Crystallization Behavior and Quality of Frozen Meat
Abstract
1. Introduction
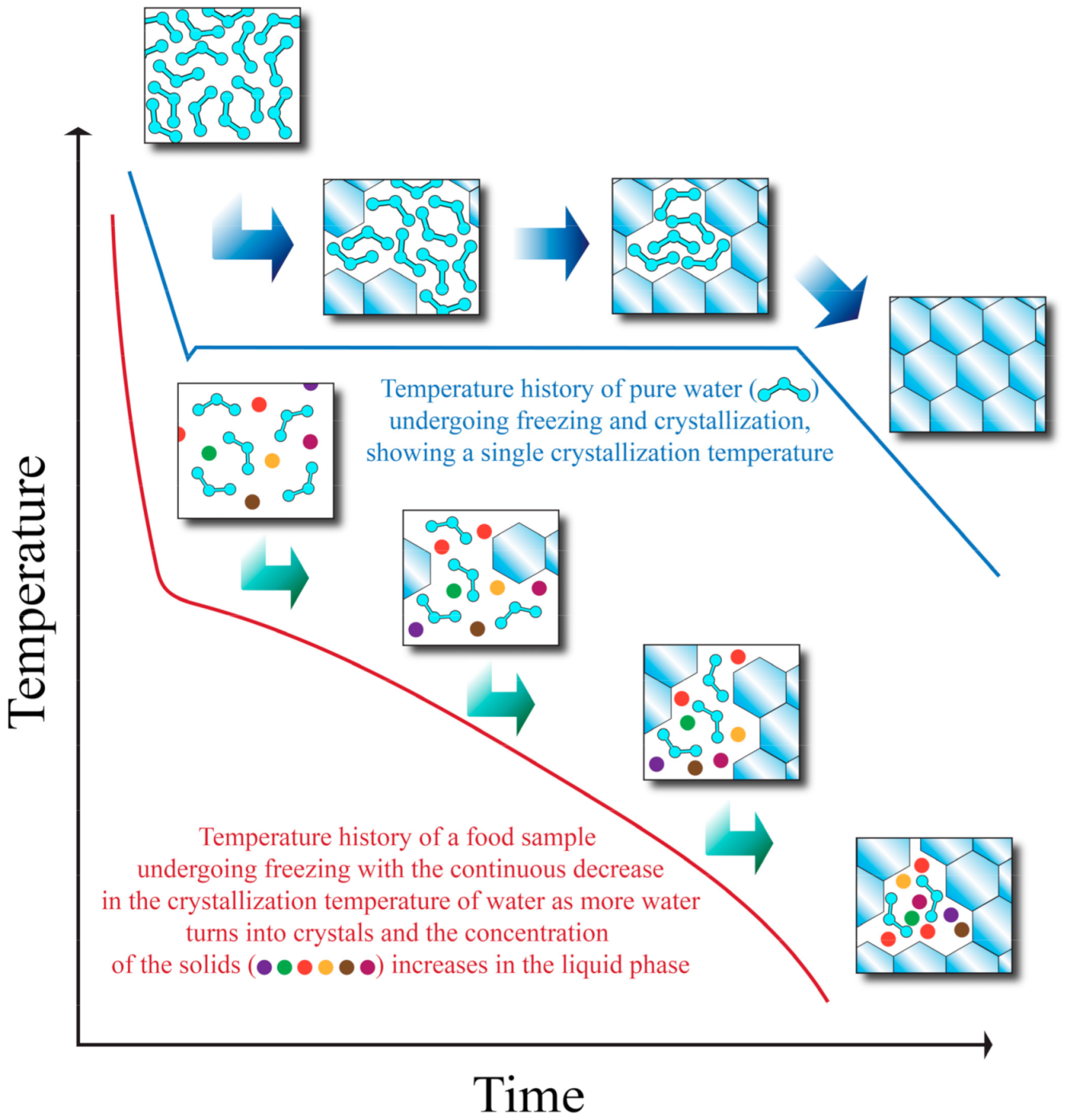
2. Freezing and Crystallization Behavior of Water
3. Energy Balance and Heat Transfer during Water Crystallization in Meat
3.1. Energy Balance
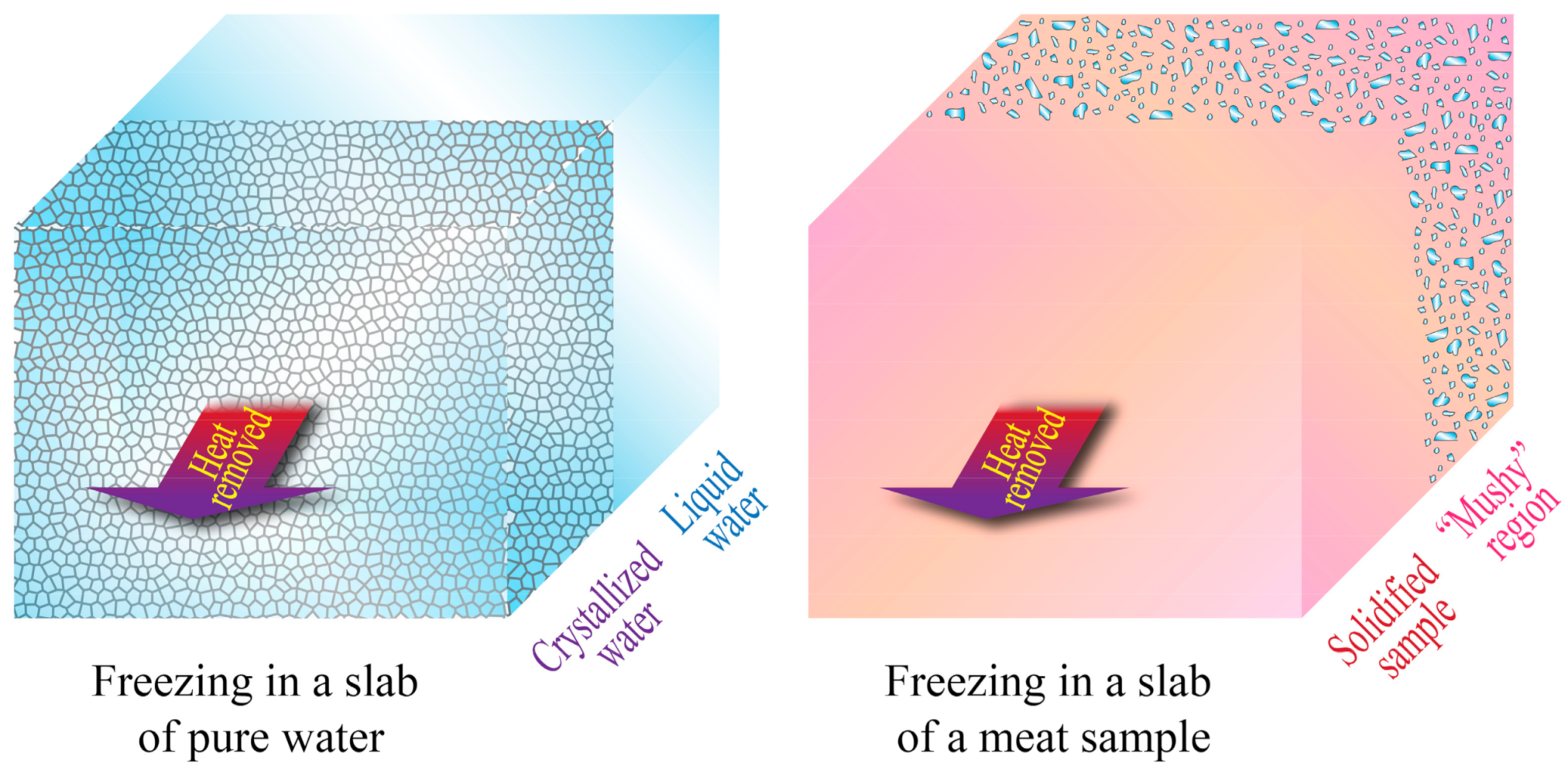
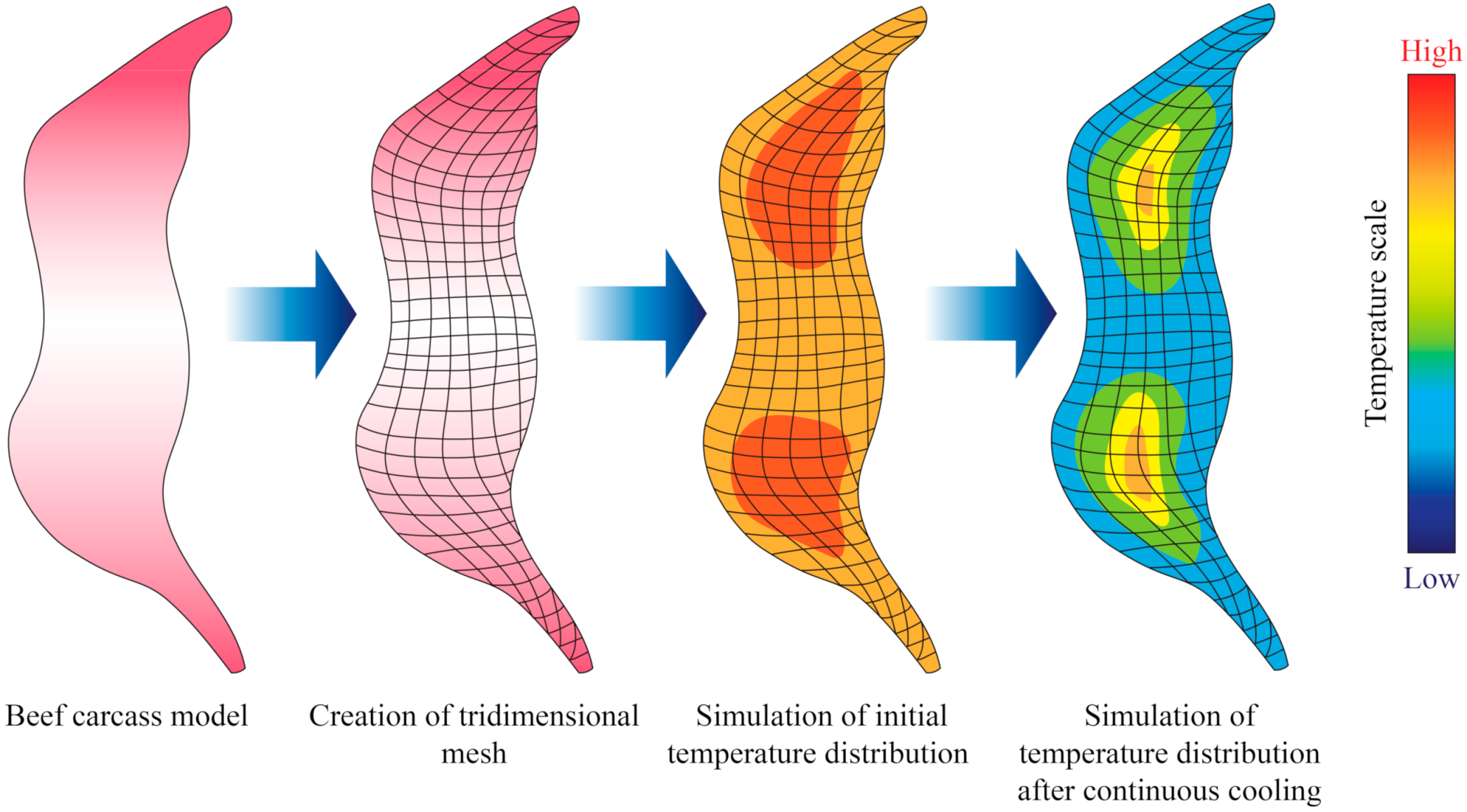
3.2. Heat Transfer and Water Crystallization in Meat
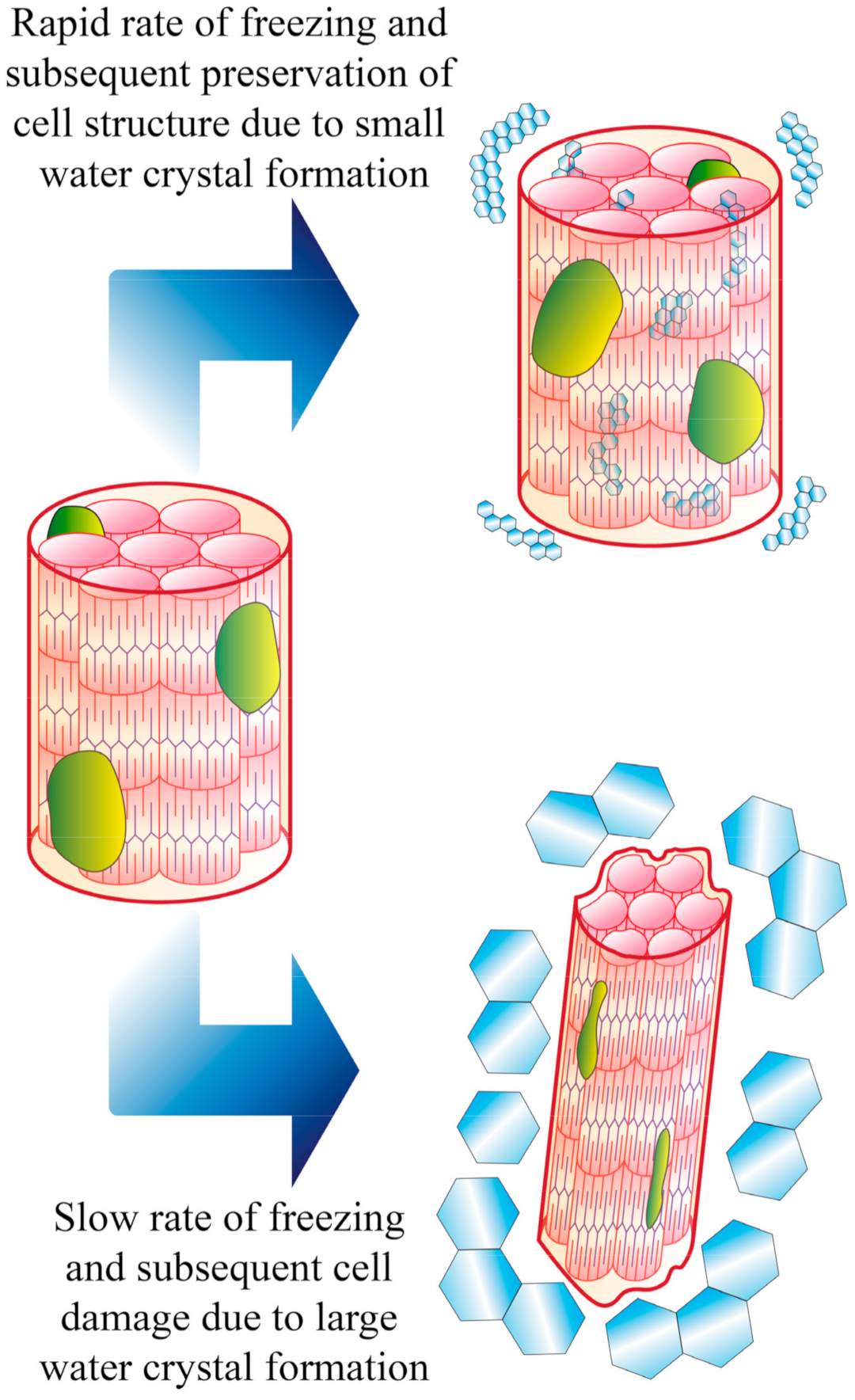
4. Freezing as a Form of Meat Preservation
5. Aspects of Meat Quality in Relation to Freezing
5.1. Tenderness
5.2. Water Holding Capacity
5.3. Color
5.4. Flavor
6. Methods for Mitigating the Effects of Freezing and Thawing of Meat Products
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Harkouss, R.; Astruc, T.; Lebert, A.; Gatellier, P.; Loison, O.; Safa, H.; Portanguen, S.; Parafita, E.; Mirade, P.S. Quantitative study of the relationships among proteolysis, lipid oxidation, structure and texture throughout the dry-cured ham process. Food Chem. 2015, 166, 522–530. [Google Scholar] [CrossRef]
- Vignolo, G.; Fontana, C.; Fadda, S. Semidry and Dry Fermented Sausages. In Handbook of Meat Processing; Toldra, F., Ed.; Wiley: London, UK, 2010; pp. 379–398. [Google Scholar]
- Grasso, S.; Brunton, N.; Lyng, J.; Lalor, F.; Monahan, F. Healthy processed meat products–Regulatory, reformulation and consumer challenges. Trends Food Sci. Technol. 2014, 39, 4–17. [Google Scholar] [CrossRef]
- Hammad, H.; Ma, M.; Damaka, A.; Elkhedir, A.; Jin, G. Effect of freeze and re-freeze on chemical composition of beef and poultry meat at storage period 4.5 months (SP4. 5). J. Food Process. Technol. 2019, 10, 2. [Google Scholar]
- Dave, D.; Ghaly, A.E. Meat spoilage mechanisms and preservation techniques: A critical review. Am. J. Agric. Biol. Sci. 2011, 6, 486–510. [Google Scholar]
- Koutsoumanis, K.; Stamatiou, A.; Skandamis, P.; Nychas, G.J. Development of a microbial model for the combined effect of temperature and pH on spoilage of ground meat, and validation of the model under dynamic temperature conditions. Appl. Environ. Microbiol. 2006, 72, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Impact of freezing and thawing on the quality of meat. Meat Sci. 2012, 91, 93–98. [Google Scholar] [CrossRef]
- Rahman, M.S.; Velez-Ruiz, J.F. Food Preservation by Freezing. In Handbook of Food Preservation; CRC Press: Boca Raton, FL, USA, 2007; pp. 653–684. [Google Scholar]
- Kiani, H.; Sun, D.W. Water crystallization and its importance to freezing of foods: A review. Trends Food Sci. Technol. 2011, 22, 407–426. [Google Scholar] [CrossRef]
- Fletcher, N.H. Active sites and ice crystal nucleation. J. Atmos. Sci. 1969, 26, 1266–1271. [Google Scholar] [CrossRef]
- Botsaris, G.D. Secondary nucleation—A review. Ind. Cryst. 1976, 1, 3–22. [Google Scholar]
- Mullin, J.W. Crystallization; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Mersmann, A. Crystallization Technology Handbook; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Delgado, A.; Sun, D.W. Heat and mass transfer models for predicting freezing processes—A review. J. Food Eng. 2001, 47, 157–174. [Google Scholar] [CrossRef]
- Cleland, D.; Cleland, A.; Earle, R.; Byrne, S. Prediction of rates of freezing, thawing or cooling in solids or arbitrary shape using the finite element method. Int. J. Refrig. 1984, 7, 6–13. [Google Scholar] [CrossRef]
- Hossain, M.M.; Cleland, D.; Cleland, A. Prediction of freezing and thawing times for foods of regular multi-dimensional shape by using an analytically derived geometric factor. Int. J. Refrig. 1992, 15, 227–234. [Google Scholar] [CrossRef]
- Eek, L. A Convenience Born of Necessity: The Growth of the Modern Food Freezing Industry. In Food Freezing; Springer: Berlin/Heidelberg, Germany, 1991; pp. 143–155. [Google Scholar]
- Chau, K.; Gaffney, J. A finite difference model for heat and mass transfer in products with internal heat generation and transpiration. J. Food Sci. 1990, 55, 484–487. [Google Scholar] [CrossRef]
- Hayakawa, K.I.; Succar, J. Heat transfer and moisture loss of spherical fresh produce. J. Food Sci. 1982, 47, 596–605. [Google Scholar] [CrossRef]
- Tocci, A.; Mascheroni, R. Numerical models for the simulation of the simultaneous heat and mass transfer during food freezing and storage. Int. Commun. Heat Mass Transf. 1995, 22, 251–260. [Google Scholar] [CrossRef]
- Heldman, D.; Singh, R. Food Process Engineering; The AVI Pub. Co. Inc.: Westport, CT, USA, 1981. [Google Scholar]
- Singh, R.P.; Heldman, D.R. Introduction to Food Engineering; Gulf Professional Publishing: Houston, TX, USA, 2001. [Google Scholar]
- Singh, R.P.; Heldman, D.R. Introduction to Food Engineering, 5th ed.; Gulf Professional Publishing: Houston, TX, USA, 2014. [Google Scholar]
- Toledo, R.T.; Singh, R.K.; Kong, F. Fundamentals of Food Process Engineering; Springer: Berlin/Heidelberg, Germany, 2007; Volume 297. [Google Scholar]
- Earle, R.L. Unit Operations in Food Processing; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Datta, A.K. Biological and Bioenvironmental Heat and Mass Transfer; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Tamilmani, P.; Pandey, M.C. Thermal analysis of meat and meat products. J. Therm. Anal. Calorim. 2016, 123, 1899–1917. [Google Scholar] [CrossRef]
- Zhao, Y.; Takhar, P.S. Freezing of foods: Mathematical and experimental aspects. Food Eng. Rev. 2017, 9, 1–12. [Google Scholar] [CrossRef]
- Hobani, A.I.; Elansari, A.M. Effect of temperature and moisture content on thermal properties of four types of meat Part Two: Specific heat & enthalpy. Int. J. Food Prop. 2008, 11, 571–584. [Google Scholar]
- Cengel, Y.; Heat, T.M. A Practical Approach; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Becker, B.R.; Fricke, B.A. Evaluation of semi-analytical/empirical freezing time estimation methods part II: Irregularly shaped food items. HVAC&R Res. 1999, 5, 171–187. [Google Scholar]
- Erdogdu, F.; Sarghini, F.; Marra, F. Mathematical modeling for virtualization in food processing. Food Eng. Rev. 2017, 9, 295–313. [Google Scholar] [CrossRef]
- Fadiji, T.; Ashtiani, S.H.M.; Onwude, D.I.; Li, Z.; Opara, U.L. Finite Element Method for Freezing and Thawing Industrial Food Processes. Foods 2021, 10, 869. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.W.; Zhu, X. Effect of heat transfer direction on the numerical prediction of beef freezing processes. J. Food Eng. 1999, 42, 45–50. [Google Scholar] [CrossRef]
- Delgado, A.E.; Sun, D.W. One-dimensional finite difference modelling of heat and mass transfer during thawing of cooked cured meat. J. Food Eng. 2003, 57, 383–389. [Google Scholar] [CrossRef]
- Trujillo, F.J.; Pham, Q.T. A computational fluid dynamic model of the heat and moisture transfer during beef chilling. Int. J. Refrig. 2006, 29, 998–1009. [Google Scholar] [CrossRef][Green Version]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Modern Food Microbiology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Sofos, J.N. Challenges to meat safety in the 21st century. Meat Sci. 2008, 78, 3–13. [Google Scholar] [CrossRef]
- Podpečan, B.; Pengov, A.; Vadnjal, S. The source of contamination of ground meat for production of meat products with bacteria Staphylococcus aureus. Slov. Vet. Res. 2007, 44, 25–30. [Google Scholar]
- Singha, P.; Muthukumarappan, K. Quality changes and freezing time prediction during freezing and thawing of ginger. Food Sci. Nutr. 2016, 4, 521–533. [Google Scholar] [CrossRef]
- Greer, G.; Murray, A. Freezing effects on quality, bacteriology and retail—Case life of pork. J. Food Sci. 1991, 56, 891–894. [Google Scholar] [CrossRef]
- Müller, K.; Aabo, S.; Birk, T.; Mordhorst, H.; Bjarnadóttir, B.; Agersø, Y. Survival and growth of epidemically successful and nonsuccessful Salmonella enterica clones after freezing and dehydration. J. Food Prot. 2012, 75, 456–464. [Google Scholar] [CrossRef]
- Eastridge, J.S.; Bowker, B.C. Effect of rapid thawing on the meat quality attributes of USDA select beef strip loin steaks. J. Food Sci. 2011, 76, S156–S162. [Google Scholar] [CrossRef] [PubMed]
- Huff-Lonergan, E.; Sosnicki, A. Water-holding capacity of fresh meat. Fact Sheet 2002, 4669, 1–8. [Google Scholar]
- Wang, Y.; Liang, H.; Xu, R.; Lu, B.; Song, X.; Liu, B. Effects of temperature fluctuations on the meat quality and muscle microstructure of frozen beef. Int. J. Refrig. 2020, 116, 1–8. [Google Scholar] [CrossRef]
- Schudel, S.; Prawiranto, K.; Defraeye, T. Comparison of freezing and convective dehydrofreezing of vegetables for reducing cell damage. J. Food Eng. 2021, 293, 110376. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, J.H.; Seo, J.K.; Setyabrata, D.; Kim, Y.H.B. Effects of aging/freezing sequence and freezing rate on meat quality and oxidative stability of pork loins. Meat Sci. 2018, 139, 162–170. [Google Scholar] [CrossRef]
- Hou, Q.; Cheng, Y.p.; Kang, D.c.; Zhang, W.g.; Zhou, G.h. Quality changes of pork during frozen storage: Comparison of immersion solution freezing and air blast freezing. Int. J. Food Sci. Technol. 2020, 55, 109–118. [Google Scholar] [CrossRef]
- Mortensen, M.; Andersen, H.J.; Engelsen, S.B.; Bertram, H.C. Effect of freezing temperature, thawing and cooking rate on water distribution in two pork qualities. Meat Sci. 2006, 72, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Lagerstedt, Å.; Enfält, L.; Johansson, L.; Lundström, K. Effect of freezing on sensory quality, shear force and water loss in beef M. longissimus dorsi. Meat Sci. 2008, 80, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kang, S.M.; Seong, P.; Kang, G.; Kim, Y.; Kim, J.; Chang, S.; Park, B. Effect of aging and freezing conditions on meat quality and storage stability of 1++ grade Hanwoo steer beef: Implications for shelf life. Korean J. Food Sci. Anim. Resour. 2017, 37, 440. [Google Scholar] [CrossRef]
- Kim, Y.H.B.; Liesse, C.; Kemp, R.; Balan, P. Evaluation of combined effects of ageing period and freezing rate on quality attributes of beef loins. Meat Sci. 2015, 110, 40–45. [Google Scholar] [CrossRef]
- Vieira, C.; Diaz, M.; Martínez, B.; García-Cachán, M. Effect of frozen storage conditions (temperature and length of storage) on microbiological and sensory quality of rustic crossbred beef at different states of ageing. Meat Sci. 2009, 83, 398–404. [Google Scholar] [CrossRef]
- Balan, P.; Kim, Y.H.B.; Stuart, A.D.; Kemp, R.; Staincliffe, M.; Craigie, C.; Farouk, M.M. Effect of fast freezing then thaw-aging on meat quality attributes of lamb M. longissimus lumborum. Anim. Sci. J. 2019, 90, 1060–1069. [Google Scholar] [CrossRef]
- Muela, E.; Sañudo, C.; Campo, M.; Medel, I.; Beltrán, J. Effect of freezing method and frozen storage duration on lamb sensory quality. Meat Sci. 2012, 90, 209–215. [Google Scholar] [CrossRef]
- Choe, J.H.; Stuart, A.; Kim, Y.H.B. Effect of different aging temperatures prior to freezing on meat quality attributes of frozen/thawed lamb loins. Meat Sci. 2016, 116, 158–164. [Google Scholar] [CrossRef]
- Kim, H.-W.; Miller, D.K.; Yan, F.; Wang, W.; Cheng, H.-W.; Kim, Y.H.B. Probiotic supplementation and fast freezing to improve quality attributes and oxidation stability of frozen chicken breast muscle. LWT 2017, 75, 34–41. [Google Scholar] [CrossRef]
- Ab Aziz, M.F.; Hayat, M.N.; Kaka, U.; Kamarulzaman, N.H.; Sazili, A.Q. Physico-chemical characteristics and microbiological quality of broiler chicken pectoralis major muscle subjected to different storage temperature and duration. Foods 2020, 9, 741. [Google Scholar] [CrossRef]
- Herring, H.; Cassens, R.; Rriskey, E. Further studies on bovine muscle tenderness as influenced by carcass position, sarcomere length, and fiber diameter. J. Food Sci. 1965, 30, 1049–1054. [Google Scholar] [CrossRef]
- Matarneh, S.K.; England, E.M.; Scheffler, T.L.; Yen, C.-N.; Wicks, J.C.; Shi, H.; Gerrard, D.E. A mitochondrial protein increases glycolytic flux. Meat Sci. 2017, 133, 119–125. [Google Scholar] [CrossRef]
- Farouk, M.; Wieliczko, K.; Merts, I. Ultra-fast freezing and low storage temperatures are not necessary to maintain the functional properties of manufacturing beef. Meat Sci. 2004, 66, 171–179. [Google Scholar] [CrossRef]
- Fernandes, R.d.P.P.; de Alvarenga Freire, M.T.; da Costa Carrer, C.; Trindade, M.A. Evaluation of physicochemical, microbiological and sensory stability of frozen stored vacuum-packed lamb meat. J. Integr. Agric. 2013, 12, 1946–1952. [Google Scholar] [CrossRef]
- Muela, E.; Monge, P.; Sañudo, C.; Campo, M.; Beltrán, J. Meat quality of lamb frozen stored up to 21 months: Instrumental analyses on thawed meat during display. Meat Sci. 2015, 102, 35–40. [Google Scholar] [CrossRef]
- Soyer, A.; Özalp, B.; Dalmış, Ü.; Bilgin, V. Effects of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat. Food Chem. 2010, 120, 1025–1030. [Google Scholar] [CrossRef]
- Bueno, M.; Resconi, V.C.; Campo, M.M.; Cacho, J.; Ferreira, V.; Escudero, A. Effect of freezing method and frozen storage duration on odor-active compounds and sensory perception of lamb. Food Res. Int. 2013, 54, 772–780. [Google Scholar] [CrossRef]
- Estévez, M.; Ventanas, S.; Heinonen, M.; Puolanne, E. Protein carbonylation and water-holding capacity of pork subjected to frozen storage: Effect of muscle type, premincing, and packaging. J. Agric. Food Chem. 2011, 59, 5435–5443. [Google Scholar] [CrossRef]
- Miller, M.F.; Carr, M.; Ramsey, C.; Crockett, K.; Hoover, L. Consumer thresholds for establishing the value of beef tenderness. J. Anim. Sci. 2001, 79, 3062–3068. [Google Scholar] [CrossRef] [PubMed]
- Van Wezemael, L.; De Smet, S.; Ueland, Ø.; Verbeke, W. Relationships between sensory evaluations of beef tenderness, shear force measurements and consumer characteristics. Meat Sci. 2014, 97, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Hergenreder, J.E.; Hosch, J.J.; Varnold, K.A.; Haack, A.L.; Senaratne, L.S.; Pokharel, S.; Beauchamp, C.; Lobaugh, B.; Calkins, C.R. The effects of freezing and thawing rates on tenderness, sensory quality, and retail display of beef subprimals. J. Anim. Sci. 2013, 91, 483–490. [Google Scholar] [CrossRef]
- Winger, R.; Fennema, O. Tenderness and water holding properties of beef muscle as influenced by freezing and subsequent storage at −3 or 15 °C. J. Food Sci. 1976, 41, 1433–1438. [Google Scholar] [CrossRef]
- Schulte, M.D.; Johnson, L.G.; Zuber, E.A.; Patterson, B.M.; Outhouse, A.C.; Fedler, C.A.; Steadham, E.M.; King, D.A.; Prusa, K.J.; Huff-Lonergan, E.; et al. Influence of postmortem aging and post-aging freezing on pork loin quality attributes. Meat Muscle Biol. 2019, 3, 313–323. [Google Scholar] [CrossRef]
- Thielke, S.; Lhafi, S.; Kühne, M. Effects of aging prior to freezing on poultry meat tenderness. Poult. Sci. 2005, 84, 607–612. [Google Scholar] [CrossRef]
- Qi, J.; Li, C.; Chen, Y.; Gao, F.; Xu, X.; Zhou, G. Changes in meat quality of ovine longissimus dorsi muscle in response to repeated freeze and thaw. Meat Sci. 2012, 92, 619–626. [Google Scholar] [CrossRef]
- Estrada-Solis, J.; Figueroa-Rodriguez, K.A.; Figueroa-Sandoval, B.; Hernandez-Rosas, F.; Hernandez-Cazares, A.S. Microstructure and physical changes in the Mexican cooked lamb meat barbacoa made with chilled and frozen meat. Meat Sci. 2016, 118, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Kemp, C.M.; Sensky, P.L.; Bardsley, R.G.; Buttery, P.J.; Parr, T. Tenderness—An enzymatic view. Meat Sci. 2010, 84, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Aroeira, C.N.; Torres Filho, R.A.; Fontes, P.R.; Gomide, L.A.M.; Ramos, A.L.; Ladeira, M.M.; Ramos, E.M. Freezing, thawing and aging effects on beef tenderness from Bos indicus and Bos taurus cattle. Meat Sci. 2016, 116, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Grayson, A.; King, D.; Shackelford, S.; Koohmaraie, M.; Wheeler, T. Freezing and thawing or freezing, thawing, and aging effects on beef tenderness. J. Anim. Sci. 2014, 92, 2735–2740. [Google Scholar] [CrossRef]
- Geesink, G.; Kuchay, S.; Chishti, A.; Koohmaraie, M. μ-Calpain is essential for postmortem proteolysis of muscle proteins. J. Anim. Sci. 2006, 84, 2834–2840. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Stromer, M.H.; Robson, R.M. Effect of electrical stimulation on postmortem titin, nebulin, desmin, and troponin-T degradation and ultrastructural changes in bovine longissimus muscle. J. Anim. Sci. 1996, 74, 1563–1575. [Google Scholar] [CrossRef]
- Marino, R.; Albenzio, M.; Della Malva, A.; Santillo, A.; Loizzo, P.; Sevi, A. Proteolytic pattern of myofibrillar protein and meat tenderness as affected by breed and aging time. Meat Sci. 2013, 95, 281–287. [Google Scholar] [CrossRef]
- Zhang, Y.; Ertbjerg, P. Effects of frozen-then-chilled storage on proteolytic enzyme activity and water-holding capacity of pork loin. Meat Sci. 2018, 145, 375–382. [Google Scholar] [CrossRef]
- Murphy, R.M.; Verburg, E.; Lamb, G.D. Ca2+ activation of diffusible and bound pools of μ-calpain in rat skeletal muscle. J. Physiol. 2006, 576, 595–612. [Google Scholar] [CrossRef]
- Koohmaraie, M. Quantification of Ca2+− dependent protease activities by hydrophobic and ion-exchange chromatography. J. Anim. Sci. 1990, 68, 659–665. [Google Scholar] [CrossRef]
- Duckett, S.; Klein, T.; Leckie, R.; Thorngate, J.; Busboom, J.; Snowder, G. Effect of freezing on calpastatin activity and tenderness of callipyge lamb. J. Anim. Sci. 1998, 76, 1869–1874. [Google Scholar] [CrossRef]
- Ingólfsson, R.; Dransfield, E. The effects of low-voltage electrical stimulation and freezing on tenderization, enzyme activities, drip losses and cooking losses of lamb. Icel. J. Agric. Sci. 1991, 5, 63–80. [Google Scholar]
- Doumit, M.; Koohmaraie, M. Immunoblot analysis of calpastatin degradation: Evidence for cleavage by calpain in postmortem muscle. J. Anim. Sci. 1999, 77, 1467–1473. [Google Scholar] [CrossRef]
- Lonergan, E.H.; Zhang, W.; Lonergan, S.M. Biochemistry of postmortem muscle—Lessons on mechanisms of meat tenderization. Meat Sci. 2010, 86, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Hamm, R. Delocalization of Mitochondrial Enzymes during Freezing and Thawing of Skeletal Muscle; ACS Publications: Washington, DC, USA, 1979. [Google Scholar]
- Lee, S.; Jo, K.; Jeong, H.G.; Yong, H.I.; Choi, Y.S.; Kim, D.; Jung, S. Freezing-then-aging treatment improved the protein digestibility of beef in an in vitro infant digestion model. Food Chem. 2021, 350, 129224. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Kunz, W.S.; Saks, V.; Usson, Y.; Mazat, J.P.; Letellier, T.; Gellerich, F.N.; Margreiter, R. Cryopreservation of mitochondria and mitochondrial function in cardiac and skeletal muscle fibers. Anal. Biochem. 2003, 319, 296–303. [Google Scholar] [CrossRef]
- Denecker, G.; Dooms, H.; Van Loo, G.; Vercammen, D.; Grooten, J.; Fiers, W.; Declercq, W.; Vandenabeele, P. Phosphatidyl serine exposure during apoptosis precedes release of cytochrome c and decrease in mitochondrial transmembrane potential. FEBS Lett. 2000, 465, 47–52. [Google Scholar] [CrossRef]
- Loeffler, M.; Kroemer, G. The mitochondrion in cell death control: Certainties and incognita. Exp. Cell Res. 2000, 256, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Feng, X.C.; Zhang, Y.Y.; Liu, X.; Zhang, W.; Li, C.; Ullah, N.; Xu, X.; Zhou, G. Effects of ultrasonic processing on caspase-3, calpain expression and myofibrillar structure of chicken during post-mortem ageing. Food Chem. 2015, 177, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.S.; Stafford, C.D.; Taylor, M.J.; Buhler, J.F.; Thornton, K.J.; Matarneh, S.K. Ultrasonication of beef improves calpain-1 autolysis and caspase-3 activity by elevating cytosolic calcium and inducing mitochondrial dysfunction. Meat Sci. 2021, 108646. [Google Scholar]
- Zhan, X.; Sun, D.W.; Zhu, Z.; Wang, Q.J. Improving the quality and safety of frozen muscle foods by emerging freezing technologies: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2925–2938. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef]
- Añón, M.C.; Calvelo, A. Freezing rate effects on the drip loss of frozen beef. Meat Sci. 1980, 4, 1–14. [Google Scholar] [CrossRef]
- Xue, S.; Wang, H.; Yang, H.; Yu, X.; Bai, Y.; Tendu, A.A.; Xu, X.; Ma, H.; Zhou, G. Effects of high-pressure treatments on water characteristics and juiciness of rabbit meat sausages: Role of microstructure and chemical interactions. Innov. Food Sci. Emerg. Technol. 2017, 41, 150–159. [Google Scholar] [CrossRef]
- Santhi, D.; Kalaikannan, A.; Sureshkumar, S. Factors influencing meat emulsion properties and product texture: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2021–2027. [Google Scholar] [CrossRef]
- Carballo, J.; Cofrades, S.; Solas, M.T.; Jiménez-Colmenero, F. High pressure/thermal treatment of meat batters prepared from freeze–thawed pork. Meat Sci. 2000, 54, 357–364. [Google Scholar] [CrossRef]
- Peng, W.; Xu, X.L.; Zhou, G.H. Effects of meat and phosphate level on water-holding capacity and texture of emulsion-type sausage during storage. Agric. Sci. China 2009, 8, 1475–1481. [Google Scholar]
- Mancini, R.; Hunt, M. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef]
- Matarneh, S.K.; Silva, S.L.; Gerrard, D.E. New insights in muscle biology that alter meat quality. Annu. Rev. Anim. Biosci. 2021, 9, 355–377. [Google Scholar] [CrossRef]
- Hughes, J.; Oiseth, S.; Purslow, P.; Warner, R. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Sci. 2014, 98, 520–532. [Google Scholar] [CrossRef]
- Aroeira, C.N.; de Almeida Torres Filho, R.; Fontes, P.R.; Ramos, A.d.L.S.; de Miranda Gomide, L.A.; Ladeira, M.M.; Ramos, E.M. Effect of freezing prior to aging on myoglobin redox forms and CIE color of beef from Nellore and Aberdeen Angus cattle. Meat Sci. 2017, 125, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdallah, M.; Marchello, J.A.; Ahmad, H.A. Effect of freezing and microbial growth on myoglobin derivatives of beef. J. Agric. Food Chem. 1999, 47, 4093–4099. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Kim, G.D.; Yang, H.S.; Joo, S.T. Effect of freeze–thaw cycles on physicochemical properties and color stability of beef semimembranosus muscle. Food Res. Int. 2011, 44, 3222–3228. [Google Scholar] [CrossRef]
- Lanari, M.; Bevilacqua, A.; Zaritzky, N. Pigments modifications during freezing and frozen storage of packaged beef. J. Food Process Eng. 1990, 12, 49–66. [Google Scholar] [CrossRef]
- Alvarenga, T.I.; Hopkins, D.L.; Ramos, E.M.; Almeida, A.K.; Geesink, G. Ageing-freezing/thaw process affects blooming time and myoglobin forms of lamb meat during retail display. Meat Sci. 2019, 153, 19–25. [Google Scholar] [CrossRef]
- Fennema, O. Reaction Kinetics in Partially Frozen Aqueous Systems. In Proceedings of the International Symposium on Water Relations of Foods; Academic Press: Glasgow, UK, 1975; pp. 440–455. [Google Scholar]
- Asghar, A. Perspectives on warmed-over flavor. Food Technol. 1988, 42, 102–108. [Google Scholar]
- Li, F.; Zhong, Q.; Kong, B.; Wang, B.; Pan, N.; Xia, X. Deterioration in quality of quick-frozen pork patties induced by changes in protein structure and lipid and protein oxidation during frozen storage. Food Res. Int. 2020, 133, 109142. [Google Scholar] [CrossRef]
- Tuell, J.R.; Seo, J.K.; Kim, Y.H.B. Combined impacts of initial freezing rate of pork leg muscles (M. biceps femoris and M. semitendinosus) and subsequent freezing on quality characteristics of pork patties. Meat Sci. 2020, 170, 108248. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Zhang, D.; Chen, X.; Li, H. Effect of multiple freeze-thaw cycles on protein and lipid oxidation in rabbit meat. Int. J. Food Sci. Technol. 2021, 56, 3004–3015. [Google Scholar] [CrossRef]
- Xia, X.; Kong, B.; Liu, Q.; Liu, J. Physicochemical change and protein oxidation in porcine longissimus dorsi as influenced by different freeze–thaw cycles. Meat Sci. 2009, 83, 239–245. [Google Scholar] [CrossRef]
- Tichivangana, J.; Morrissey, P. The influence of pH on lipid oxidation in cooked meats from several species. Ir. J. Food Sci. Technol. 1985, 9, 99–106. [Google Scholar]
- Keeton, J.T.; Dikeman, M.E. ‘Red’and ‘white’meats—terms that lead to confusion. Anim. Front. 2017, 7, 29–33. [Google Scholar] [CrossRef]
- Ali, S.; Zhang, W.; Rajput, N.; Khan, M.A.; Li, C.B.; Zhou, G.H. Effect of multiple freeze–thaw cycles on the quality of chicken breast meat. Food Chem. 2015, 173, 808–814. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, X.; Li, R.; Yang, H.; Wang, H.; Wang, H.; Tan, M. Influence of multiple freeze-thaw cycles on quality characteristics of beef semimembranous muscle: With emphasis on water status and distribution by LF-NMR and MRI. Meat Sci. 2019, 147, 44–52. [Google Scholar] [CrossRef]
- Thanonkaew, A.; Benjakul, S.; Visessanguan, W.; Decker, E.A. The effect of metal ions on lipid oxidation, colour and physicochemical properties of cuttlefish (Sepia pharaonis) subjected to multiple freeze–thaw cycles. Food Chem. 2006, 95, 591–599. [Google Scholar] [CrossRef]
- Rahman, M.; Hossain, M.; Rahman, S.; Amin, M.; Oh, D.H. Evaluation of physicochemical deterioration and lipid oxidation of beef muscle affected by freeze-thaw cycles. Korean J. Food Sci. Anim. Resour. 2015, 35, 772. [Google Scholar] [CrossRef]
- Benjakul, S.; Bauer, F. Biochemical and physicochemical changes in catfish (Silurus glanis Linne) muscle as influenced by different freeze–thaw cycles. Food Chem. 2001, 72, 207–217. [Google Scholar] [CrossRef]
- Estévez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef]
- Zhang, Y.; Holman, B.W.; Ponnampalam, E.N.; Kerr, M.G.; Bailes, K.L.; Kilgannon, A.K.; Collins, D.; Hopkins, D.L. Understanding beef flavour and overall liking traits using two different methods for determination of thiobarbituric acid reactive substance (TBARS). Meat Sci. 2019, 149, 114–119. [Google Scholar] [CrossRef]
- Zhao, Y.; Flores, R.A.; Olson, D.G. High hydrostatic pressure effects on rapid thawing of frozen beef. J. Food Sci. 1998, 63, 272–275. [Google Scholar] [CrossRef]
- Li, B.; Sun, D.W. Novel methods for rapid freezing and thawing of foods—A review. J. Food Eng. 2002, 54, 175–182. [Google Scholar] [CrossRef]
- Jia, G.; Orlien, V.; Liu, H.; Sun, A. Effect of high pressure processing of pork (Longissimus dorsi) on changes of protein structure and water loss during frozen storage. LWT 2021, 135, 110084. [Google Scholar] [CrossRef]
- Witte, F.; Smetana, S.; Heinz, V.; Terjung, N. High-pressure processing of usually discarded dry aged beef trimmings for subsequent processing. Meat Sci. 2020, 170, 108241. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Sheen, S.; Sommers, C.; Sheen, L.-Y. Modeling the survival of Escherichia coli O157: H7 under hydrostatic pressure, process temperature, time and allyl isothiocyanate stresses in ground chicken meat. Front. Microbiol. 2018, 9, 1871. [Google Scholar] [CrossRef]
- Cartagena, L.; Puértolas, E.; Martínez de Marañón, I. High-pressure pretreatment in albacore (Thunnus alalunga) for reducing freeze-driven weight losses with minimal quality changes. J. Sci. Food Agric. 2021, 101, 2704–2711. [Google Scholar] [CrossRef]
- Kalichevsky, M.; Knorr, D.; Lillford, P. Potential food applications of high-pressure effects on ice-water transitions. Trends Food Sci. Technol. 1995, 6, 253–259. [Google Scholar] [CrossRef]
- Fallah-Joshaqani, S.; Hamdami, N.; Keshavarzi, E.; Keramat, J.; Dalvi-Isfahan, M. Evaluation of the static electric field effects on freezing parameters of some food systems. Int. J. Refrig. 2019, 99, 30–36. [Google Scholar] [CrossRef]
- Orlowska, M.; Havet, M.; Le-Bail, A. Controlled ice nucleation under high voltage DC electrostatic field conditions. Food Res. Int. 2009, 42, 879–884. [Google Scholar] [CrossRef]
- Xanthakis, E.; Havet, M.; Chevallier, S.; Abadie, J.; Le-Bail, A. Effect of static electric field on ice crystal size reduction during freezing of pork meat. Innov. Food Sci. Emerg. Technol. 2013, 20, 115–120. [Google Scholar] [CrossRef]
- Dalvi-Isfahan, M.; Hamdami, N.; Le-Bail, A. Effect of freezing under electrostatic field on the quality of lamb meat. Innov. Food Sci. Emerg. Technol. 2016, 37, 68–73. [Google Scholar] [CrossRef]
- Patist, A.; Bates, D. Ultrasonic innovations in the food industry: From the laboratory to commercial production. Innov. Food Sci. Emerg. Technol. 2008, 9, 147–154. [Google Scholar] [CrossRef]
- Feng, H.; Yang, W.; Hielscher, T. Power ultrasound. Innov. Food Sci. Emerg. Technol. 2008, 14, 433–436. [Google Scholar] [CrossRef]
- Zheng, L.; Sun, D.W. Ultrasonic Assistance of Food Freezing. In Emerging Technologies for Food Processing; Elsevier: Amsterdam, The Netherlands, 2005; pp. 603–626. [Google Scholar]
- Astráin-Redín, L.; Abad, J.; Rieder, A.; Kirkhus, B.; Raso, J.; Cebrián, G.; Álvarez, I. Direct contact ultrasound assisted freezing of chicken breast samples. Ultrason. Sonochem. 2021, 70, 105319. [Google Scholar] [CrossRef]
- Zhang, M.; Haili, N.; Chen, Q.; Xia, X.; Kong, B. Influence of ultrasound-assisted immersion freezing on the freezing rate and quality of porcine longissimus muscles. Meat Sci. 2018, 136, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, C.; Li, Q.; Xia, X.; Kong, B. Changes in functional properties of common carp (Cyprinus carpio) myofibrillar protein as affected by ultrasound-assisted freezing. J. Food Sci. 2020, 85, 2879–2888. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Ge, X.; Yang, L.; Ma, G.; Ma, J.; Yu, Q.L.; Han, L. Ultrasound-assisted thawing of frozen white yak meat: Effects on thawing rate, meat quality, nutrients, and microstructure. Ultrason. Sonochem. 2021, 70, 105345. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, Q.; Chen, Q.; Liu, Q.; Kong, B. Effectiveness of ultrasound-assisted immersion thawing on the thawing rate and physicochemical properties of chicken breast muscle. J. Food Sci. 2021, 86, 1692–1703. [Google Scholar] [CrossRef]
| Species | Muscle | Rate of Freezing | Storage Temperature & Duration | Thawing and Aging | Outcome (SF vs. FF or NF) | Ref. |
|---|---|---|---|---|---|---|
| Porcine | Longissimus steaks 24 h postmortem | SF: −20 °C blast freezer FF: −80 °C liquid nitrogen | Stored at −20 °C for 6-8 weeks | Thawed at 1 °C for 2 days Aging time: 19 days | ↓ WHC ↑ Myofibrillar damage No difference WBSF No difference LO | [47] |
| Porcine | Longissimus steaks 24 h postmortem | SF: −22 °C blast freezer FF: −22 °C immersion freezing | Stored at −18 °C for up to 91 days | Thawed at 4 °C for 12 h No aging | ↓ WHC ↑ Myofibrillar damage No difference in L* a* b* ↓ WBSF ↑ LO | [48] |
| Porcine | Longissimus steaks 24 h postmortem | † SF: −20 °C † FF: −80 °C | Stored at −20 °C for 30 months | Thawed at 5 °C for 16 h No aging | ↑ WHC ↓ Myofibrillar damage | [49] |
| Bovine | Longissimus steaks 48 h postmortem | SF: −20 °C blast freezer NF | Stored at −20 °C for 8 weeks | Thawed at 4 °C for 16 h Aging time: Up to 7 days | ↓ WHC ↓ WBSF ↓ Juiciness | [50] |
| Bovine | Longissimus muscle 24 h postmortem | SF: −18 °C air freezer NF | Stored at −18°C for up to 9 months | ‡ Aging time: 2 weeks | ↓ WHC ↓ WBSF ↓ L* No difference in a* & b* | [51] |
| Bovine | Longissimus steaks 24 h postmortem | SF: −18 °C air freezer FF: −18 °C immersion tank | Stored at −18 °C for 2 weeks | Thawed at 3 °C Aging time: Up to 4 weeks | ↓ WHC No effect on WBSF | [52] |
| Bovine | Longissimus steaks 24 h postmortem | † SF: −20 °C NF | Stored at −20 °C for up to 90 days | Thawed at 4 °C for 48 h Aging time: 3 & 10 days | ↓ WHC ↑ Tenderness (trained sensory panelists) ↓ L* a* b* | [53] |
| Ovine | Longissimus muscle 24 h postmortem | SF: −18 °C air freezer FF: −18 °C immersion tank | Stored at −18 °C for 2 weeks | Thawed at 3 °C Aging time: 2 weeks | ↓ WHC ↑ WBSF SF & FF ↓ L* a* b* | [54] |
| Ovine | Longissimus steaks 18 h postmortem | SF: −30 °C air freezer FF: −80 °C liquid nitrogen | Stored at −18 °C up to 6 months | Thawed at 3 °C Aging time: 72 h | Consumer panelists did not detect any sensory differences | [55] |
| Ovine | Longissimus steaks 24 h postmortem | SF: −18 °C air freezer NF | Stored at −18 °C for 1 week | Thawed at 3 °C Aging time: Up to 14 days | ↓ WHC No difference in WBSF ↓ L* & b* No difference a* | [56] |
| Broiler | Pectoralis minor 24 h postmortem | SF: −30 °C air freezer FF: −70 °C liquid nitrogen | Stored at −30 °C for 12 months | Thawed at 2 °C for 24 h No aging | ↓ WHC No differences in L* a* b* | [57] |
| Broiler | Pectoralis major immediately after harvest | † SF: −18 °C † FF: −40 °C | −18°C & −40 °C for 24 h | Thawed at 4 °C No aging | ↑ WHC No difference in WBSF No difference in L* & b* ↑ a* | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, D.S.; Bastarrachea, L.J.; Martini, S.; Matarneh, S.K. Crystallization Behavior and Quality of Frozen Meat. Foods 2021, 10, 2707. https://doi.org/10.3390/foods10112707
Dang DS, Bastarrachea LJ, Martini S, Matarneh SK. Crystallization Behavior and Quality of Frozen Meat. Foods. 2021; 10(11):2707. https://doi.org/10.3390/foods10112707
Chicago/Turabian StyleDang, David S., Luis J. Bastarrachea, Silvana Martini, and Sulaiman K. Matarneh. 2021. "Crystallization Behavior and Quality of Frozen Meat" Foods 10, no. 11: 2707. https://doi.org/10.3390/foods10112707
APA StyleDang, D. S., Bastarrachea, L. J., Martini, S., & Matarneh, S. K. (2021). Crystallization Behavior and Quality of Frozen Meat. Foods, 10(11), 2707. https://doi.org/10.3390/foods10112707







