Effects of Calcium Salts on the Physicochemical Quality of Cured Beef Sausages during Manufacturing and Storage: A Potential Calcium Application for Sausages with Alginate Casings
Abstract
:1. Introduction
2. Materials and Methods
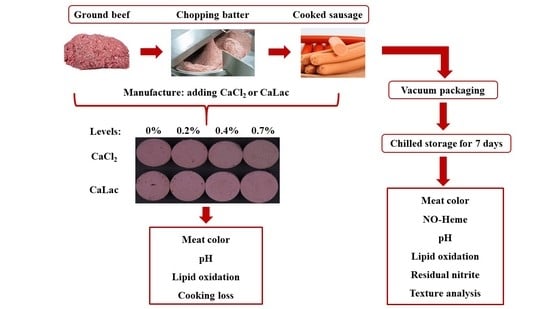
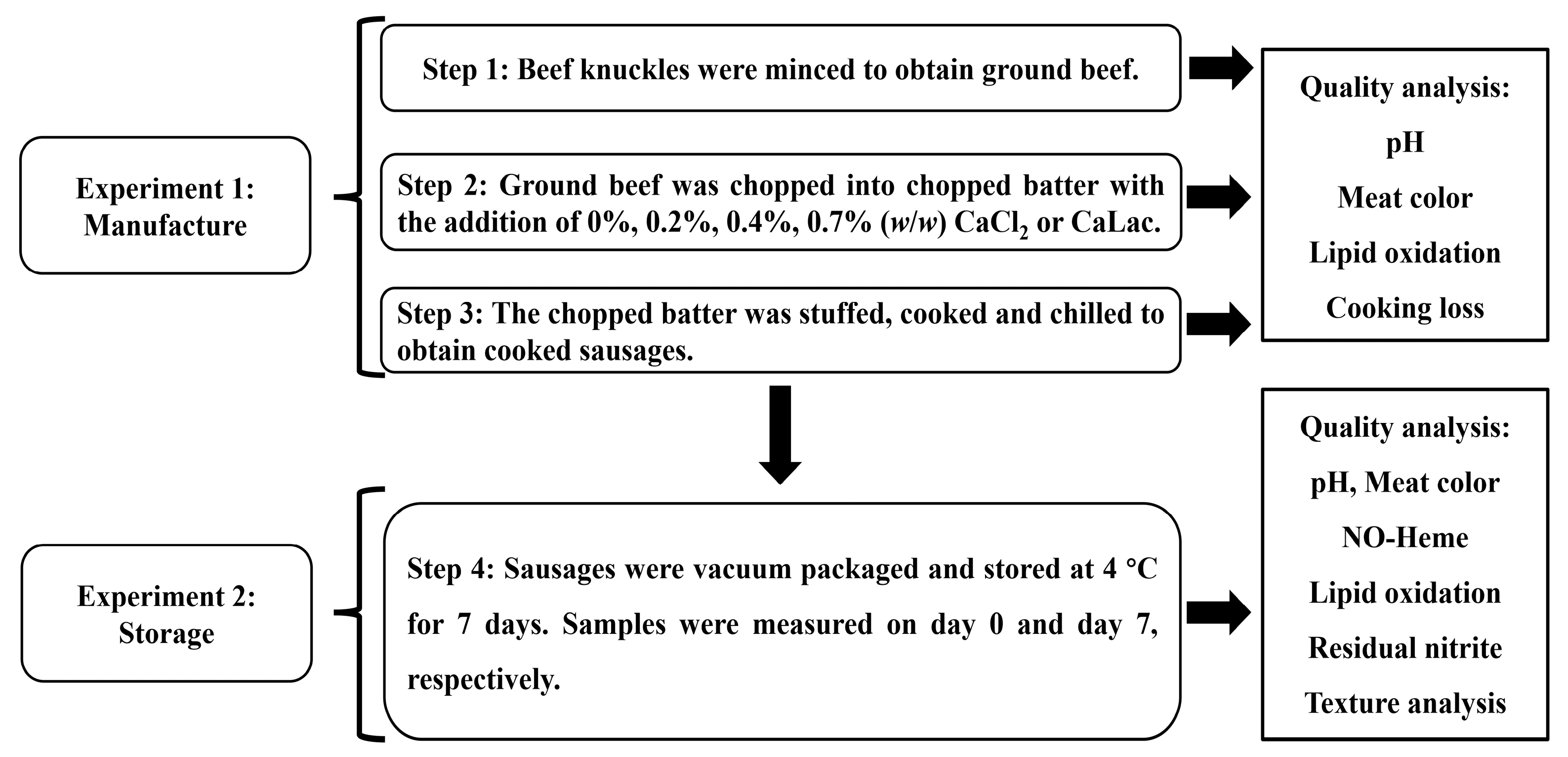
2.1. Experimental Design and Sausage Manufacture
2.2. Methods
2.2.1. pH
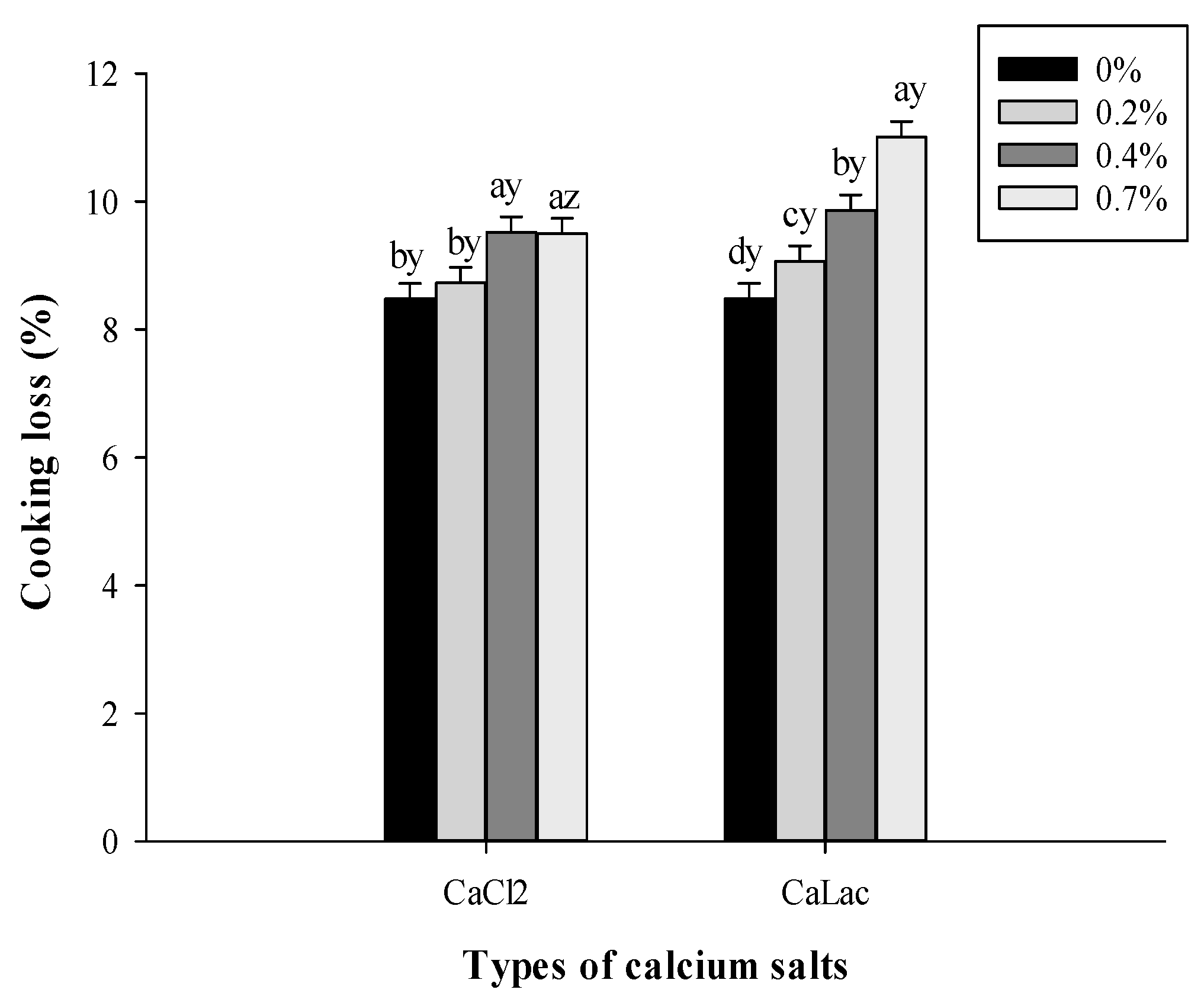
2.2.2. Cooking Loss
2.2.3. Instrumental Color
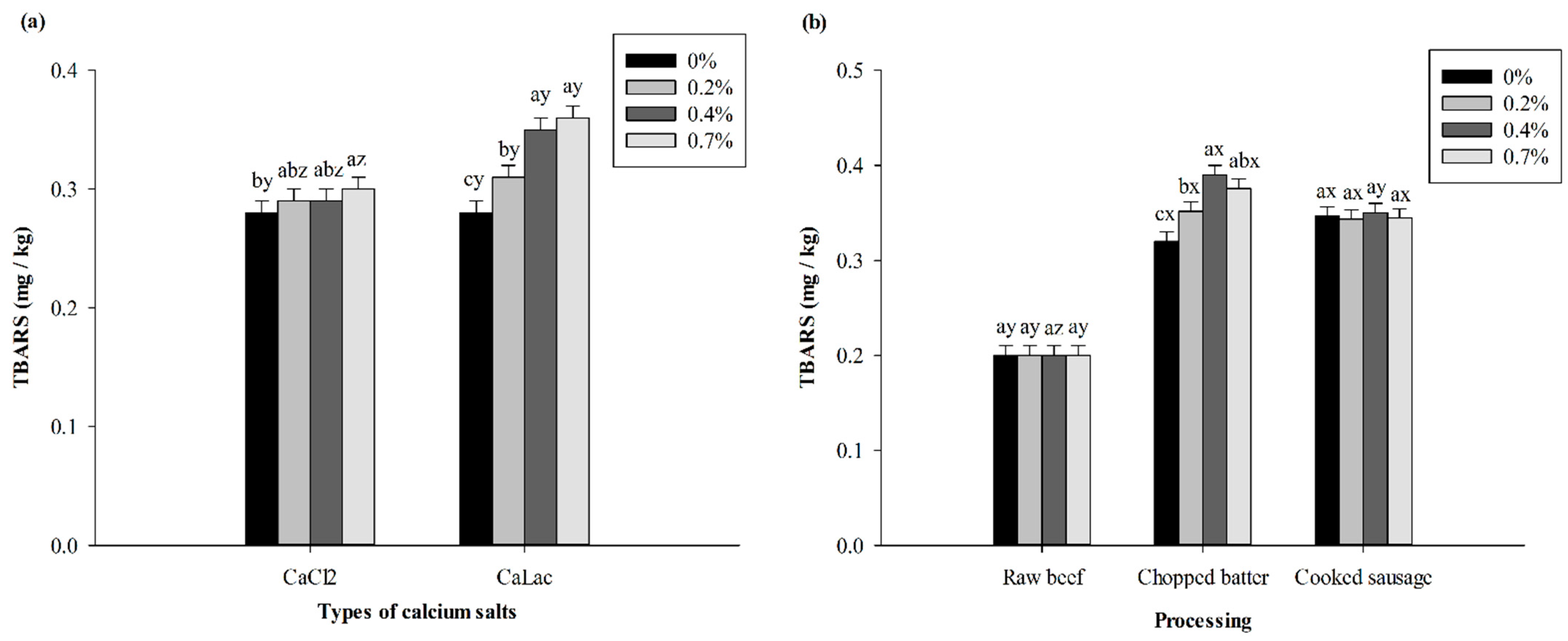
2.2.4. Thiobarbituric Reactive Substances (TBARS)
2.2.5. Residual Nitrite
2.2.6. Measurement of Nitrosylhemochrome (NO-heme)
2.2.7. Texture Analysis (TPA)
2.3. Statistical Analysis
3. Results and Discussion
3.1. Experiment 1: Effects of Calcium Salts on the Quality of Beef Sausages during Processing
3.1.1. pH and Cooking Loss Changes
3.1.2. Color Analysis
3.1.3. Lipid Oxidation
3.2. Experiment 2: Effects of Calcium Salts on the Quality of Beef Sausages during Storage
3.2.1. pH Changes
3.2.2. Color Analysis
3.2.3. TBARS Values and Residual Nitrite
3.2.4. Texture-profile Analysis (TPA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kameník, J. Co-extrusion technology for comminuted meat products: Alginate as a “casing” of casingless meat products. Maso Int. 2014, 1, 49–51. [Google Scholar]
- Suurs, P.; Barbut, S. Collagen use for co-extruded sausage casings—A review. Trends Food Sci. Technol. 2020, 102, 91–101. [Google Scholar] [CrossRef]
- Harper, B.A.; Barbut, S.; Lim, L.T.; Marcone, M.F. Characterization of ‘wet’ alginate and composite films containing gelatin, whey or soy protein. Food Res. Int. 2013, 52, 452–459. [Google Scholar] [CrossRef]
- Suman, S.P.; Nair, M.N.; Joseph, P.; Hunt, M.C. Factors influencing internal color of cooked meats. Meat Sci. 2016, 120, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Hilbig, J.; Hartlieb, K.; Herrmann, K.; Weiss, J.; Gibis, M. Influence of calcium on white efflorescence formation on dry fermented sausages with co-extruded alginate casings. Food Res. Int. 2020, 131, 109012. [Google Scholar] [CrossRef] [PubMed]
- Wenther, J.B.; Wachholtz, G. Co-extrusion systems using alginate. In Proceedings of the AMSA 67th RMC, Madison, WI, USA, 15–18 June 2014. [Google Scholar]
- Cáceres, E.; Garcia, M.L.; Selgas, M.D. Design of a new cooked meat sausage enriched with calcium. Meat Sci. 2006, 73, 368–377. [Google Scholar] [CrossRef]
- Daengprok, W.; Garnjanagoonchorn, W.; Mine, Y. Fermented pork sausage fortified with commercial or hen eggshell calcium lactate. Meat Sci. 2002, 62, 199–204. [Google Scholar] [CrossRef]
- Kim, G.D.; Hur, S.J.; Park, T.S.; Jin, S.K. Quality characteristics of fat-reduced emulsion-type pork sausage by partial substitution of sodium chloride with calcium chloride, potassium chloride and magnesium chloride. LWT Food Sci. Technol. 2018, 89, 140–147. [Google Scholar] [CrossRef]
- Bunmee, T.; Jaturasitha, S.; Kreuzer, M.; Wicke, M. Can calcium chloride injection facilitate the ageing-derived improvement in the quality of meat from culled dairy cows? Meat Sci. 2014, 96, 1440–1445. [Google Scholar] [CrossRef]
- Jaturasitha, S.; Thirawong, P.; Leangwunta, V.; Kreuzer, M. Reducing toughness of beef from Bos indicus draught steers by injection of calcium chloride: Effect of concentration and time postmortem. Meat Sci. 2004, 68, 61–69. [Google Scholar] [CrossRef]
- Lawrence, T.E.; Dikeman, M.E.; Hunt, M.C.; Kastner, C.L.; Johnson, D.E. Effects of calcium salts on beef longissimus quality. Meat Sci. 2003, 64, 299–308. [Google Scholar] [CrossRef]
- Lawrence, T.E.; Dikeman, M.E.; Hunt, M.C.; Kastner, C.L.; Johnson, D.E. Effects of enhancing beef longissimus with phosphate plus salt, or calcium lactate plus non-phosphate water binders plus rosemary extract. Meat Sci. 2004, 67, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Seyfert, M.; Hunt, M.C.; Ahnström, M.L.; Johnson, D.E. Efficacy of lactic acid salts and sodium acetate on ground beef colour stability and metmyoglobin-reducing activity. Meat Sci. 2007, 75, 134–142. [Google Scholar] [CrossRef]
- Irshad, A.; Sharma, B.D.; Ahmed, S.R.; Talukder, S.; Malav, O.P.; Kumar, A. Effect of incorporation of calcium lactate on physico-chemical, textural, and sensory properties of restructured buffalo meat loaves. Vet World 2016, 9, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.G.; Marwan, A.; Samaraweera, H.; Lee, E.J.; Ahn, D.U. Breast meat quality of broiler chickens can be affected by managing the level of nitric oxide. Poultry Sci. 2013, 92, 3044–3049. [Google Scholar] [CrossRef]
- Patton, B.A. Evaluation of Celery Powder and Cherry Powder as Alternatives to Sodium Nitrite and Sodium Erythorbate in Restructured Beef Jerky. Ph.D. Thesis, Angelo State University, San Angelo, TX, USA, 2018. [Google Scholar]
- Hornsey, H.C. The colour of cooked cured pork. J. Sci. Food Agric. 1956, 7, 534–540. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Hou, Q.; Zhuang, X.B.; Wang, Y.; Zhou, G.G.; Zhang, W.G. Effect of regenerated cellulose fiber on the physicochemical properties and sensory characteristicts of fat-reduced emulsified sausage. LWT Food Sci. Technol. 2018, 97, 157–163. [Google Scholar] [CrossRef]
- Horita, C.N.; Morgano, M.A.; Celeghini, R.M.S.; Pollonio, M.A.R. Physicochemical and sensory properties of reduced-fat mortadella prepared with blends of calcium, magnesium and potassium chloride as partial substitutes for sodium chloride. Meat Sci. 2011, 89, 426–433. [Google Scholar] [CrossRef]
- Laakkonen, E.; Wellington, G.H.; Sherbon, J.N. Low-temperature, long-time heating of bovine muscle 1. Changes in tenderness, water-binding capacity, pH and amount of water-soluble components. J. Food Sci. 1970, 35, 175–177. [Google Scholar] [CrossRef]
- Hamm, R. Biochemistry of meat hydration. In Advances in Food Research; Academic Press: Kulmbach, Germany, 1961; Volume 10, pp. 355–463. [Google Scholar]
- Naveena, B.M.; Sen, A.R.; Muthukumar, M.; Vaithiyanathan, S.; Babji, Y. The effect of lactates on the quality of microwave-cooked chicken patties during storage. J. Food Sci. 2006, 71, S603–S608. [Google Scholar] [CrossRef]
- AMSA. Meat Color Measurement Guidelines; American Meat Science Association (AMSA): Champaign, IL, USA, 2012. [Google Scholar]
- Honikel, H.O. The use and control of nitrate and nitrite for processing of meat products. Meat Sci. 2008, 78, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, T.L.; Koohmaraie, M.; Lansdell, J.L.; Siragusa, G.R.; Miller, M.F. Effects of postmortem injection time, injection level, and concentration of calcium chloride on beef quality traits. J. Anim. Sci. 1993, 71, 2965–2974. [Google Scholar] [CrossRef] [Green Version]
- Devatkal, S.; Mendiratta, S.K. Use of calcium lactate with salt-phosphate and alginate-calcium gels in restructured pork rolls. Meat Sci. 2001, 58, 371–379. [Google Scholar] [CrossRef]
- Walsh, K.A.; Rose, D. Meat pigments, factors affecting the oxidation of nitric oxide myoglobin. J. Agric. Food Chem. 1956, 4, 352–355. [Google Scholar] [CrossRef]
- Boyle, E.A.E.; Addis, P.B.; Epley, R.J. Calcium fortified reduced fat beef emulsion product. J. Food Sci. 1994, 59, 928–932. [Google Scholar] [CrossRef]
- Yu, C.S.; Jiao, J.Z.; Ma, L.Z.; Sun, W.Q. Effect of pH on the stability and molecular structure of nitrosylhemochromogen. Food Chem. 2016, 196, 503–508. [Google Scholar] [CrossRef]
- Mariutti, L.R.; Bragagnolo, N. Influence of salt on lipid oxidation in meat and seafood products: A review. Food Res. Int. 2017, 94, 90–100. [Google Scholar] [CrossRef]
- Hopkins, D.L.; Ponnampalam, E.N.; Van De Ven, R.J.; Warner, R.D. The effect of pH decline rate on the meat and eating quality of beef carcasses. Anim. Prod. Sci. 2014, 54, 407–413. [Google Scholar] [CrossRef]
- Hamm, R. Functional properties of the myofibrillar system and their measurements. In Muscle as Food; Bechtel, P.J., Ed.; Academic Press: Orlando, FL, USA, 1986; pp. 135–199. [Google Scholar]
- Damodaran, S. Amino acids, peptides and proteins. In Fennema Food Chemistry; Damodaran, S., Parkin, K.L., Fenemma, O.R., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 217–329. [Google Scholar]
- Horita, C.N.; Messias, V.C.; Morgano, M.A.; Hayakawa, F.M.; Pollonio, M.A.R. Textural, microstructural and sensory properties of reduced sodium frankfurter sausages containing mechanically deboned poultry meat and blends of chloride salts. Food Res. Int. 2014, 66, 29–35. [Google Scholar] [CrossRef] [Green Version]


| Calcium Salts | Processing | Salt Concentrations (%) | SE e | p-Value | |||
|---|---|---|---|---|---|---|---|
| 0 | 0.2 | 0.4 | 0.7 | ||||
| CaCl2 | Ground beef | 5.63 aly | 5.63 alx | 5.63 alx | 5.63 alx | 0.01 | <0.001 |
| Chopped batter | 5.55 alz | 5.50 bmz | 5.49 bmy | 5.41 cmy | |||
| Cooked sausage | 5.83 alx | 5.56 bmy | 5.46 cmz | 5.31 dmz | |||
| CaLac | Ground beef | 5.63 aly | 5.63 aly | 5.63 aly | 5.63 alx | ||
| Chopped batter | 5.55 alz | 5.52 blz | 5.51 blz | 5.50 clz | |||
| Cooked sausage | 5.83 alx | 5.83 alx | 5.69 blx | 5.54 cly | |||
| Traits | Calcium Salts | Processing | Salt Concentrations (%) | SE d | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.2 | 0.4 | 0.7 | |||||
| L* | CaCl2 | Ground beef | 51.61 alz | 51.61 alz | 51.61 alz | 51.61 alz | 0.33 | 0.007 |
| Chopped batter | 56.48 aly | 56.18 amy | 56.04 aly | 56.08 aly | ||||
| Cooked sausage | 62.33 bclx | 63.30 alx | 62.92 ablx | 61.95 clx | ||||
| CaLac | Ground beef | 51.61 alz | 51.61 alz | 51.61 alz | 51.61 alz | |||
| Chopped batter | 56.48 bly | 57.30 aly | 55.47 cly | 55.79 bcly | ||||
| Cooked sausage | 62.33 ablx | 61.82 bmx | 62.81 alx | 62.21 ablx | ||||
| a* | CaCl2 | Ground beef | 24.43 alx | 24.43 alx | 24.43 alx | 24.43 alx | 0.19 | <0.001 |
| Chopped batter | 9.07 alz | 8.34 cmz | 8.42 bclz | 8.77 ablz | ||||
| Cooked sausage | 13.55 aly | 13.40 aly | 12.57 bmy | 11.48 cmy | ||||
| CaLac | Ground beef | 24.43 alx | 24.43 alx | 24.43 alx | 24.43 alx | |||
| Chopped batter | 9.07 alz | 8.85 alz | 7.96 bmz | 7.66 bmz | ||||
| Cooked sausage | 13.55 aly | 13.65 aly | 13.41 aly | 13.40 aly | ||||
| b* | CaCl2 | Ground beef | 24.70 alx | 24.70 alx | 24.70 alx | 24.70 alx | 0.22 | 0.020 |
| Chopped batter | 20.21 aly | 19.75 aly | 19.96 aly | 19.75 aly | ||||
| Cooked sausage | 12.40 alz | 12.45 alz | 12.34 alz | 12.37 alz | ||||
| CaLac | Ground beef | 24.70 alx | 24.70 alx | 24.70 alx | 24.70 alx | |||
| Chopped batter | 20.21 bly | 20.11 bly | 18.85 amy | 18.61 amy | ||||
| Cooked sausage | 12.40 alz | 12.53 alz | 12.47 alz | 12.32 alz | ||||
| Chroma | CaCl2 | Ground beef | 34.74 alx | 34.74 alx | 34.74 alx | 34.74 alx | 0.28 | <0.001 |
| Chopped batter | 22.15 aly | 21.44 bly | 21.66 bly | 21.61 bly | ||||
| Cooked sausage | 18.37 alz | 18.29 alz | 17.62 bmz | 16.88 cmz | ||||
| CaLac | Ground beef | 34.74 alx | 34.74 alx | 34.74 alx | 34.74 alx | |||
| Chopped batter | 22.15 aly | 21.97 aly | 20.47 bmy | 20.12 bmy | ||||
| Cooked sausage | 18.37 alz | 18.53 alz | 18.32 alz | 18.21 alz | ||||
| Hue | CaCl2 | Ground beef | 45.31 aly | 45.31 aly | 45.31 aly | 45.31 alz | 0.23 | <0.001 |
| Chopped batter | 65.85 blx | 67.10 alx | 67.14 alx | 66.08 bmx | ||||
| Cooked sausage | 42.46 clz | 42.89 clz | 44.50 blz | 47.18 aly | ||||
| CaLac | Ground beef | 45.31 aly | 45.31 aly | 45.31 aly | 45.31 aly | |||
| Chopped batter | 65.85 blx | 66.25 bmx | 67.11 alx | 67.63 alx | ||||
| Cooked sausage | 42.46 alz | 42.55 alz | 42.92 amz | 42.60 amz | ||||
| Traits | Calcium Salts | Storage Time | Salt Concentrations (%) | SE e | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.2 | 0.4 | 0.7 | |||||
| L* | CaCl2 | Day0 | 62.33 clz | 63.30 alz | 62.92 blz | 61.95 dlz | 0.12 | <0.001 |
| Day7 | 64.05 bly | 64.20 bly | 64.65 aly | 64.11 bly | ||||
| CaLac | Day0 | 62.33 blz | 61.82 cmz | 62.81 alz | 62.21 blz | |||
| Day7 | 64.05 bly | 63.79 bmy | 64.63 aly | 63.78 bmy | ||||
| a* | CaCl2 | Day0 | 13.55 aly | 13.40 amy | 12.57 bmz | 11.48 cmz | 0.09 | 0.047 |
| Day7 | 13.76 aly | 13.63 amy | 13.11 bmy | 12.33 cmy | ||||
| CaLac | Day0 | 13.55 alz | 13.65 alz | 13.41 alz | 13.40 alz | |||
| Day7 | 13.76 ably | 13.92 aly | 13.87 ably | 13.65 bly | ||||
| b* | CaCl2 | Day0 | 12.40 alz | 12.45 aly | 12.35 aly | 12.37 aly | 0.05 | 0.012 |
| Day7 | 12.62 aly | 12.49 abmy | 12.41 bmy | 12.26 cmy | ||||
| CaLac | Day0 | 12.40 ablz | 12.53 alz | 12.47 alz | 12.32 blz | |||
| Day7 | 12.62 bly | 12.77 aly | 12.78 aly | 12.68 ably | ||||
| Chroma | CaCl2 | Day0 | 18.37 | 18.29 | 17.62 | 16.88 | 0.06 | |
| Day7 | 18.67 | 18.49 | 18.05 | 17.39 | ||||
| Means | 18.52 al | 18.39 am | 17.84 bm | 17.14 cm | <0.001 | |||
| CaLac | Day0 | 18.37 | 18.53 | 18.32 | 18.21 | |||
| Day7 | 18.67 | 18.89 | 18.71 | 18.80 | ||||
| Means | 18.52 bl | 18.71 al | 18.52 bl | 18.51 bl | 0.06 | <0.001 | ||
| Hue | CaCl2 | Day0 | 42.46 cly | 42.89 cly | 44.50 bly | 47.18 aly | 0.21 | 0.002 |
| Day7 | 42.53 cly | 42.51 cly | 43.43 blz | 44.83 alz | ||||
| CaLac | Day0 | 42.46 aly | 42.55 aly | 42.92 amy | 42.60 amy | |||
| Day7 | 42.53 bly | 42.53 bly | 43.12 aly | 42.44 bmy | ||||
| Traits | Calcium Salts | Storage Time | Salt Concentrations (%) | SE c | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.2 | 0.4 | 0.7 | |||||
| TBARS (mg MDA/kg) | CaCl2 | Day0 | 0.35 aly | 0.33 aly | 0.30 amy | 0.32 alz | 0.02 | 0.037 |
| Day7 | 0.31 bly | 0.30 bly | 0.34 bly | 0.42 aly | ||||
| CaLac | Day0 | 0.35 aly | 0.35 aly | 0.35 aly | 0.37 aly | |||
| Day7 | 0.31 aly | 0.32 aly | 0.33 aly | 0.32 amz | ||||
| Residual nitrite (mg/kg) | CaCl2 | Day0 | 60.61 aly | 40.49 bmy | 24.84 cmy | 13.77 dmy | 1.10 | 0.014 |
| Day7 | 52.60 alz | 32.65 bmz | 16.73 cmz | 12.29 dmy | ||||
| CaLac | Day0 | 60.61 aly | 52.81 bly | 42.94 cly | 28.86 dly | |||
| Day7 | 52.60 alz | 47.82 blz | 36.78 clz | 22.10 dlz | ||||
| Traits | Calcium Salts | Salt Concentrations (%) | SE e | p-Value | |||
|---|---|---|---|---|---|---|---|
| 0 | 0.2 | 0.4 | 0.7 | ||||
| Hardness (N) | CaCl2 | 70.48 dy | 83.05 cy | 94.03 by | 111.79 ay | 1.79 | <0.001 |
| CaLac | 70.48 cy | 80.63 by | 79.89 bz | 98.58 az | |||
| Springiness (mm) | CaCl2 | 0.86 cy | 0.87 by | 0.88 by | 0.89 ay | <0.01 | 0.001 |
| CaLac | 0.86 ay | 0.87 ay | 0.86 az | 0.87 az | |||
| Cohesiveness | CaCl2 | 0.54 dy | 0.57 cy | 0.63 by | 0.69 ay | <0.01 | <0.001 |
| CaLac | 0.55 cy | 0.57 aby | 0.56 bcz | 0.59 az | |||
| Gumminess (N) | CaCl2 | 39.47 dy | 48.05 cy | 58.97 by | 77.84 ay | 1.44 | <0.001 |
| CaLac | 39.47 cy | 47.77 by | 45.84 bz | 60.32 az | |||
| Chewiness (N) | CaCl2 | 34.56 dy | 41.93 cy | 52.07 by | 69.31 ay | 1.22 | <0.001 |
| CaLac | 34.56 dy | 41.95 by | 38.80 cz | 53.37 az | |||
| Resilience | CaCl2 | 0.25 dy | 0.26 cy | 0.30 by | 0.35 ay | <0.01 | <0.001 |
| CaLac | 0.25 cy | 0.26 aby | 0.26 bz | 0.27 az | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Sebranek, J.G.; Luo, X.; Zhang, W.; Zhang, M.; Xu, B.; Zhang, Y.; Liang, R. Effects of Calcium Salts on the Physicochemical Quality of Cured Beef Sausages during Manufacturing and Storage: A Potential Calcium Application for Sausages with Alginate Casings. Foods 2021, 10, 2783. https://doi.org/10.3390/foods10112783
Yang X, Sebranek JG, Luo X, Zhang W, Zhang M, Xu B, Zhang Y, Liang R. Effects of Calcium Salts on the Physicochemical Quality of Cured Beef Sausages during Manufacturing and Storage: A Potential Calcium Application for Sausages with Alginate Casings. Foods. 2021; 10(11):2783. https://doi.org/10.3390/foods10112783
Chicago/Turabian StyleYang, Xiaoyin, Joseph G. Sebranek, Xin Luo, Wangang Zhang, Mengmeng Zhang, Baochen Xu, Yimin Zhang, and Rongrong Liang. 2021. "Effects of Calcium Salts on the Physicochemical Quality of Cured Beef Sausages during Manufacturing and Storage: A Potential Calcium Application for Sausages with Alginate Casings" Foods 10, no. 11: 2783. https://doi.org/10.3390/foods10112783
APA StyleYang, X., Sebranek, J. G., Luo, X., Zhang, W., Zhang, M., Xu, B., Zhang, Y., & Liang, R. (2021). Effects of Calcium Salts on the Physicochemical Quality of Cured Beef Sausages during Manufacturing and Storage: A Potential Calcium Application for Sausages with Alginate Casings. Foods, 10(11), 2783. https://doi.org/10.3390/foods10112783