Fatty Acid Composition and Volatile Profile of longissimus thoracis et lumborum Muscle from Burguete and Jaca Navarra Foals Fattened with Different Finishing Diets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Animal Management
2.2. Animal Slaughter and Sample Collection
2.3. Analysis of Fatty Acid Methyl Esters
2.4. Volatile Compound Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effect of Breed and Finishing Diet on Fatty Acids Profile
3.2. Effect of Breed and Finishing Diet on Volatile Compounds
3.2.1. Hydrocarbons: Lineal, Branched and Cyclic
3.2.2. Acids
3.2.3. Alcohols
3.2.4. Aldehydes
3.2.5. Ketones
3.2.6. Esters, Ethers, Furans, Nitrogen Compounds and Sulfur Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ursin, L. The ethics of the meat paradox. Environ. Ethics 2016, 38, 131–144. [Google Scholar] [CrossRef]
- Belaunzaran, X.; Bessa, R.J.B.; Lavín, P.; Mantecón, A.R.; Kramer, J.K.G.; Aldai, N. Horse-meat for human consumption—Current research and future opportunities. Meat Sci. 2015, 108, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Cittadini, A.; Sarriés, M.V.; Domínguez, R.; Indurain, G.; Lorenzo, J.M. Effect of breed and finishing diet on growth parameters and carcass quality characteristics of navarre autochthonous foals. Animals 2021, 11, 488. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Sarriés, M.V.; Tateo, A.; Polidori, P.; Franco, D.; Lanza, M. Carcass characteristics, meat quality and nutritional value of horsemeat: A review. Meat Sci. 2014, 96, 1478–1488. [Google Scholar] [CrossRef]
- Insausti, K.; Beldarrain, L.R.; Lavín, M.P.; Aldai, N.; Mantecón, Á.R.; Sáez, J.L.; Canals, R.M. Horse meat production in northern Spain: Ecosystem services and sustainability in High Nature Value farmland. Anim. Front. 2021, 11, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Crecente, S.; Borrajo, P.; Agregán, R.; Lorenzo, J.M. Effect of slaughter age on foal carcass traits and meat quality. Animal 2015, 9, 1713–1720. [Google Scholar] [CrossRef] [Green Version]
- Domínguez, R.; Pateiro, M.; Crecente, S.; Ruiz, M.; Sarriés, M.V.; Lorenzo, J.M. Effect of linseed supplementation and slaughter age on meat quality of grazing cross-bred Galician x Burguete foals. J. Sci. Food Agric. 2018, 98, 266–273. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Campagnol, P.C.B.; Zhu, Z.; Alpas, H.; Barba, F.J.; Tomasevic, I. Technological aspects of horse meat products—A review. Food Res. Int. 2017, 102, 176–183. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Fuciños, C.; Purriños, L.; Franco, D. Intramuscular fatty acid composition of “Galician Mountain” foals breed. Effect of sex, slaughtered age and livestock production system. Meat Sci. 2010, 86, 825–831. [Google Scholar] [CrossRef]
- Beldarrain, L.R.; Morán, L.; Sentandreu, M.A.; Insausti, K.; Barron, L.J.R.; Aldai, N. Muscle and Subcutaneous Fatty Acid Composition and the Hispano-Bretón Horse Breed. Animals 2021, 11, 1421. [Google Scholar] [CrossRef]
- Weylandt, K.H. Docosapentaenoic acid derived metabolites and mediators—The new world of lipid mediator medicine in a nutshell. Eur. J. Pharmacol. 2016, 785, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Lombardi-Boccia, G.; Lanzi, S.; Aguzzi, A. Aspects of meat quality: Trace elements and B vitamins in raw and cooked meats. J. Food Compos. Anal. 2005, 18, 39–46. [Google Scholar] [CrossRef]
- Tateo, A.; De Palo, P.; Ceci, E.; Centoducati, P. Physicochemical properties of meat of Italian Heavy Draft horses slaughtered at the age of eleven months. J. Anim. Sci. 2008, 86, 1205–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzo, J.M.; Sarriés, M.V.; Franco, D. Sex effect on meat quality and carcass traits of foals slaughtered at 15 months of age. Animal 2013, 7, 1199–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pateiro, M.; Munekata, P.E.S.; Domínguez, R.; Lorenzo, J.M. Ganadería extensiva frente al cambio climático en España. ITEA 2020, 116, 444–460. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Domestic Animal Diversity Information System. Available online: https://www.fao.org/dad-is/en/ (accessed on 10 September 2021).
- Gómez, M.D.; Azor, P.J.; Alonso, M.E.; Jordana, J.; Valera, M. Morphological and genetic characterization of Spanish heavy horse breeds: Implications for their conservation. Livest. Sci. 2012, 144, 57–66. [Google Scholar] [CrossRef]
- Juárez, M.; Polvillo, O.; Gómez, M.D.; Alcalde, M.J.; Romero, F.; Valera, M. Breed effect on carcass and meat quality of foals slaughtered at 24 months of age. Meat Sci. 2009, 83, 224–228. [Google Scholar] [CrossRef]
- Sarriés, M.V.; Beriain, M.J. Carcass characteristics and meat quality of male and female foals. Meat Sci. 2005, 70, 141–152. [Google Scholar] [CrossRef]
- Sarriés, M.V.; Murray, B.E.; Troy, D.; Beriain, M.J. Intramuscular and subcutaneous lipid fatty acid profile composition in male and female foals. Meat Sci. 2006, 72, 475–485. [Google Scholar] [CrossRef]
- Aaslyng, M.D.; Meinert, L. Meat flavour in pork and beef—From animal to meal. Meat Sci. 2017, 132, 112–117. [Google Scholar] [CrossRef]
- Domínguez, R.; Purriños, L.; Pérez-Santaescolástica, C.; Pateiro, M.; Barba, F.J.; Tomasevic, I.; Campagnol, P.C.B.; Lorenzo, J.M. Characterization of volatile compounds of dry-cured meat products using HS-SPME-GC/MS technique. Food Anal. Methods 2019, 12, 1263–1284. [Google Scholar] [CrossRef]
- Tateo, A.; Maggiolino, A.; Domínguez, R.; Lorenzo, J.M.; Dinardo, F.R.; Ceci, E.; Marino, R.; della Malva, A.; Bragaglio, A.; De Palo, P. Volatile organic compounds, oxidative and sensory patterns of vacuum aged foal meat. Animals 2020, 10, 1495. [Google Scholar] [CrossRef]
- Maggiolino, A.; Lorenzo, J.M.; Marino, R.; della Malva, A.; Centoducati, P.; De Palo, P. Foal meat volatile compounds: Effect of vacuum ageing on semimembranosus muscle. J. Sci. Food Agric. 2019, 99, 1660–1667. [Google Scholar] [CrossRef]
- Domínguez, R.; Gómez, M.; Fonseca, S.; Lorenzo, J.M. Influence of thermal treatment on formation of volatile compounds, cooking loss and lipid oxidation in foal meat. LWT—Food Sci. Technol. 2014, 58, 439–445. [Google Scholar] [CrossRef]
- Domínguez, R.; Gómez, M.; Fonseca, S.; Lorenzo, J. Effect of different cooking methods on lipid oxidation and formation of volatile compounds in foal meat. Meat Sci. 2014, 97, 223–230. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Domínguez, R. Cooking losses, lipid oxidation and formation of volatile compounds in foal meat as affected by cooking procedure. Flavour Fragr. J. 2014, 29, 240–248. [Google Scholar] [CrossRef]
- Arshad, M.S.; Sohaib, M.; Ahmad, R.S.; Nadeem, M.T.; Imran, A.; Arshad, M.U.; Kwon, J.H.; Amjad, Z. Ruminant meat flavor influenced by different factors with special reference to fatty acids. Lipids Health Dis. 2018, 17, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Lanza, M.; Landi, C.; Scerra, M.; Galofaro, V.; Pennisi, P. Meat quality and intramuscular fatty acid composition of Sanfratellano and Haflinger foals. Meat Sci. 2009, 81, 142–147. [Google Scholar] [CrossRef]
- De Palo, P.; Tateo, A.; Maggiolino, A.; Centoducati, P. Effect of nutritive level on carcass traits and meat quality of IHDH foals. Anim. Sci. J. 2014, 85, 780–786. [Google Scholar] [CrossRef] [Green Version]
- Franco, D.; Rodríguez, E.; Purriños, L.; Crecente, S.; Bermúdez, R.; Lorenzo, J.M. Meat quality of “Galician Mountain” foals breed. Effect of sex, slaughter age and livestock production system. Meat Sci. 2011, 88, 292–298. [Google Scholar] [CrossRef]
- Franco, D.; Crecente, S.; Vázquez, J.A.; Gómez, M.; Lorenzo, J.M. Effect of cross breeding and amount of finishing diet on growth parameters, carcass and meat composition of foals slaughtered at 15months of age. Meat Sci. 2013, 93, 547–556. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Crecente, S.; Franco, D.; Sarriés, M.V.; Gómez, M. The effect of livestock production system and concentrate level on carcass traits and meat quality of foals slaughtered at 18 months of age. Animal 2014, 8, 494–503. [Google Scholar] [CrossRef] [Green Version]
- Kosowska, M.; Majcher, M.A.; Fortuna, T. Volatile compounds in meat and meat products. Food Sci. Technol. 2017, 37, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, M.; Sarriés, M.V.; Beriain, M.J.; Crecente, S.; Domínguez, R.; Lorenzo, J.M. Relationship between carcass traits, prime cuts and carcass grading from foals slaughtered at the age of 13 and 26 months and supplemented with standard and linseed-rich feed. Animal 2018, 12, 1084–1092. [Google Scholar] [CrossRef]
- Council of the European Union. Council Regulation (EC) No 1/2005 of 22 December 2004, on the protection of animals during transport and related operations and amending Directives 64/432/EEC and 93/119/EC and Regulation (EC) No 1255/97. OJEU 2005, L3, 1–44. [Google Scholar]
- Council of the European Union. Council Regulation (EC) No No 1099/2009 of 24 September 2009 on the protection of animals at the time of killing. OJEU 2009, L303, 1–30. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Barros, J.C.; Munekata, P.E.S.; De Carvalho, F.A.L.; Pateiro, M.; Barba, F.J.; Domínguez, R.; Trindade, M.A.; Lorenzo, J.M. Use of tiger nut (Cyperus esculentus L.) oil emulsion as animal fat replacement in beef burgers. Foods 2020, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Fernández, M.; Ordóñez, J.A.; Cambero, I.; Santos, C.; Pin, C.; Hoz, L. de la Fatty acid compositions of selected varieties of Spanish dry ham related to their nutritional implications. Food Chem. 2007, 101, 107–112. [Google Scholar] [CrossRef]
- Franco, D.; Lorenzo, J.M. Effect of muscle and intensity of finishing diet on meat quality of foals slaughtered at 15months. Meat Sci. 2014, 96, 327–334. [Google Scholar] [CrossRef]
- Seong, P.N.; Park, K.M.; Kang, G.H.; Cho, S.H.; Park, B.Y.; Chae, H.S.; Van Ba, H. The differences in chemical composition, physical quality traits and nutritional values of horse meat as affected by various retail cut types. Asian-Australas. J. Anim. Sci. 2016, 29, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Trombetta, M.F.; Nocelli, F.; Pasquini, M. Meat quality and intramuscular fatty acid composition of Catria Horse. Anim. Sci. J. 2017, 88, 1107–1112. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Maggiolino, A.; Sarriés, M.V.; Polidori, P.; Franco, D.; Lanza, M.; Palo, P. De More than Beef, Pork and Chicken—The Production, Processing, and Quality Traits of Other Sources of Meat for Human Diet; Springer: Cham, Swizterland, 2019; ISBN 9783030054847. [Google Scholar]
- Simopoulos, A.P. Omega-6/omega-3 essential fatty acid ratio and chronic diseases. Food Rev. Int. 2004, 20, 77–90. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Pateiro, M. Influence of type of muscles on nutritional value of foal meat. Meat Sci. 2013, 93, 630–638. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Pateiro, M.; Franco, D. Influence of muscle type on physicochemical and sensory properties of foal meat. Meat Sci. 2013, 94, 77–83. [Google Scholar] [CrossRef]
- Sahaka, M.; Amara, S.; Wattanakul, J.; Gedi, M.A.; Aldai, N.; Parsiegla, G.; Lecomte, J.; Christeller, J.T.; Gray, D.; Gontero, B.; et al. The digestion of galactolipids and its ubiquitous function in Nature for the uptake of the essential α-linolenic acid. Food Funct. 2020, 11, 6710–6744. [Google Scholar] [CrossRef]
- Belaunzaran, X.; Lavín, P.; Barron, L.J.R.; Mantecón, A.R.; Kramer, J.K.G.; Aldai, N. An assessment of the fatty acid composition of horse-meat available at the retail level in northern Spain. Meat Sci. 2017, 124, 39–47. [Google Scholar] [CrossRef]
- Roberts, D.D.; Pollien, P.; Antille, N.; Lindinger, C.; Yeretzian, C. Comparison of nosespace, headspace, and sensory intensity ratings for the evaluation of flavor absorption by fat. J. Agric. Food Chem. 2003, 51, 3636–3642. [Google Scholar] [CrossRef]
- Echegaray, N.; Pateiro, M.; Gonzales-barron, U.; Lorenzo, M. Influence of feeding system on Longissimus thoracis et lumborum volatile compounds of an Iberian local lamb breed. Small Rumin. Res. 2021, 201, 1064017. [Google Scholar] [CrossRef]
- Flores, M. Understanding the implications of current health trends on the aroma of wet and dry cured meat products. Meat Sci. 2018, 144, 53–61. [Google Scholar] [CrossRef]
- Coppa, M.; Martin, B.; Pradel, P.; Leotta, B.; Priolo, A.; Vasta, V. Effect of a Hay-Based Diet or Different Upland Grazing Systems on Milk Volatile Compounds. J. Agric. Food Chem 2011, 59, 4947–4954. [Google Scholar] [CrossRef]
- Montanari, C.; Gatto, V.; Torriani, S.; Barbieri, F.; Bargossi, E.; Lanciotti, R.; Grazia, L.; Magnani, R.; Tabanelli, G.; Gardini, F. Effects of the diameter on physico-chemical, microbiological and volatile profile in dry fermented sausages produced with two different starter cultures. Food Biosci. 2018, 22, 9–18. [Google Scholar] [CrossRef]
- Calkins, C.R.; Hodgen, J.M. A fresh look at meat flavor. Meat Sci. 2007, 77, 63–80. [Google Scholar] [CrossRef]
- Théron, L.; Tournayre, P.; Kondjoyan, N.; Abouelkaram, S.; Santé-Lhoutellier, V.; Berdagué, J.L. Analysis of the volatile profile and identification of odour-active compounds in Bayonne ham. Meat Sci. 2010, 85, 453–460. [Google Scholar] [CrossRef]
- Vasta, V.; Luciano, G.; Dimauro, C.; Röhrle, F.; Priolo, A.; Monahan, F.J.; Moloney, A.P. The volatile profile of longissimus dorsi muscle of heifers fed pasture, pasture silage or cereal concentrate: Implication for dietary discrimination. Meat Sci. 2011, 87, 282–289. [Google Scholar] [CrossRef]
- Karabagias, I.K. Volatile profile of raw lamb meat stored at 4 ± 1 °C: The potential of specific aldehyde ratios as indicators of lamb meat quality. Foods 2018, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- Selli, S.; Cayhan, G.G. Analysis of volatile compounds of wild gilthead sea bream (Sparus aurata) by simultaneous distillation-extraction (SDE) and GC-MS. Microchem. J. 2009, 93, 232–235. [Google Scholar] [CrossRef]
- Nieto, G.; Bañón, S.; Garrido, M.D. Effect of supplementing ewes’ diet with thyme (Thymus zygis ssp. gracilis) leaves on the lipid oxidation of cooked lamb meat. Food Chem. 2011, 125, 1147–1152. [Google Scholar] [CrossRef]
- Echegaray, N.; Domínguez, R.; Cadavez, V.A.P.; Bermúdez, R.; Purriños, L.; Gonzales-Barron, U.; Hoffman, E.; Lorenzo, J.M. Influence of the production system (Intensive vs. extensive) at farm level on proximate composition and volatile compounds of portuguese lamb meat. Foods 2021, 10, 1450. [Google Scholar] [CrossRef]
- Vasta, V.; Priolo, A. Ruminant fat volatiles as affected by diet. A review. Meat Sci. 2006, 73, 218–228. [Google Scholar] [CrossRef]
- Descalzo, A.M.; Insani, E.M.; Biolatto, A.; Sancho, A.M.; García, P.T.; Pensel, N.A.; Josifovich, J.A. Influence of pasture or grain-based diets supplemented with vitamin E on antioxidant/oxidative balance of Argentine beef. Meat Sci. 2005, 70, 35–44. [Google Scholar] [CrossRef]
- Fruet, A.P.B.; Trombetta, F.; Stefanello, F.S.; Speroni, C.S.; Donadel, J.Z.; De Souza, A.N.M.; Rosado Júnior, A.; Tonetto, C.J.; Wagner, R.; De Mello, A.; et al. Effects of feeding legume-grass pasture and different concentrate levels on fatty acid profile, volatile compounds, and off-flavor of the M. longissimus thoracis. Meat Sci. 2018, 140, 112–118. [Google Scholar] [CrossRef]
- Chaijan, M.; Panpipat, W. Mechanism of oxidation in foods of animal origin. In Natural Antioxidants. Applications in Foods of Animal Origin; Banerjee, R., Verma, A.K., Siddiqui, M.W., Eds.; Apple Academic Press, Inc.: Boca Raton, FL, USA, 2017; pp. 1–38. ISBN 978-1-315-36591-6. [Google Scholar]
- Lindqvist, H.; Nadeau, E.; Jensen, S.K. Alpha-tocopherol and β-carotene in legume-grass mixtures as influenced by wilting, ensiling and type of silage additive. Grass Forage Sci. 2012, 67, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Stefanello, F.S.; dos Santos, C.O.; Bochi, V.C.; Fruet, A.P.B.; Soquetta, M.B.; Dörr, A.C.; Nörnberg, J.L. Analysis of polyphenols in brewer’s spent grain and its comparison with corn silage and cereal brans commonly used for animal nutrition. Food Chem. 2018, 239, 385–401. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.; Liu, M.; Zhao, X.; Luo, H. Effect of breed on the volatile compound precursors and odor profile attributes of lamb meat. Foods 2020, 9, 1178. [Google Scholar] [CrossRef]
- Pastorelli, G.; Magni, S.; Rossi, R.; Pagliarini, E.; Baldini, P.; Dirinck, P.; Van Opstaele, F.; Corino, C. Influence of dietary fat, on fatty acid composition and sensory properties of dry-cured Parma ham. Meat Sci. 2003, 65, 571–580. [Google Scholar] [CrossRef]
- Bosse (née Danz), R.; Wirth, M.; Konstanz, A.; Becker, T.; Weiss, J.; Gibis, M. Determination of volatile marker compounds in raw ham using headspace-trap gas chromatography. Food Chem. 2017, 219, 249–259. [Google Scholar] [CrossRef]
- Petričević, S.; Marušić Radovčić, N.; Lukić, K.; Listeš, E.; Medić, H. Differentiation of dry-cured hams from different processing methods by means of volatile compounds, physico-chemical and sensory analysis. Meat Sci. 2018, 137, 217–227. [Google Scholar] [CrossRef]
- Sidira, M.; Kandylis, P.; Kanellaki, M.; Kourkoutas, Y. Effect of immobilized Lactobacillus casei on volatile compounds of heat treated probiotic dry-fermented sausages. Food Chem. 2015, 178, 201–207. [Google Scholar] [CrossRef]
- Echegaray, N.; Domínguez, R.; Bodas, R.; Montañés, M.; García, J.J.; Benito, A.; Bermúdez, R.; Purriños, L.; Lorenzo, J.M. Characterization of volatile profile of longissimus thoracis et lumborum muscle from Castellana and INRA 401 lambs reared under commercial conditions. Small Rumin. Res. 2021, 200, 106396. [Google Scholar] [CrossRef]
- Elmore, J.S.; Cooper, S.L.; Enser, M.; Mottram, D.S.; Sinclair, L.A.; Wilkinson, R.G.; Wood, J.D. Dietary manipulation of fatty acid composition in lamb meat and its effect on the volatile aroma compounds of grilled lamb. Meat Sci. 2005, 69, 233–242. [Google Scholar] [CrossRef]
- Urbach, G. Effect of feed on flavor in dairy foods. J. Dairy Sci. 1990, 73, 3639–3650. [Google Scholar] [CrossRef]
- Gargouri, M.; Drouet, P.; Legoy, M.D. Synthesis of a novel macrolactone by lipase-catalyzed intra-esterification of hydroxy-fatty acid in organic media. J. Biotechnol. 2002, 92, 259–266. [Google Scholar] [CrossRef]
- Morand-Fehr, P.; Tran, G. La fraction lipidique des aliments et les corps gras utilisés en alimentation animale. Prod. Anim. 2001, 14, 285–302. [Google Scholar] [CrossRef]
- Resconi, V.C.; Campo, M.M.; Montossi, F.; Ferreira, V.; Sañudo, C.; Escudero, A. Relationship between odour-active compounds and flavour perception in meat from lambs fed different diets. Meat Sci. 2010, 85, 700–706. [Google Scholar] [CrossRef]
- Van Ba, H.; Park, K.; Dashmaa, D.; Hwang, I. Effect of muscle type and vacuum chiller ageing period on the chemical compositions, meat quality, sensory attributes and volatile compounds of Korean native cattle beef. Anim. Sci. J. 2014, 85, 164–173. [Google Scholar]
- Insausti, K.; Beriain, M.; Gorraiz, C.; Purroy, A. Volatile compounds of raw beef from 5 local spanish cattle breeds stored under modified atmosphere. Sens. Nutr. Qual. Food 2002, 67, 1580–1589. [Google Scholar] [CrossRef]
- Narváez-Rivas, M.; Gallardo, E.; León-Camacho, M. Analysis of volatile compounds from Iberian hams: A review. Grasa Aceites 2012, 63, 432–454. [Google Scholar]
- Saraiva, C.; Oliveira, I.; Silva, J.A.; Martins, C.; Ventanas, J.; García, C. Implementation of multivariate techniques for the selection of volatile compounds as indicators of sensory quality of raw beef. J. Food Sci. Technol. 2015, 52, 3887–3898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontou, S.; Tsipi, D.; Tzia, C. Stability of the dithiocarbamate pesticide maneb in tomato homogenates during cold storage and thermal processing. Food Addit. Contam. 2004, 21, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
| Oats | Starter Feed | Finisher Feed | Straw | Silage | Organic Feed | |
|---|---|---|---|---|---|---|
| Chemical composition (%) | ||||||
| Fat | 5.58 | 3.40 | 6.00 | 1.95 | 3.71 | 4.80 |
| Protein | 8.78 | 12.90 | 12.80 | 2.00 | 16.22 | 8.50 |
| Ash | 2.59 | 8.50 | 4.50 | 3.88 | 8.81 | 5.00 |
| Fatty acids (g/100 g of fatty acids) | ||||||
| C8:0 | 0.02 | 0.17 | 0.00 | 0.91 | 0.00 | 0.00 |
| C10:0 | 0.01 | 0.17 | 0.04 | 0.78 | 0.13 | 0.00 |
| C12:0 | 0.02 | 2.17 | 0.18 | 0.98 | 0.14 | 0.01 |
| C14:0 | 0.19 | 1.11 | 0.73 | 3.74 | 0.20 | 0.14 |
| C15:0 | 0.01 | 0.09 | 0.07 | 0.50 | 0.12 | 0.01 |
| C16:0 | 15.96 | 22.58 | 22.00 | 29.25 | 16.98 | 14.05 |
| C16:1n-7 | 0.19 | 0.22 | 0.80 | 0.74 | 0.15 | 0.14 |
| C17:0 | 0.06 | 0.15 | 0.18 | 0.21 | 0.32 | 0.10 |
| C18:0 | 1.21 | 2.67 | 3.83 | 6.39 | 1.52 | 2.42 |
| 9t-C18:1 | 0.02 | 0.03 | 0.13 | 0.51 | 0.20 | 0.00 |
| 11t-C18:1 | 0.00 | 0.00 | 0.23 | 0.00 | 0.00 | 0.00 |
| C18:1n-9 | 37.74 | 26.08 | 34.88 | 14.53 | 2.73 | 28.90 |
| C18:1n-7 | 1.25 | 1.20 | 1.74 | 0.97 | 0.46 | 1.26 |
| C18:2n-6 | 40.09 | 36.80 | 31.26 | 19.64 | 17.12 | 47.24 |
| C18:3n-3 | 1.35 | 4.85 | 2.22 | 13.10 | 56.63 | 3.98 |
| C20:0 | 0.15 | 0.29 | 0.20 | 1.73 | 0.37 | 0.29 |
| C20:1n-9 | 0.83 | 0.47 | 0.57 | 0.00 | 0.19 | 0.55 |
| C21:0 | 0.02 | 0.03 | 0.02 | 0.69 | 0.26 | 0.04 |
| C22:0 | 0.13 | 0.23 | 0.00 | 2.10 | 0.61 | 0.00 |
| C20:5n-3 (EPA) | 0.00 | 0.00 | 0.14 | 0.00 | 0.00 | 0.27 |
| C22:1n-9 | 0.51 | 0.17 | 0.22 | 0.31 | 0.10 | 0.26 |
| C22:2n-6 | 0.01 | 0.04 | 0.00 | 0.00 | 0.77 | 0.00 |
| C24:0 | 0.09 | 0.23 | 0.10 | 2.09 | 0.98 | 0.17 |
| C22:6n-3 (DHA) | 0.08 | 0.08 | 0.11 | 0.00 | 0.00 | 0.07 |
| SFA | 17.90 | 29.98 | 27.37 | 50.20 | 21.63 | 17.28 |
| MUFA | 40.55 | 28.18 | 38.60 | 17.06 | 3.83 | 31.11 |
| PUFA | 41.55 | 41.84 | 34.04 | 32.75 | 74.54 | 51.62 |
| JN | BU | Sig. | ||||||
|---|---|---|---|---|---|---|---|---|
| Fatty Acids | D1 | D2 | D1 | D2 | SEM | B | D | B × D |
| C10:0 | 0.08 b | 0.05 a | 0.08 b | 0.05 a | 0.002 | ns | *** | ns |
| C12:0 | 0.18 c | 0.12 a | 0.20 c | 0.14 b | 0.006 | ** | *** | ns |
| C14:0 | 3.29 b | 2.49 a | 3.50 b | 2.64 a | 0.078 | ns | *** | ns |
| C14:1n-5 | 0.38 b | 0.22 a | 0.50 c | 0.27 a | 0.018 | *** | *** | ns |
| C15:0 | 0.23 b | 0.15 a | 0.24 b | 0.14 a | 0.007 | ns | *** | ns |
| C16:0 | 26.48 b | 25.32 ab | 25.63 ab | 24.73 a | 0.245 | ns | * | ns |
| C16:1n-7 | 6.84 b | 5.32 a | 8.21 c | 5.58 a | 0.218 | * | *** | ns |
| C17:0 | 0.26 c | 0.22 b | 0.25 c | 0.20 a | 0.005 | * | *** | ns |
| C18:0 | 3.84 c | 4.01 c | 3.10 a | 3.49 b | 0.069 | *** | ** | ns |
| 9t-C18:1 | 0.13 c | 0.12 b | 0.11 b | 0.10 a | 0.002 | *** | *** | ns |
| C18:1n-9 | 28.73 bc | 26.00 a | 29.56 c | 26.96 ab | 0.391 | ns | *** | ns |
| C18:1n-7 | 1.63 b | 1.35 a | 1.84 c | 1.33 a | 0.038 | * | *** | * |
| C18:2n-6 | 10.24 b | 9.84 b | 7.42 a | 8.28 a | 0.256 | *** | ns | ns |
| C18:3n-3 | 3.16 a | 4.41 b | 2.64 a | 4.51 b | 0.174 | ns | *** | ns |
| C20:1n-9 | 0.31 b | 0.28 ab | 0.30 b | 0.25 a | 0.007 | ns | ** | ns |
| C20:2n-6 | 0.19 b | 0.19 b | 0.13 a | 0.15 a | 0.006 | *** | ns | ns |
| C20:3n-6 | 0.16 b | 0.20 c | 0.11 a | 0.15 b | 0.006 | *** | *** | ns |
| C20:4n-6 | 0.68 bc | 0.80 c | 0.40 a | 0.56 b | 0.030 | *** | ** | ns |
| C20:3n-3 | 0.13 b | 0.19 d | 0.10 a | 0.16 c | 0.006 | ** | *** | ns |
| C20:5n-3 (EPA) | 0.05 a | 0.09 b | 0.05 a | 0.08 b | 0.004 | ns | *** | ns |
| C22:5n-3 (DPA) | 0.29 b | 0.48 d | 0.21 a | 0.39 c | 0.020 | ** | *** | ns |
| C22:6n-3 (DHA) | 0.06 ab | 0.10 c | 0.06 a | 0.09 bc | 0.006 | ns | ** | ns |
| SFA | 34.45 b | 32.45 a | 33.08 ab | 31.46 a | 0.311 | * | ** | ns |
| MUFA | 38.04 b | 33.30 a | 40.53 b | 34.49 a | 0.613 | ns | *** | ns |
| PUFA | 14.99 b | 16.32 b | 11.14 a | 14.38 b | 0.419 | *** | ** | ns |
| n-3 | 3.69 a | 5.27 b | 3.05 a | 5.23 b | 0.197 | ns | *** | ns |
| n-6 | 11.29 b | 11.05 b | 8.08 a | 9.15 a | 0.289 | *** | ns | ns |
| LC n-3 | 0.40 a | 0.67 c | 0.31 a | 0.56 b | 0.028 | * | *** | ns |
| n-6/n-3 | 3.12 c | 2.17 b | 2.77 c | 1.77 a | 0.102 | ** | *** | ns |
| PUFA/SFA | 0.44 b | 0.51 b | 0.34 a | 0.46 b | 0.015 | ** | *** | ns |
| TI | 0.94 b | 0.84 a | 0.95 b | 0.81 a | 0.015 | ns | *** | ns |
| AI | 0.75 ab | 0.71 a | 0.77 b | 0.73 a | 0.008 | ns | ** | ns |
| h/H | 1.50 a | 1.54 a | 1.44 a | 1.53 a | 0.017 | ns | ns | ns |
| Volatile Compounds | LRI | m/z | JN | BU | Sig. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D1 | D2 | SEM | B | D | B × D | |||
| Isobutane | 488 | 57 | 0.24 ab | 0.19 a | 0.30 b | 0.22 ab | 0.015 | ns | * | ns |
| Pentane, 2-methyl- | 516 | 71 | 0.25 a | 0.21 a | 0.51 b | 0.58 b | 0.029 | *** | ns | ns |
| Pentane, 3-methyl- | 525 | 57 | 2.24 b | 1.41 a | 3.67 c | 3.30 c | 0.173 | *** | * | ns |
| Pentane | 500 | 43 | 1.30 b | 0.87 a | 1.14 ab | 0.92 a | 0.060 | ns | ** | ns |
| Cyclopentane, methyl- | 564 | 56 | 1.47 a | 1.13 a | 3.13 b | 2.75 b | 0.160 | *** | ns | ns |
| Bicyclo[3.2.0]hepta-2,6-diene | 790 | 91 | 2670 b | 2210 a | 2981 b | 2801 b | 86.640 | ** | * | ns |
| Octane | 800 | 85 | 1.95 ab | 1.63 a | 2.18 b | 1.44 a | 0.091 | ns | ** | ns |
| Nonane | 900 | 57 | 0.65 b | 0.43 a | 0.76 b | 0.75 b | 0.033 | *** | * | ns |
| Cyclopentane, 1,1-dimethyl- | 927 | 69 | 0.70 c | 0.34 a | 0.59 bc | 0.50 b | 0.032 | ns | *** | * |
| Cyclopropane | 1041 | 55 | 25.64 b | 16.03 a | 19.75 a | 20.20 a | 1.027 | ns | * | ** |
| Undecane, 5,5-dimethyl- | 1069 | 71 | 0.27 a | 1.53 b | 0.13 a | 0.07 a | 0.102 | *** | *** | *** |
| Cyclobutane, butyl- | 1119 | 84 | 0.86 b | 0.36 a | 0.74 b | 0.40 a | 0.039 | ns | *** | ns |
| Dodecane | 1200 | 71 | 0.20 b | 0.40 c | 0.22 b | 0.07 a | 0.023 | *** | ns | *** |
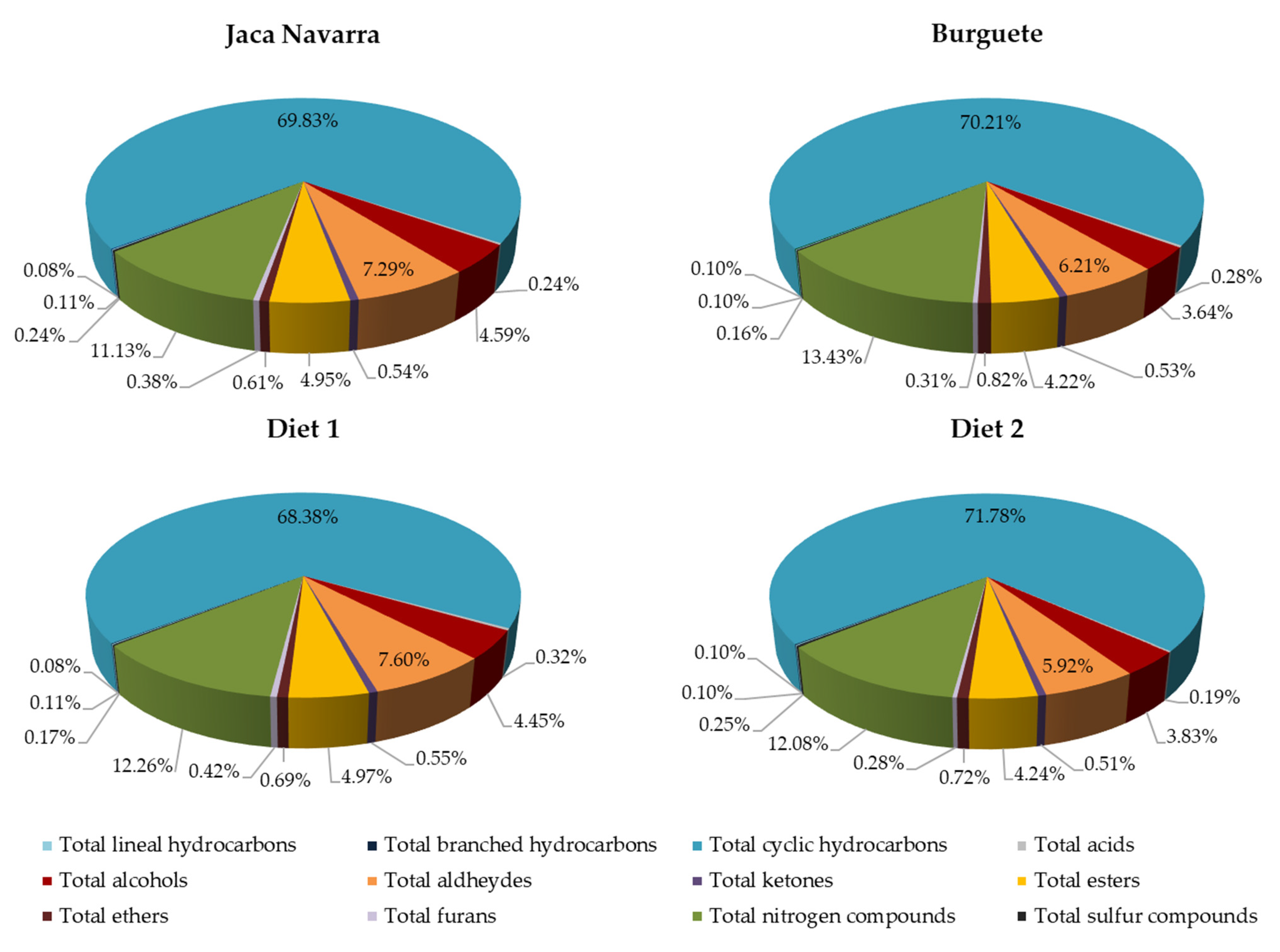
| Total lineal hydrocarbons | 4.35 b | 3.53 a | 4.60 b | 3.40 a | 0.135 | ns | *** | ns | ||
| Total branched hydrocarbons | 2.76 a | 3.15 a | 4.31 b | 3.95 b | 0.156 | *** | ns | ns | ||
| Total cyclic hydrocarbons | 2698 b | 2228 a | 3006 b | 2825 b | 86.732 | ** | * | ns | ||
| Total hydrocarbons | 2705 b | 2235 a | 3015 b | 2832 b | 86.831 | ** | * | ns | ||
| Butanoic acid, 4-hydroxy- | 1038 | 86 | 4.49 b | 3.98 b | 4.20 b | 2.95 a | 0.170 | * | ** | ns |
| Hexanoic acid | 1074 | 60 | 5.73 b | 0.76 a | 9.46 c | 4.36 b | 0.524 | *** | *** | ns |
| 2-Furancarboxylic acid, tetrahydro-3-methyl-5-oxo- | 1131 | 99 | 1.44 c | 0.45 a | 1.81 d | 1.02 b | 0.092 | *** | *** | ns |
| Total acids | 11.65 c | 5.20 a | 15.48 d | 8.33 b | 0.642 | *** | *** | ns | ||
| Glycidol | 472 | 44 | 3.52 b | 1.78 a | 2.93 b | 2.05 a | 0.165 | ns | *** | ns |
| Cyclobutanemethanol | 716 | 57 | 19.47 b | 11.47 a | 15.75 ab | 19.01 b | 0.942 | ns | ns | ** |
| 1-Pentanol | 834 | 70 | 46.05 c | 26.67 a | 35.05 b | 29.94 ab | 1.750 | ns | *** | * |
| Prenol | 843 | 71 | 0.72 b | 0.54 a | 0.55 a | 0.68 b | 0.024 | ns | ns | ** |
| Cis-2-Pentenol | 843 | 57 | 2.15 b | 1.57 a | 2.28 b | 2.48 b | 0.098 | ** | ns | * |
| 2,3-Butanediol | 906 | 45 | 0.68 a | 0.70 a | 1.55 b | 0.85 a | 0.071 | *** | ** | ** |
| 1-Hexanol | 943 | 56 | 10.71 c | 4.94 a | 11.62 c | 7.60 b | 0.511 | ** | *** | ns |
| 5-Methyl-1-heptanol | 1029 | 83 | 6.05 b | 2.94 a | 5.60 b | 4.93 b | 0.283 | ns | *** | ** |
| 1-Heptanol | 1036 | 70 | 3.36 b | 1.79 a | 3.54 b | 1.92 a | 0.165 | ns | *** | ns |
| 1-Octen-3-ol | 1042 | 57 | 103.5 b | 69.38 a | 73.66 a | 71.70 a | 3.599 | * | ** | * |
| Diethylene glycol | 1080 | 45 | 1.19 b | 0.39 a | 1.03 b | 0.25 a | 0.080 | ns | *** | ns |
| 2-Ethyl-1-hexanol | 1085 | 57 | 1.06 b | 0.71 a | 1.32 c | 1.08 b | 0.043 | *** | *** | ns |
| 1-Octanol | 1119 | 56 | 1.26 b | 0.68 a | 1.20 b | 0.66 a | 0.058 | ns | *** | ns |
| 6-Undecanol | 1156 | 83 | 1.78 b | 0.45 a | 2.52 c | 0.54 a | 0.157 | * | *** | ns |
| Total alcohols | 201.5 c | 124.0 a | 158.6 b | 143.7 ab | 6.285 | ns | *** | ** | ||
| Volatile Compounds | LRI | m/z | JN | BU | Sig. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D1 | D2 | SEM | B | D | B × D | |||
| Propanal | 499 | 58 | 9.70 b | 5.42 a | 10.29 b | 11.74 b | 0.531 | *** | ns | ** |
| 2-Propynal | 537 | 53 | 7.47 a | 7.41 a | 13.43 b | 12.60 b | 0.583 | *** | ns | ns |
| Pentanal | 714 | 57 | 15.66 b | 8.05 a | 11.07 a | 15.28 b | 0.802 | ns | ns | *** |
| Hexanal | 853 | 56 | 294.0 c | 140.0 a | 207.1 b | 198.4 ab | 13.513 | ns | *** | ** |
| Heptanal | 963 | 70 | 7.83 b | 2.07 a | 8.91 b | 3.78 a | 0.517 | * | *** | ns |
| 2-Heptenal, (Z)- | 1029 | 83 | 6.70 c | 3.29 a | 4.27 ab | 4.90 b | 0.296 | ns | ** | *** |
| Octanal | 1057 | 84 | 1.98 c | 0.80 a | 1.98 c | 1.27 b | 0.107 | ns | *** | ns |
| 2-Octenal, (E)- | 1116 | 83 | 2.05 c | 0.99 a | 1.88 bc | 1.47 ab | 0.106 | ns | *** | ns |
| Nonanal | 1141 | 98 | 4.21 c | 1.78 a | 3.69 bc | 3.03 b | 0.198 | ns | *** | ** |
| Total aldehydes | 349.6 c | 169.9 a | 262.6 b | 252.4 b | 15.017 | ns | *** | *** | ||
| 2,3-Pentanedione | 722 | 100 | 5.28 ab | 4.08 a | 5.80 b | 8.29 c | 0.309 | *** | ns | *** |
| Acetoin | 776 | 45 | 5.41 a | 5.74 a | 9.60 b | 4.07 a | 0.438 | ns | *** | *** |
| 2-Heptanone | 956 | 58 | 3.03 b | 1.35 a | 2.73 b | 1.55 a | 0.167 | ns | *** | ns |
| Butyrolactone | 1038 | 86 | 4.49 b | 3.98 b | 4.20 b | 2.95 a | 0.170 | * | ** | ns |
| 5-Hexen-3-one | 1075 | 69 | 0.55 b | 0.41 b | 0.87 c | 0.22 a | 0.039 | ns | *** | *** |
| 3-Octen-2-one | 1099 | 111 | 2.34 b | 1.30 a | 2.05 b | 1.73 ab | 0.122 | ns | ** | ns |
| Total ketones | 21.10 b | 16.86 a | 25.26 c | 18.80 ab | 0.696 | ** | *** | ns | ||
| Dibutyl sulphate | 525 | 56 | 1.65 a | 1.24 a | 3.35 b | 2.91 b | 0.163 | *** | ns | ns |
| Vinyl butyrate | 1041 | 71 | 102.55 b | 60.71 a | 77.61 a | 77.90 a | 3.717 | ns | ** | ** |
| Caproic acid, vinyl ester | 1041 | 99 | 115.3 b | 61.33 a | 93.08 b | 89.63 b | 5.121 | ns | ** | ** |
| Glycerol 1,2-diacetate | 1333 | 145 | 0.37 b | 0.26 a | 0.34 ab | 0.55 c | 0.021 | *** | ns | *** |
| Butyl isobutyrate | 1355 | 89 | 0.62 b | 0.16 a | 0.24 a | 0.20 a | 0.036 | *** | *** | *** |
| 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | 1439 | 71 | 4.45 b | 2.90 a | 2.11 a | 2.38 a | 0.206 | *** | ns | ** |
| Total esters | 224.9 c | 126.5 a | 176.7 b | 173.5 b | 8.673 | ns | *** | ** | ||
| Dimethyl ether | 491 | 45 | 5.80 a | 7.03 a | 14.52 c | 9.53 b | 0.618 | *** | * | *** |
| Tetrahydrofuran | 587 | 72 | 2.69 a | 6.08 b | 8.18 c | 4.69 b | 0.433 | ** | ns | *** |
| Methane, oxybis[dichloro- | 591 | 83 | 10.45 ab | 9.04 a | 16.68 c | 13.59 bc | 0.737 | *** | ns | ns |
| Oxirane, tetramethyl- | 883 | 59 | 1.21 c | 0.45 a | 0.73 b | 0.42 a | 0.064 | ** | *** | * |
| Total ethers | 20.15 a | 22.60 a | 40.11 c | 28.23 b | 1.408 | *** | * | *** | ||
| Furan, 2-ethyl- | 688 | 81 | 2.82 b | 1.86 a | 2.90 b | 2.60 ab | 0.155 | ns | * | ns |
| Furan, 2-pentyl- | 1027 | 81 | 15.79 c | 6.72 a | 12.19 b | 8.33 a | 0.775 | ns | *** | * |
| Total furans | 18.61 b | 8.58 a | 15.08 b | 10.94 a | 0.893 | ns | *** | * | ||
| Diazene, dimethyl- | 502 | 58 | 2.95 b | 4.61 c | 1.91 a | 2.08 a | 0.205 | *** | ** | ** |
| 2-Propen-1-amine | 535 | 56 | 235.3 a | 154.1 a | 390.2 b | 367.7 b | 20.813 | *** | ns | ns |
| 2-Propanamine | 714 | 58 | 7.02 b | 3.02 a | 3.91 a | 3.53 a | 0.380 | * | *** | ** |
| Propane, 2-nitro- | 1041 | 43 | 218.0 b | 162.7 a | 172.6 ab | 173.0 ab | 8.762 | ns | ns | ns |
| Total nitrogen compounds | 463.3 b | 324.5 a | 568.7 b | 546.4 b | 22.754 | *** | * | ns | ||
| Carbon disulfide | 506 | 76 | 7.31 a | 9.59 b | 6.27 a | 6.93 a | 0.412 | * | ns | ns |
| Total sulfur compounds | 7.31 a | 9.59 b | 6.27 a | 6.93 a | 0.412 | * | ns | ns | ||
| Total volatile compounds | 4024 b | 3043 a | 4284 b | 4022 b | 109.963 | *** | *** | * | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cittadini, A.; Domínguez, R.; Pateiro, M.; Sarriés, M.V.; Lorenzo, J.M. Fatty Acid Composition and Volatile Profile of longissimus thoracis et lumborum Muscle from Burguete and Jaca Navarra Foals Fattened with Different Finishing Diets. Foods 2021, 10, 2914. https://doi.org/10.3390/foods10122914
Cittadini A, Domínguez R, Pateiro M, Sarriés MV, Lorenzo JM. Fatty Acid Composition and Volatile Profile of longissimus thoracis et lumborum Muscle from Burguete and Jaca Navarra Foals Fattened with Different Finishing Diets. Foods. 2021; 10(12):2914. https://doi.org/10.3390/foods10122914
Chicago/Turabian StyleCittadini, Aurora, Rubén Domínguez, Mirian Pateiro, María V. Sarriés, and José M. Lorenzo. 2021. "Fatty Acid Composition and Volatile Profile of longissimus thoracis et lumborum Muscle from Burguete and Jaca Navarra Foals Fattened with Different Finishing Diets" Foods 10, no. 12: 2914. https://doi.org/10.3390/foods10122914
APA StyleCittadini, A., Domínguez, R., Pateiro, M., Sarriés, M. V., & Lorenzo, J. M. (2021). Fatty Acid Composition and Volatile Profile of longissimus thoracis et lumborum Muscle from Burguete and Jaca Navarra Foals Fattened with Different Finishing Diets. Foods, 10(12), 2914. https://doi.org/10.3390/foods10122914










