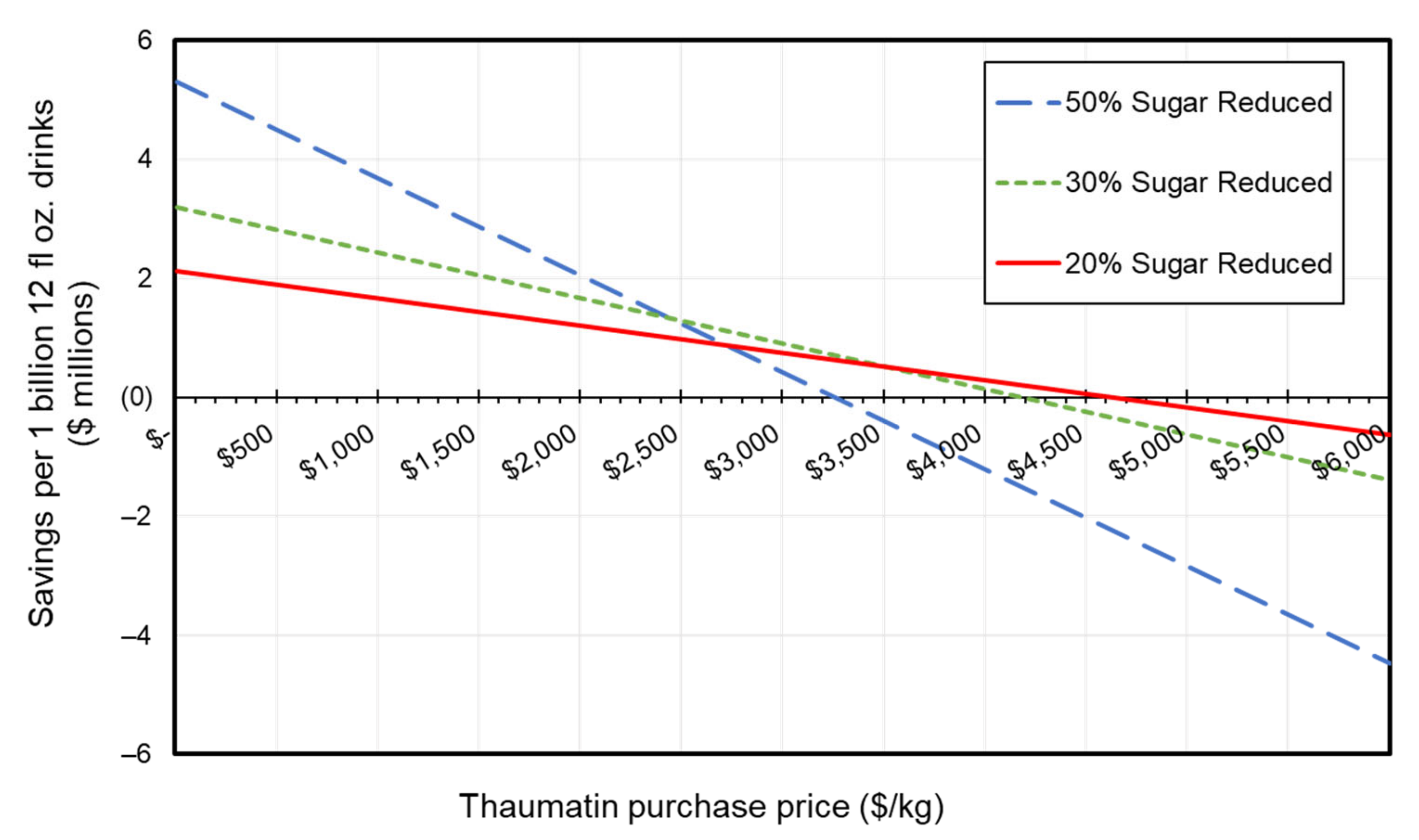
| Scenario | Thaumatin II Requirement (kg) Per 1 Billion 12 fl Oz Drinks (Per Day) | Annual Thaumatin Requirement (MT) |
|---|---|---|
| 20% sugar reduced | 460 | 170 |
| 30% sugar reduced | 770 | 280 |
| 50% sugar reduced | 1600 | 600 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelada, K.D.; Tusé, D.; Gleba, Y.; McDonald, K.A.; Nandi, S. Process Simulation and Techno-Economic Analysis of Large-Scale Bioproduction of Sweet Protein Thaumatin II. Foods 2021, 10, 838. https://doi.org/10.3390/foods10040838
Kelada KD, Tusé D, Gleba Y, McDonald KA, Nandi S. Process Simulation and Techno-Economic Analysis of Large-Scale Bioproduction of Sweet Protein Thaumatin II. Foods. 2021; 10(4):838. https://doi.org/10.3390/foods10040838
Chicago/Turabian StyleKelada, Kirolos D., Daniel Tusé, Yuri Gleba, Karen A. McDonald, and Somen Nandi. 2021. "Process Simulation and Techno-Economic Analysis of Large-Scale Bioproduction of Sweet Protein Thaumatin II" Foods 10, no. 4: 838. https://doi.org/10.3390/foods10040838
APA StyleKelada, K. D., Tusé, D., Gleba, Y., McDonald, K. A., & Nandi, S. (2021). Process Simulation and Techno-Economic Analysis of Large-Scale Bioproduction of Sweet Protein Thaumatin II. Foods, 10(4), 838. https://doi.org/10.3390/foods10040838








