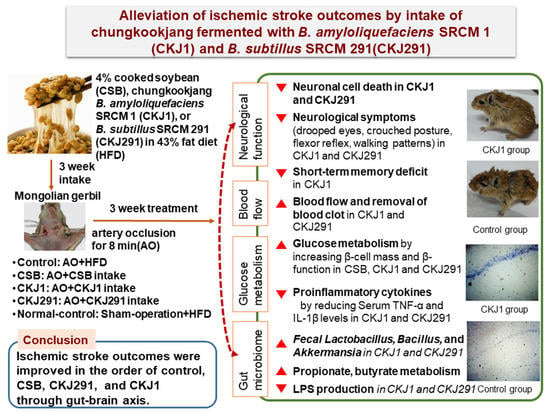
Alleviation of Neuronal Cell Death and Memory Deficit with Chungkookjang Made with Bacillus amyloliquefaciens and Bacillus subtilis Potentially through Promoting Gut–Brain Axis in Artery-Occluded Gerbils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation of B. subtilus SCDB 291 and B. amyloliquefaciens SCGB 1 and Their Probiotic Properties
2.2. Chungkookjang Preparation and Isoflavonoid Contents
2.3. Animals and Diets
2.4. Transient Forebrain Ischemia Induction
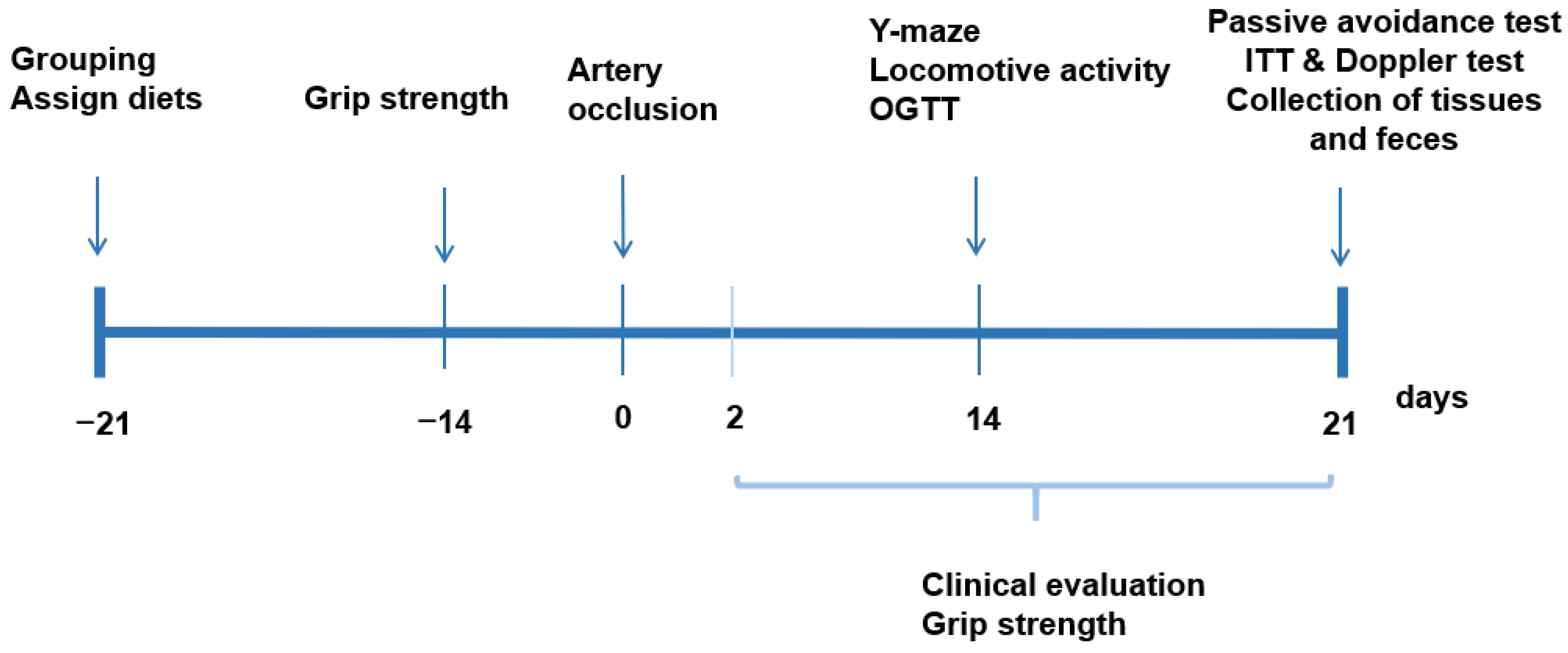
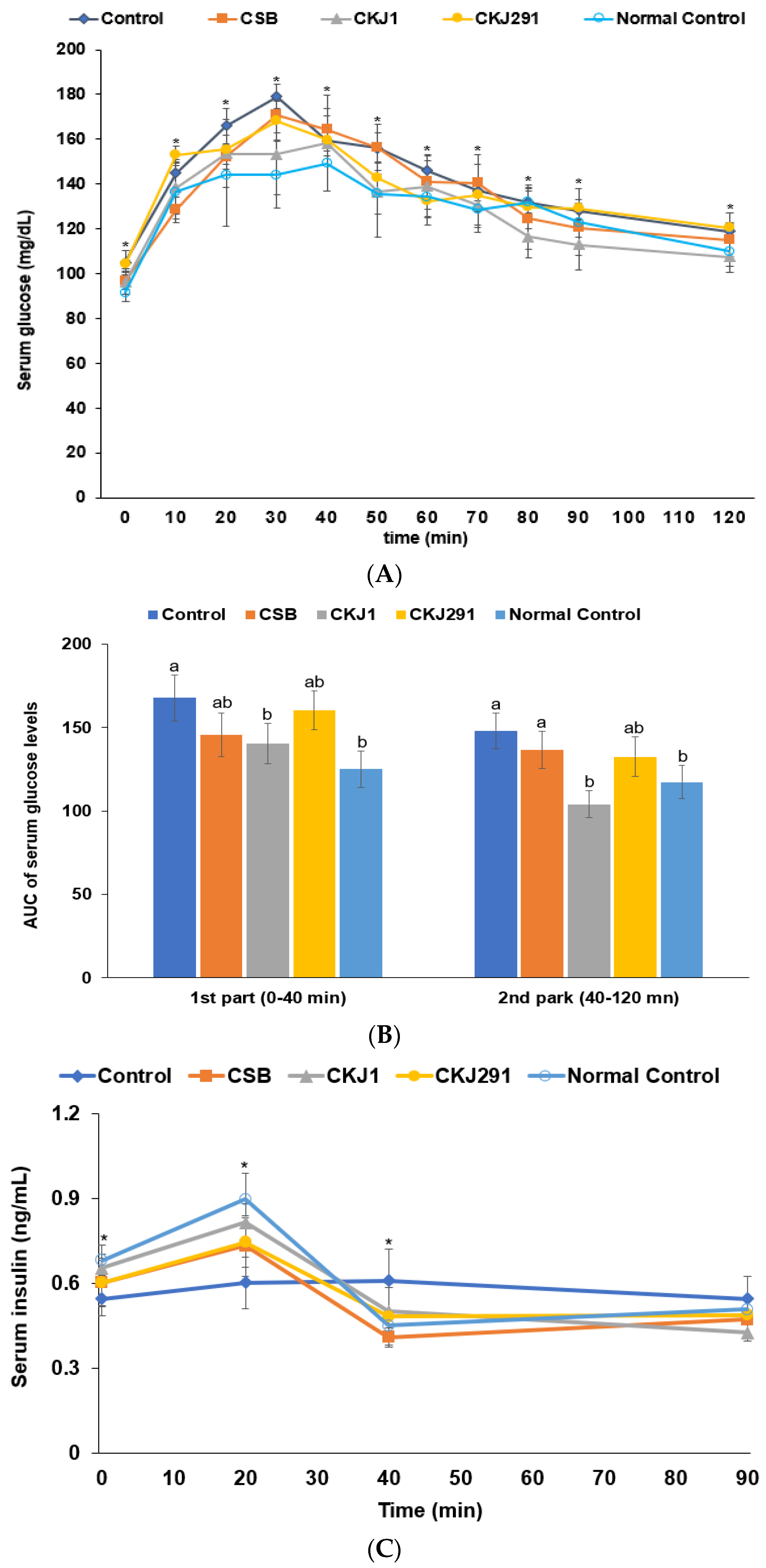
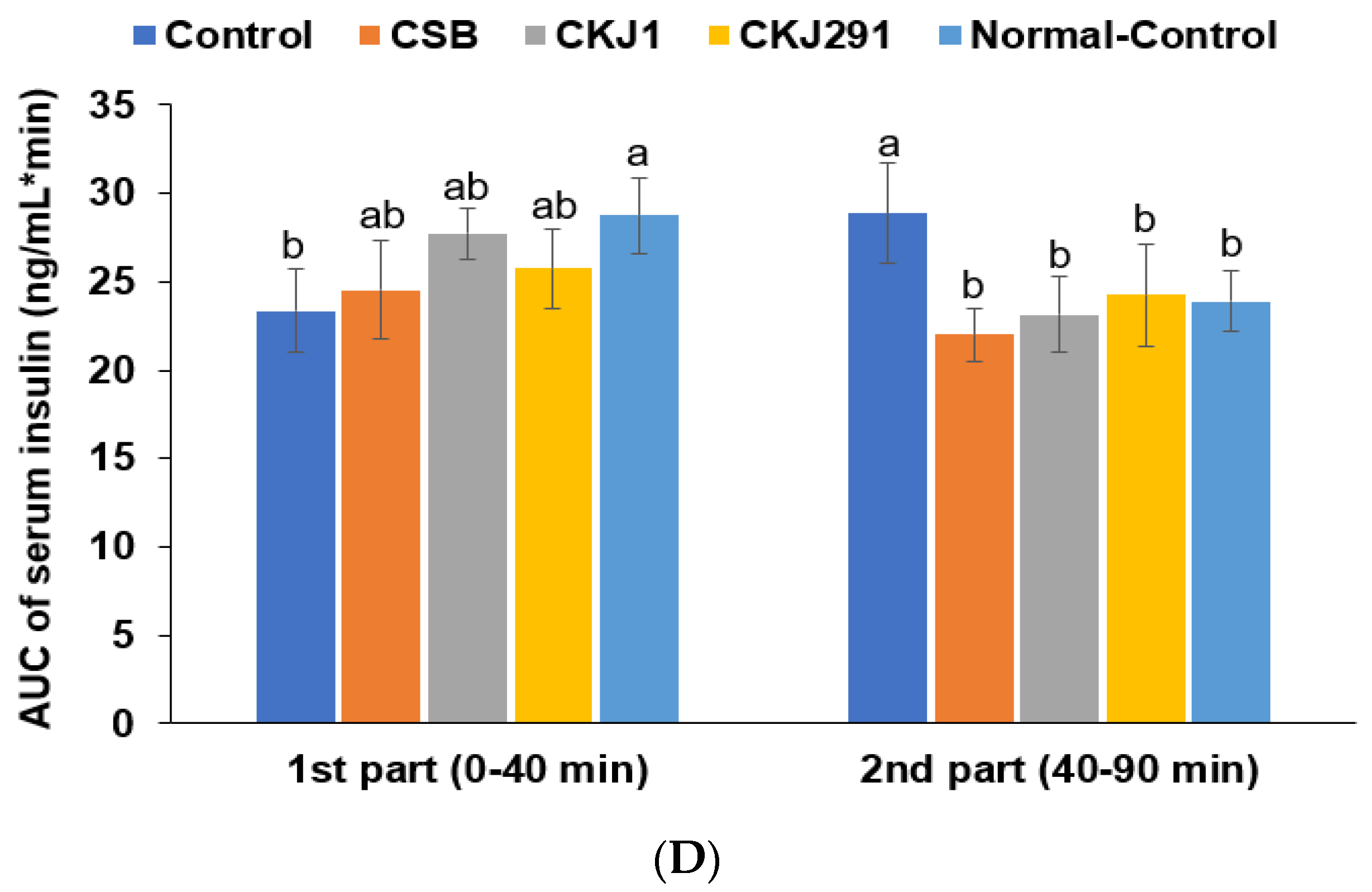
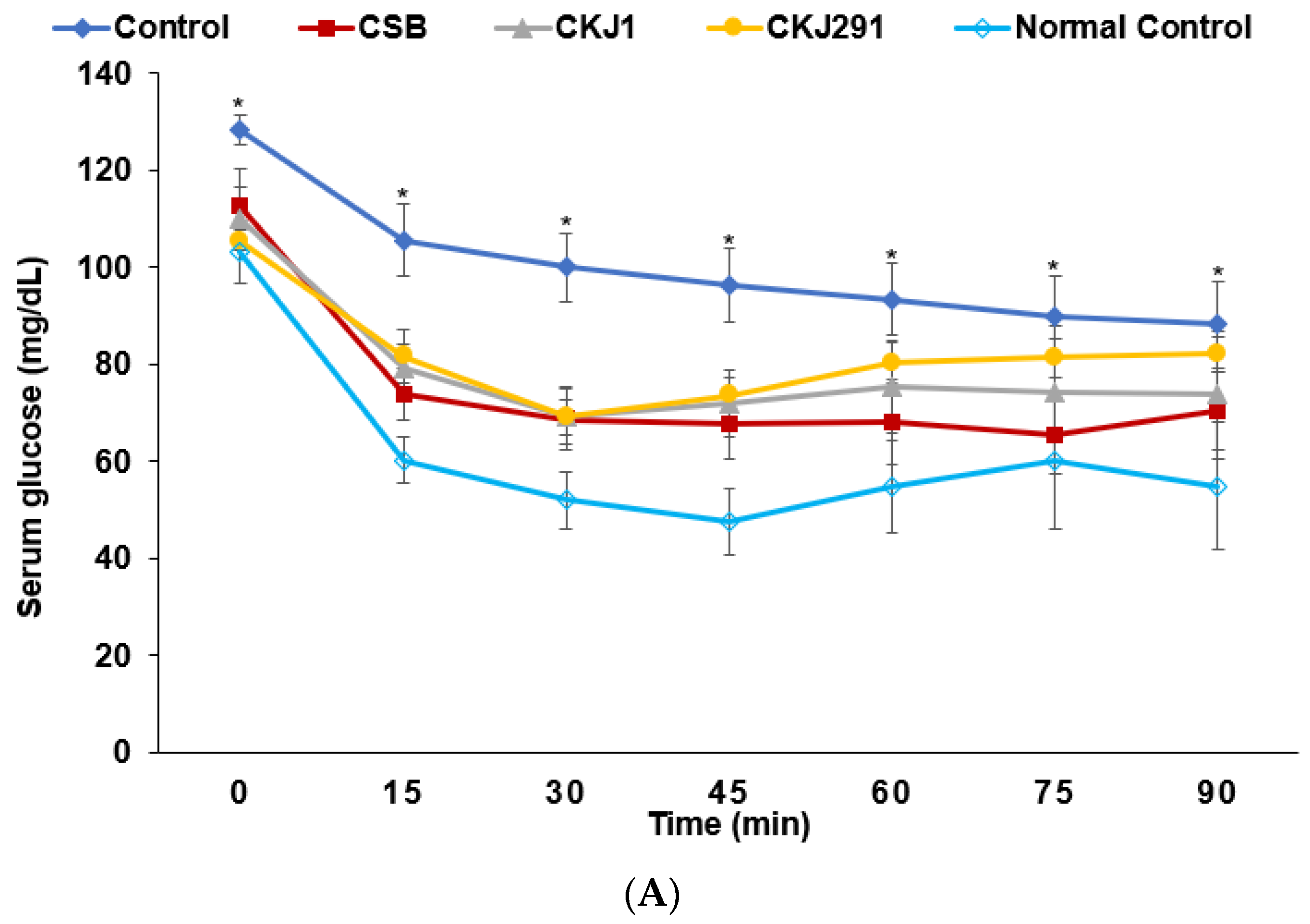
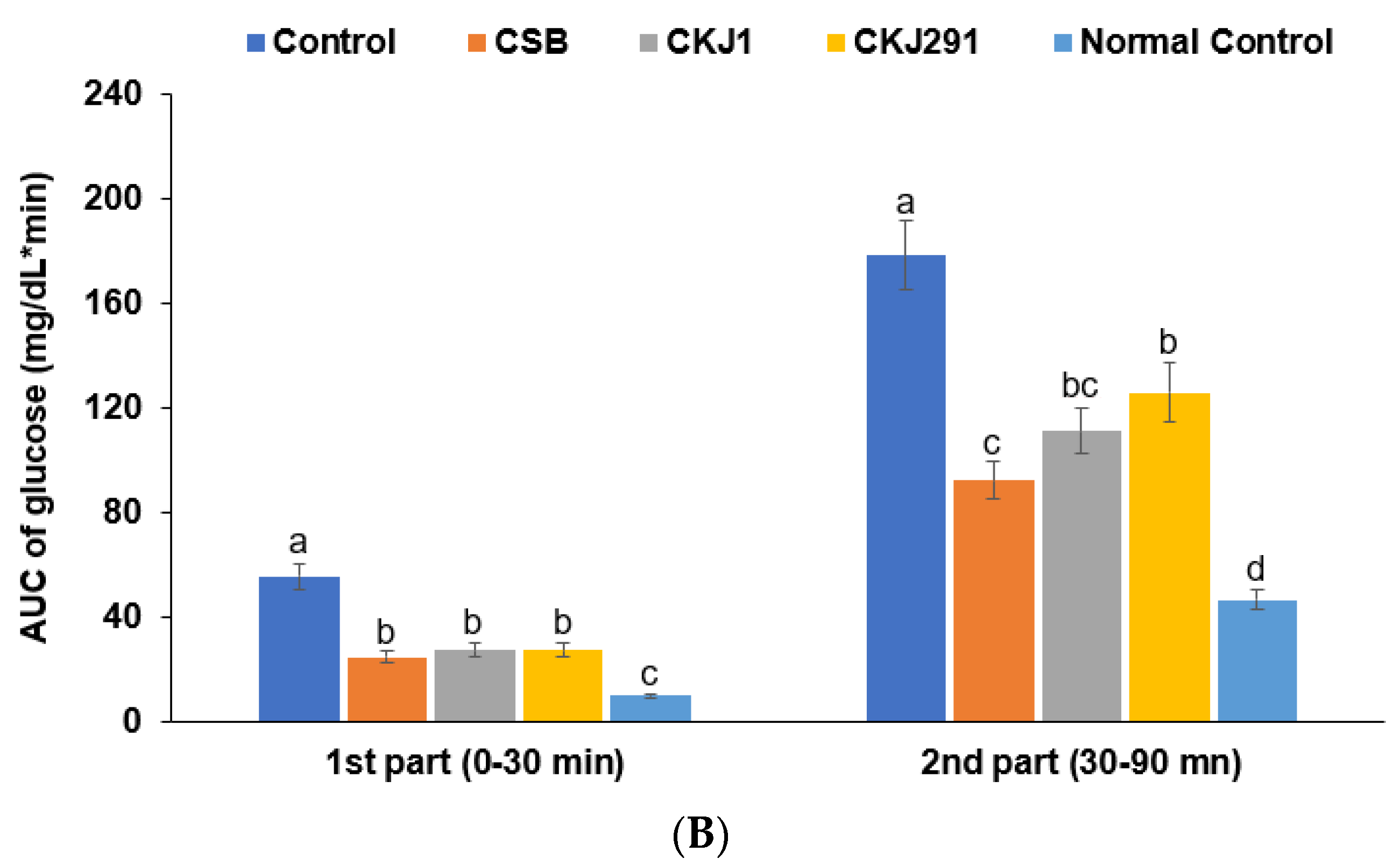
2.5. Experimental Design, Glucose Metabolism, Memory Impairment, and Grip Strength
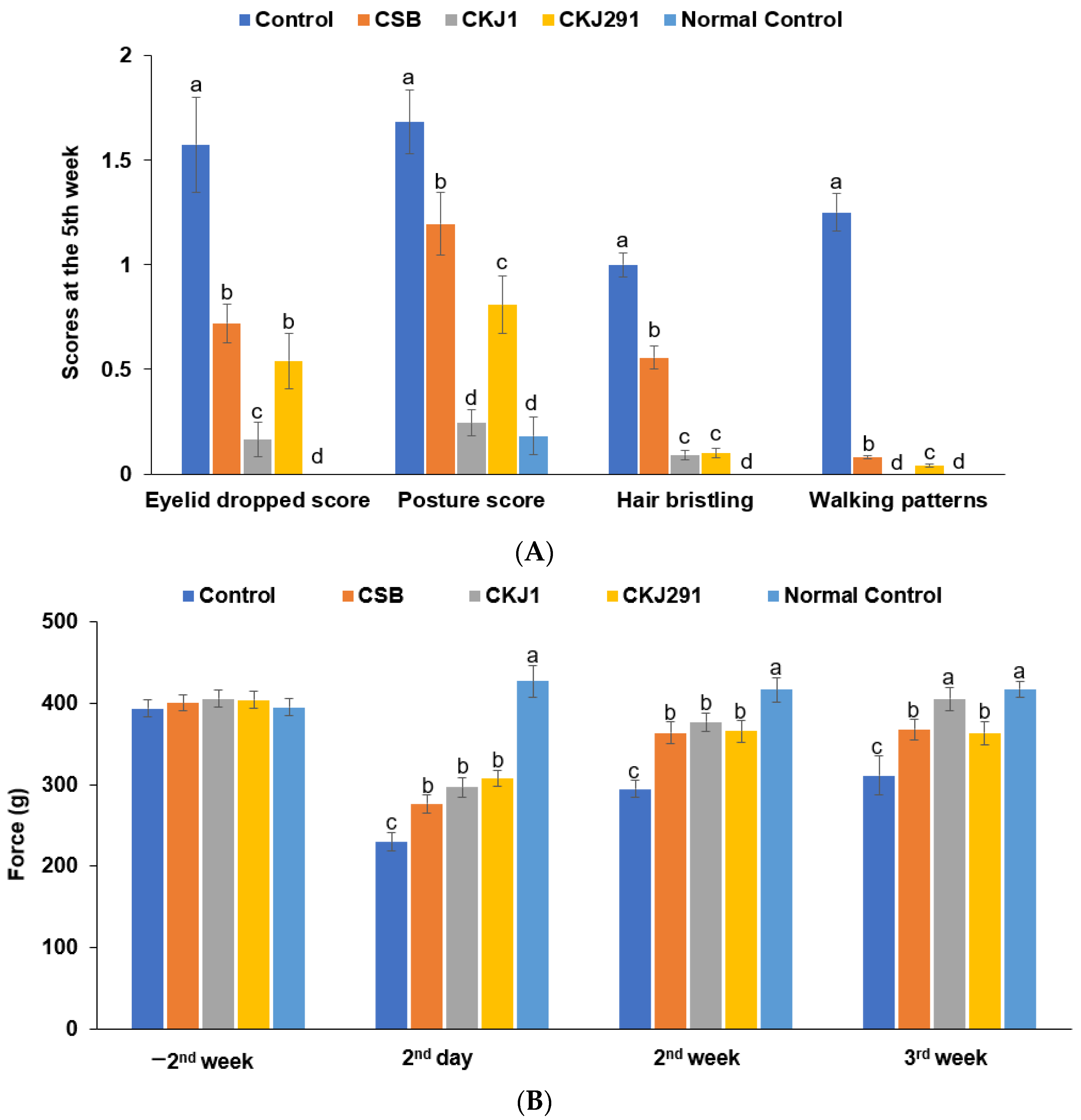
2.6. Neurological Severity Score and Grip Strength
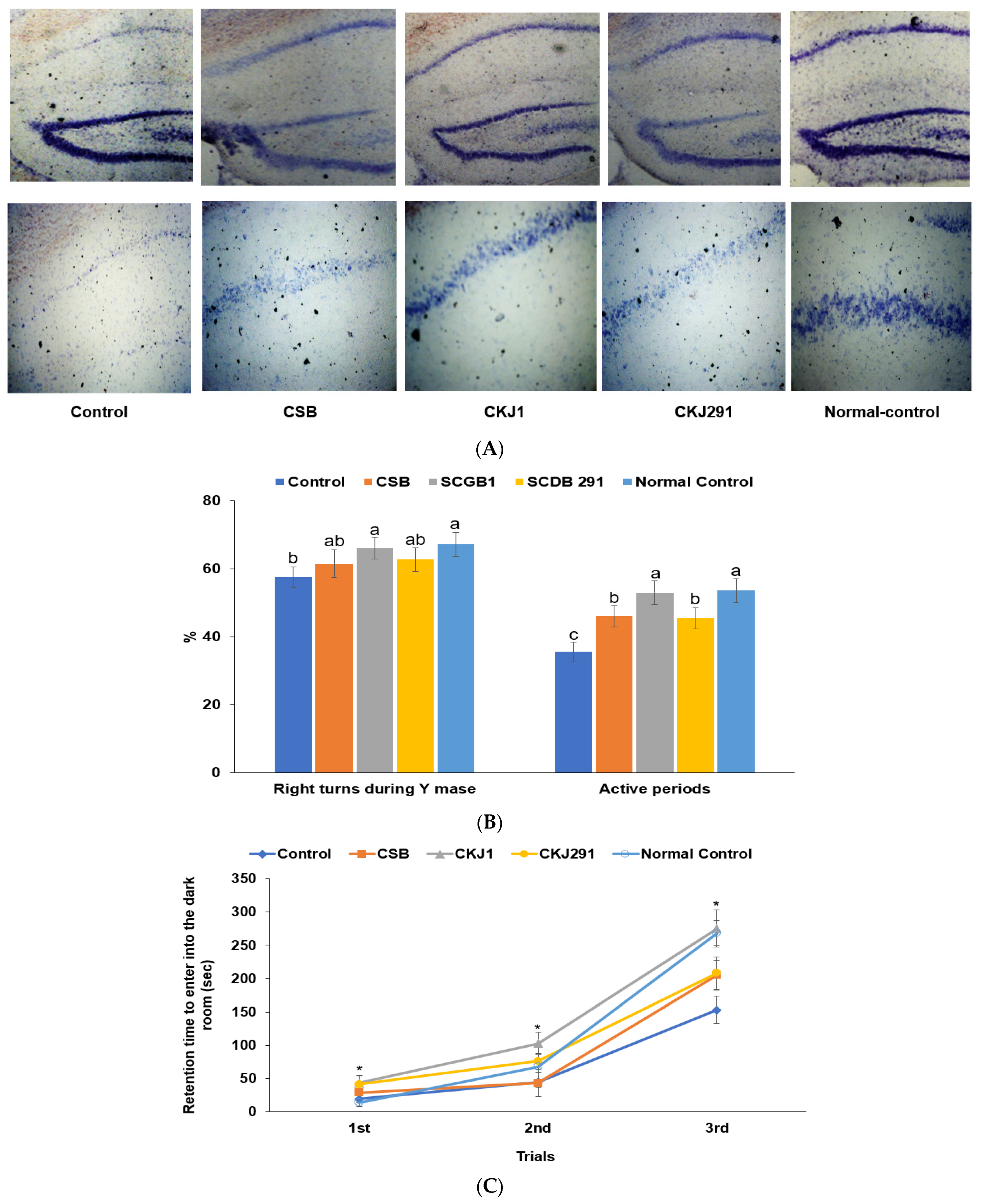
2.7. Short-Term Memory Impairment Measurement Using the Y maze and Passive Avoidance Tests
2.8. Lipid Peroxidation Contents
2.9. Cresyl Violet Staining in the Hippocampus
2.10. Islet Morphometry by Immunohistochemistry
2.11. Serum Short-Chain Fatty Acid (SCFA) Concentrations by Gas Chromatography (GC)
2.12. Next-Generation Sequencing (NGS) of gut Microbiomes
2.13. Statistical Analysis
3. Results
3.1. Characteristics of B. amyloliquefaciens SCGB 1 and B. Subtilis SCDB 291
3.2. Energy Metabolism
3.3. Neuronal Cell Death in the Hippocampus and Memory Dysfunction
3.4. Clinical Neurological Symptom Evaluation
3.5. Blood Flow and Lipid Profiles
3.6. Glucose Metabolism
3.7. β-Cell Mass
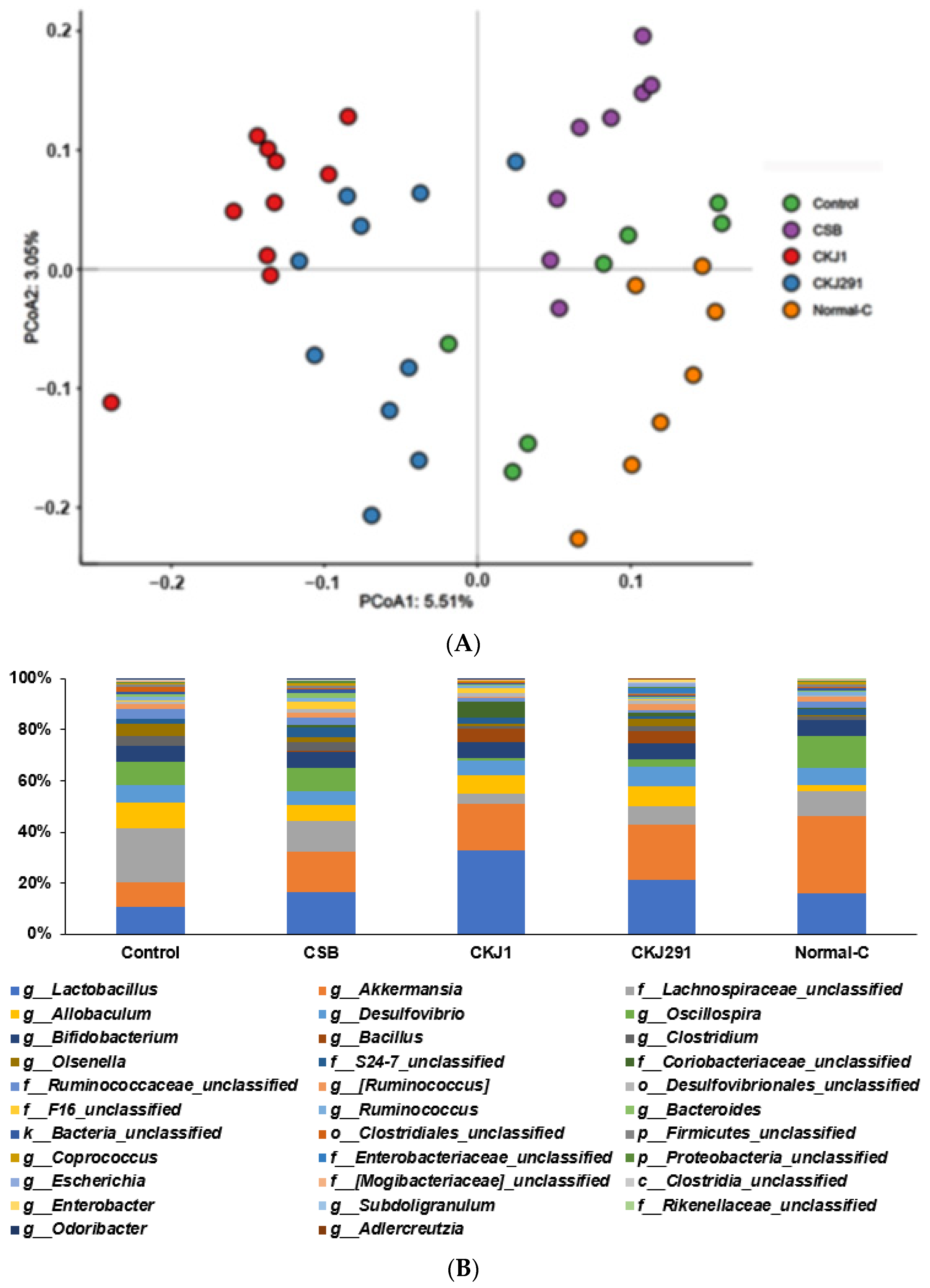
3.8. Serum SCFA Concentrations and Gut Microbiome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Somnath, P. Stroke Prevalence and Risk Factors. US Pharm. 2021, 46, 14. [Google Scholar]
- Turana, Y.; Tengkawan, J.; Chia, Y.C.; Nathaniel, M.; Wang, J.-G.; Sukonthasarn, A.; Chen, C.-H.; Minh, H.V.; Buranakitjaroen, P.; Shin, J.; et al. Hypertension and stroke in Asia: A comprehensive review from HOPE Asia. J. Clin. Hypertens. 2021, 23, 513–521. [Google Scholar] [CrossRef]
- Denorme, F.; Portier, I.; Kosaka, Y.; Campbell, R.A. Hyperglycemia exacerbates ischemic stroke outcome independent of platelet glucose uptake. J. Thromb. Haemost. 2021, 19, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.; Moretti, A.; Villa, R.F. Hyperglycemia in acute ischemic stroke: Physiopathological and therapeutic complexity. Neural. Regen. Res. 2022, 17, 292–299. [Google Scholar] [PubMed]
- Kwon, D.Y.; Jang, J.S.; Lee, J.E.; Kim, Y.S.; Shin, D.H.; Park, S. The isoflavonoid aglycone-rich fractions of Chungkookjang, fermented unsalted soybeans, enhance insulin signaling and peroxisome proliferator-activated receptor-gamma activity in vitro. Biofactors 2006, 26, 245–258. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Daily, J.W., 3rd; Kim, H.J.; Park, S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr. Res. 2010, 30, 1–13. [Google Scholar] [CrossRef]
- Ajayeoba, T.A.; Dula, S.; Ijabadeniyi, O.A. Properties of Poly-γ-Glutamic Acid Producing-Bacillus Species Isolated From Ogi Liquor and Lemon-Ogi Liquor. Front. Microbiol. 2019, 10, 771. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Jeong, D.Y.; Kim, D.S.; Park, S. Chungkookjang with High Contents of Poly-γ-Glutamic Acid Improves Insulin Sensitizing Activity in Adipocytes and Neuronal Cells. Nutrients 2018, 10, 1588. [Google Scholar] [CrossRef] [Green Version]
- Byun, M.S.; Yu, O.K.; Cha, Y.S.; Park, T.S. Korean traditional Chungkookjang improves body composition, lipid profiles and atherogenic indices in overweight/obese subjects: A double-blind, randomized, crossover, placebo-controlled clinical trial. Eur. J. Clin. Nutr. 2016, 70, 1116–1122. [Google Scholar] [CrossRef]
- Back, H.I.; Kim, S.R.; Yang, J.A.; Kim, M.G.; Chae, S.W.; Cha, Y.S. Effects of Chungkookjang supplementation on obesity and atherosclerotic indices in overweight/obese subjects: A 12-week, randomized, double-blind, placebo-controlled clinical trial. J. Med. Food 2011, 14, 532–537. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Jang, J.S.; Hong, S.M.; Lee, J.E.; Sung, S.R.; Park, H.R.; Park, S. Long-term consumption of fermented soybean-derived Chungkookjang enhances insulinotropic action unlike soybeans in 90% pancreatectomized diabetic rats. Eur. J. Nutr. 2007, 46, 44–52. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.S.; Kang, S.; Moon, B.R. Fermented soybeans, Chungkookjang, prevent hippocampal cell death and β-cell apoptosis by decreasing pro-inflammatory cytokines in gerbils with transient artery occlusion. Exp. Biol. Med. 2016, 241, 296–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, M.S.; Yang, H.S.; Kim, J.W.; Jeong, S.J.; Jeong, S.Y.; Eom, J.S.; Jeong, D.Y. Potential probiotics activity of Bacillus spp. from traditional soybean pastes and fermentation characteristics of Cheonggukjang. Korean J. Food Preserv. 2017, 12, 1168–1179. [Google Scholar] [CrossRef] [Green Version]
- Ryu, M.S.; Yang, H.S.; Jeong, S.J.; Seo, J.W.; Ha, G.; Jeong, S.Y.; Jeong, D.Y. Characteristic study and optimization of culture conditions for Bacillus amyloliquefaciens SRCM 100731 as probiotic resource for companion animal. Korean J. Microbiol. 2018, 54, 384–397. [Google Scholar]
- Li, M.; Zhang, Z.; Li, S.; Tian, Z.; Ma, X. Study on the mechanism of production of γ-PGA and nattokinase in Bacillus subtilis natto based on RNA-seq analysis. Microb. Cell Factories 2021, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- Back, H.I.; Ha, K.C.; Kim, H.M.; Kim, M.G.; Yu, O.K.; Byun, M.S.; Jeong, D.Y.; Jeong, S.Y.; Cha, Y.S.; Park, T.S. The influence of the Korean traditional Chungkookjang on variables of metabolic syndrome in overweight/obese subjects: Study protocol. BMC Complement. Altern. Med. 2013, 13, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, D.Y.; Daily, J.W.; Lee, G.H.; Ryu, M.S.; Yang, H.J.; Jeong, S.Y.; Qiu, J.Y.; Zhang, T.; Park, S. Short-Term Fermented Soybeans with Bacillus amyloliquefaciens Potentiated Insulin Secretion Capacity and Improved Gut Microbiome Diversity and Intestinal Integrity To Alleviate Asian Type 2 Diabetic Symptoms. J. Agric. Food Chem. 2020, 68, 13168–13178. [Google Scholar] [CrossRef]
- Jeong, D.Y.; Jeong, S.Y.; Zhang, T.; Wu, X.; Qiu, J.Y.; Park, S. Chungkookjang, a soy food, fermented with Bacillus amyloliquefaciens protects gerbils against ishcmeic stroke injury, and post-stroke hyperglycemia. Food Res. Int. 2020, 128, 108769. [Google Scholar] [CrossRef]
- Jeong, D.-Y.; Ryu, M.S.; Yang, H.-J.; Park, S. γ-PGA-Rich Chungkookjang, Short-Term Fermented Soybeans: Prevents Memory Impairment by Modulating Brain Insulin Sensitivity, Neuro-Inflammation, and the Gut-Microbiome-Brain Axis. Foods 2021, 10, 221. [Google Scholar] [CrossRef]
- Jeong, S.-J.; Ryu, M.-S.; Yang, H.-J.; Wu, X.-H.; Jeong, D.-Y.; Park, S.-M. Bacterial Distribution, Biogenic Amine Contents, and Functionalities of Traditionally Made Doenjang, a Long-Term Fermented Soybean Food, from Different Areas of Korea. Microorganisms 2021, 9, 1348. [Google Scholar] [CrossRef]
- Eom, J.S.; Song, J.; Choi, H.S. Protective Effects of a Novel Probiotic Strain of Lactobacillus plantarum JSA22 from Traditional Fermented Soybean Food Against Infection by Salmonella enterica Serovar Typhimurium. J. Microbiol. Biotechnol. 2015, 25, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.O.; Shin, J.Y.; Kim, J.S.; Che, D.N.; Kang, H.J.; Jeong, D.Y.; Jang, S.I. Soybean Fermented with Bacillus amyloliquefaciens (Cheonggukjang) Ameliorates Atopic Dermatitis-Like Skin Lesion in Mice by Suppressing Infiltration of Mast Cells and Production of IL-31 Cytokine. J. Microbiol. Biotechnol. 2019, 29, 827–837. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, R.; Shehzad, A.; Bilal, S.; Lee, I.J. Bacillus amyloliquefaciens RWL-1 as a New Potential Strain for Augmenting Biochemical and Nutritional Composition of Fermented Soybean. Molecules 2020, 25, 2346. [Google Scholar] [CrossRef]
- Kondo, T.; Yoshida, S.; Nagai, H.; Takeshita, A.; Mino, M.; Morioka, H.; Nakajima, T.; Kusakabe, K.T.; Okada, T. Transient forebrain ischemia induces impairment in cognitive performance prior to extensive neuronal cell death in Mongolian gerbil (Meriones unguiculatus). J. Vet. Sci. 2018, 19, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Mankin, E.A.; Thurley, K.; Chenani, A.; Haas, O.V.; Debs, L.; Henke, J.; Galinato, M.; Leutgeb, J.K.; Leutgeb, S.; Leibold, C. The hippocampal code for space in Mongolian gerbils. Hippocampus 2019, 29, 787–801. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.S.; Kang, S.; Kwon, D.Y. Ischemic hippocampal cell death induces glucose dysregulation by attenuating glucose-stimulated insulin secretion which is exacerbated by a high fat diet. Life Sci. 2011, 88, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kang, S.; Kim, D.S.; Shin, B.K.; Moon, N.R.; Daily, J.W., 3rd. Ebselen pretreatment attenuates ischemia/reperfusion injury and prevents hyperglycemia by improving hepatic insulin signaling and beta-cell survival in gerbils. Free Radic. Res. 2014, 48, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, D.S.; Kang, E.S.; Kim, D.B.; Kang, S. Low-dose brain estrogen prevents menopausal syndrome while maintaining the diversity of the gut microbiomes in estrogen-deficient rats. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E99–E109. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, H.; Yamamoto, K.; Nozato, S.; Inagaki, T.; Tsuchimochi, H.; Shirai, M.; Yamamoto, R.; Imaizumi, Y.; Hongyo, K.; Yokoyama, S.; et al. Modified forelimb grip strength test detects aging-associated physiological decline in skeletal muscle function in male mice. Sci. Rep. 2017, 7, 42323. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.-S.; Ryuk, J.A.; Hwang, J.T.; Zhang, T.; Wu, X.; Park, S. Ojayeonjonghwan, an oriental medicine composed of five seeds, protects against vasomotor and neurological disorders in estrogen-deficient rats. Exp. Biol. Med. 2019, 244, 193–206. [Google Scholar] [CrossRef]
- Yang, H.J.; Hwang, J.T.; Kwon, D.Y.; Kim, M.J.; Kang, S.; Moon, N.R.; Park, S. Yuzu extract prevents cognitive decline and impaired glucose homeostasis in beta-amyloid-infused rats. J. Nutr. 2013, 143, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Cunningham, M.W.; Pabbidi, M.R.; Wang, S.; Booz, G.W.; Fan, F. Targeting vascular inflammation in ischemic stroke: Recent developments on novel immunomodulatory approaches. Eur. J. Pharmacol. 2018, 833, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Khoshnam, S.E.; Winlow, W.; Farzaneh, M.; Farbood, Y.; Moghaddam, H.F. Pathogenic mechanisms following ischemic stroke. Neurol. Sci. 2017, 38, 1167–1186. [Google Scholar] [CrossRef] [PubMed]
- Li, W.A.; Moore-Langston, S.; Chakraborty, T.; Rafols, J.A.; Conti, A.C.; Ding, Y. Hyperglycemia in stroke and possible treatments. Neurol. Res. 2013, 35, 479–491. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.S.; Kang, S. Exercise training attenuates cerebral ischemic hyperglycemia by improving hepatic insulin signaling and β-cell survival. Life Sci. 2013, 93, 153–160. [Google Scholar] [CrossRef]
- Shin, S.K.; Kwon, J.H.; Jeong, Y.J.; Jeon, S.M.; Choi, J.Y.; Choi, M.S. Supplementation of cheonggukjang and red ginseng cheonggukjang can improve plasma lipid profile and fasting blood glucose concentration in subjects with impaired fasting glucose. J. Med. Food 2011, 14, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Hwang, N.; Ko, G.; Tatsuya, U. Effects of digested Cheonggukjang on human microbiota assessed by in vitro fecal fermentation. J. Microbiol. 2021, 59, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.H.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, Y.; Fei, X. Effect of probiotics on glucose metabolism in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Medicina 2016, 52, 28–34. [Google Scholar] [CrossRef]
- Yan, F.; Li, N.; Shi, J.; Li, H.; Yue, Y.; Jiao, W.; Wang, N.; Song, Y.; Huo, G.; Li, B. Lactobacillus acidophilus alleviates type 2 diabetes by regulating hepatic glucose, lipid metabolism and gut microbiota in mice. Food Funct. 2019, 10, 5804–5815. [Google Scholar] [CrossRef]
- Farida, E.; Nuraida, L.; Giriwono, P.E.; Jenie, B.S.L. Lactobacillus rhamnosus Reduces Blood Glucose Level through Downregulation of Gluconeogenesis Gene Expression in Streptozotocin-Induced Diabetic Rats. Int J. Food Sci. 2020, 2020, 6108575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaller, B.J.; Graf, R.; Jacobs, A.H. Pathophysiological changes of the gastrointestinal tract in ischemic stroke. Am. J. Gastroenterol. 2006, 101, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Nyavor, Y.; Brands, C.R.; May, G.; Kuther, S.; Nicholson, J.; Tiger, K.; Tesnohlidek, A.; Yasuda, A.; Starks, K.; Litvinenko, D.; et al. High-fat diet-induced alterations to gut microbiota and gut-derived lipoteichoic acid contributes to the development of enteric neuropathy. Neurogastroenterol. Motil. 2020, 32, e13838. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, P.M.; Anwar, A.; Saleem, S.; Nasir, S.; Inayat, A. Traumatic brain injury causing intestinal dysfunction: A review. J. Clin. Neurosci. 2020, 79, 237–240. [Google Scholar] [CrossRef]
- Daily, J.W.; Kang, S.; Park, S. Protection against Alzheimer’s disease by luteolin: Role of brain glucose regulation, anti-inflammatory activity, and the gut microbiota-liver-brain axis. Biofactors 2021, 47, 218–231. [Google Scholar] [CrossRef] [PubMed]
| B. amyloliquefaciens SCGB 1 | B. subtilis SCDB 291 | |
|---|---|---|
| Protease activity (diameter, cm) | 2.3 ± 0.2 | 2.1 ± 0.1 |
| Cellulase activity (diameter, cm) | 1.2 ± 0.1 | 1.2 ± 0.1 |
| Amylase activity (diameter, cm) | 2.5 ± 0.2 | 2.1 ± 0.1 * |
| Thrombolytic activity (diameter, cm) | 1.4 ± 0.0 | 1.6 ± 0.0 * |
| DPPH free removal activity (%) | 15.0 ± 0.5 | 29.7 ± 1.2 * |
| Survival cells at 0.5% oxgall (%) | 54.6 ± 1.1 | 72.9 ± 1.7 * |
| Survival cells at pH 2.0 (%) | 52.1 ± 1.4 | 57.1 ± 1.2 * |
| Survival cells at 80°C (%) | 55.7 ± 1.8 | 94.2 ± 1.6 * |
| Bile salt hydrolase activity (%) | 0.0 ± 0.0 | 0.0 ± 0.0 |
| CCD-18Co Cell adhesion (%) | 62.1 ± 1.4 | 82.1 ± 0.9 * |
| γ-PGA (cm) | 31 ± 0.9 | 22 ± 0.5 * |
| Control (n = 10) | Cooked Soybeans (n = 10) | CKJ1 (n = 10) | CKJ291 (n = 10) | Normal-Control (n = 10) | |
|---|---|---|---|---|---|
| Body weight gain during 1–3 week period before artery occlusion (g) | 2.87 ± 0.55 b | 3.05 ± 0.76 b | 7.27 ± 0.86 a | 7.16 ± 0.63 a | 2.57 ± 0.70 b |
| Body weight gain during 1–3 week period after artery occlusion (g) | −3.20 ± 0.81 d | 1.52 ± 0.69 c | 5.82 ± 0.75 a | 3.04 ± 0.67 b | 2.71 ± 0.72 b |
| Food intake during 1–3 week period before artery occlusion (g/day) | 4.1 ± 0.51 b | 4.5 ± 0.53 b | 5.8 ± 0.50 a | 6.4 ± 0.57 a | 4.3 ± 0.55 b |
| Food intake during 1–3 week period after artery occlusion (g/day) | 4.1 ± 0.58 | 4.0 ± 0.48 | 3.9 ± 0.62 | 4.6 ± 0.63 | 4.1 ± 0.52 |
| Isoflavonoid aglycones during 1–3 week period after artery occlusion (μg/day) | 0 ± 0 d | 211 ± 24.2 c | 630 ± 99 b | 892 ± 122 a | 0 ± 0 d |
| Isoflavonoid glycones during 1–3 week period after artery occlusion (μg/day) | 0 ± 0 d | 1443 ± 173 a | 369 ± 59 b | 252 ± 34 c | 0 ± 0 d |
| Control (n = 10) | Cooked Soybeans (n = 10) | CKJ1 (n = 10) | CKJ291 (n = 10) | Normal-Control (n = 10) | |
|---|---|---|---|---|---|
| Serum IL-1β levels (pg/mL) | 10.7 ± 1.17 a | 7.84 ± 0.88 b | 7.94 ± 0.84 b | 8.08 ± 0.91 b | 7.76 ± 0.79 b |
| Serum TNF-α (pg/mL) | 26.9 ± 2.15 a | 21.8 ± 2.05 b | 18.5 ± 2.03 c | 20.6 ± 1.98 bc | 17.9 ± 1.87 c |
| Hippocampal lipid peroxides (MDA μmol/g tissue) | 0.54 ± 0.08 a | 0.38 ± 0.05 b | 0.26 ± 0.05 c | 0.32 ± 0.04 b | 0.25 ± 0.05 c |
| Relative mRNA expression of hippocampal TNF-α (AU) | 2.2 ± 1.1 a | 1.0 ± 0.2 b | 0.98 ± 0.11 b | 1.1 ± 0.2 b | 1.0 ± 0.0 b |
| Relative mRNA expression of hippocampal IL-1β (AU) | 1.9 ± 0 a | 1.3 ± 0.2 b | 0.9 ± 0.2 c | 1.0 ± 0.2 c | 1.0 ± 0.0 c |
| Relative mRNA expression of BDNF (AU) | 0.54 ± 0.08 c | 0.81 ± 0.10 b | 0.93 ± 0.11 a | 0.86 ± 0.11 ab | 1.0 ± 0.0 a |
| Control (n = 10) | Cooked Soybeans (n = 10) | CKJ1 (n = 10) | CKJ291 (n = 10) | Normal-Control (n = 10) | |
|---|---|---|---|---|---|
| Peak blood perfusion unit (BPU) | 11.0 ± 3.94 d | 18.7 ± 3.73 c | 24.6 ± 4.01 b | 33.1 ± 4.89 a | 35.8 ± 4.44 a |
| Periods to remove the clog (min) | 3.23 ± 0.59 a | 2.51 ± 0.37 b | 1.51 ± 0.40 c | 1.42 ± 0.35 c | 1.54 ± 0.36 c |
| Total cholesterol | 181.2 ± 22.5 a | 149.1 ± 17.4 a | 151.4 ± 19.2 a | 146.2 ± 19.4 b | 139.4 ± 16.2 c |
| HDL | 33.5 ± 3.89 c | 33.6 ± 4.26 c | 50.1 ± 5.59 a | 47.7 ± 4.75 a | 42.2 ± 4.24 b |
| LDL | 105.5 ± 12.1 a | 92.0 ± 9.91 b | 71.6 ± 8.41 c | 69.7 ± 7.38 c | 69.4 ± 7.55 c |
| Triglyceride | 211.3 ± 22.0 a | 117.8 ± 9.39 c | 148.4 ± 16.8 b | 144.2 ± 12.3 b | 138.7 ± 14.2 b |
| Fasting serum glucose levels (mg/dL) | 111.1 ± 3.92 a | 94.7 ± 5.18 c | 92.2 ± 1.71 c | 102.2 ± 5.31 b | 91.6 ± 3.78 c |
| Serum insulin levels (ng/mL) | 0.52 ± 0.06 c | 0.60 ± 0.08 b | 0.65 ± 0.05 ab | 0.60 ± 0.08 b | 0.68 ± 0.05 a |
| Fasting serum glucose at the 3rd week before artery occlusion (mg/dL) | 95.4 ± 2.15 a | 89.5 ± 1.95 c | 88.8 ± 1.67 c | 92.2 ± 2.03 b | 95.5 ± 1.94 a |
| Control (n = 10) | Cooked Soybeans (n = 10) | CKJ1 (n = 10) | CKJ291 (n = 10) | Normal-Control (n = 10) | |
|---|---|---|---|---|---|
| Individual β-cell size (μm2) | 8.09 ± 0.92 a | 6.65 ± 0.73 b | 6.81 ± 0.71 b | 7.16 ± 0.74 ab | 6.24 ± 0.75 b |
| β-cell area (%) | 21.7 ± 2.76 c | 25.1 ± 3.02 b | 24.8 ± 2.95 b | 26.4 ± 3.04 a | 26.9 ± 3.12 a |
| Total β-cell mass (mg) | 0.98 ± 0.13 c | 1.24 ± 0.17 ab | 1.32 ± 0.19 a | 1.19 ± 0.12 b | 1.36 ± 0.15 a |
| BrdU+ cells (% BrdU+ cells of islets) | 5.03 ± 0.62 b | 5.83 ± 0.71 ab | 6.07 ± 0.69 a | 5.64 ± 0.65 ab | 5.96 ± 0.72 a |
| Apoptosis (% apoptotic bodies of islets) | 23.4 ± 2.61 a | 16.33 ± 1.94 c | 15.5 ± 1.82 c | 19.2 ± 2.25 b | 15.3 ± 1.67 c |
| Control (n = 10) | Cooked Soybeans (n = 10) | CKJ1 (n = 10) | CKJ291 (n = 10) | Normal-Control (n = 10) | |
|---|---|---|---|---|---|
| Serum acetate | 0.89 ± 0.06 | 0.87 ± 0.07 | 0.94 ± 0.07 | 0.92 ± 0.09 | 0.88 ± 0.05 |
| Serum propionate | 0.27 ± 0.05 | 0.27 ± 0.03 | 0.27 ± 0.01 | 0.27 ± 0.02 | 0.26 ± 0.04 |
| Serum butyrate | 0.14 ± 0.02 b | 0.18 ± 0.02 a | 0.19 ± 0.02 a | 0.16 ± 0.02 ab | 0.16 ± 0.02 ab |
| Chao | 6897 ± 440 c | 9753 ± 574 a | 9259 ± 615 ab | 8957 ± 709 b | 9201 ± 657 ab |
| Shannon index | 4.8 ± 0.5 b | 5.6 ± 0.5 a | 5.5 ± 0.4 a | 5.6 ± 0.4 a | 5.6 ± 0.4 a |
| Butanoate metabolism | 1.08 ± 0.03 b | 1.13 ± 0.04 a | 1.19 ± 0.0.02 a | 1.10 ± 0.02 ab | 1.18 ± 0.04 a |
| LPS biosynthesis | 0.50 ± 0.07 a | 0.28 ± 0.02 b | 0.25 ± 0.06 b | 0.29 ± 0.05 b | 0.25 ± 0.03 b |
| Starch and sucrose metabolism | 1.94 ± 0.05 c | 2.35 ± 0.08 b | 2.80 ± 0.13 a | 2.67 ± 0.08 a | 2.32 ± 0.10 b |
| Fatty acid metabolism | 0.88 ± 0.03 a | 0.88 ± 0.03 a | 0.74 ± 0.03 b | 0.74 ± 0.01 b | 0.91 ± 0.04 a |
| Branched-chain amino acid synthesis | 0.85 ± 0.06 a | 0.73 ± 0.08 b | 0.53 ± 0.06 c | 0.67 ± 0.09 b | 0.71 ± 0.05 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Ryu, M.-S.; Wu, X.; Yang, H.-J.; Jeong, S.J.; Seo, J.-W.; Jeong, D.-Y.; Park, S. Alleviation of Neuronal Cell Death and Memory Deficit with Chungkookjang Made with Bacillus amyloliquefaciens and Bacillus subtilis Potentially through Promoting Gut–Brain Axis in Artery-Occluded Gerbils. Foods 2021, 10, 2697. https://doi.org/10.3390/foods10112697
Zhang T, Ryu M-S, Wu X, Yang H-J, Jeong SJ, Seo J-W, Jeong D-Y, Park S. Alleviation of Neuronal Cell Death and Memory Deficit with Chungkookjang Made with Bacillus amyloliquefaciens and Bacillus subtilis Potentially through Promoting Gut–Brain Axis in Artery-Occluded Gerbils. Foods. 2021; 10(11):2697. https://doi.org/10.3390/foods10112697
Chicago/Turabian StyleZhang, Ting, Myeong-Seon Ryu, Xuangao Wu, Hee-Jong Yang, Su Ji Jeong, Ji-Won Seo, Do-Yeon Jeong, and Sunmin Park. 2021. "Alleviation of Neuronal Cell Death and Memory Deficit with Chungkookjang Made with Bacillus amyloliquefaciens and Bacillus subtilis Potentially through Promoting Gut–Brain Axis in Artery-Occluded Gerbils" Foods 10, no. 11: 2697. https://doi.org/10.3390/foods10112697
APA StyleZhang, T., Ryu, M.-S., Wu, X., Yang, H.-J., Jeong, S. J., Seo, J.-W., Jeong, D.-Y., & Park, S. (2021). Alleviation of Neuronal Cell Death and Memory Deficit with Chungkookjang Made with Bacillus amyloliquefaciens and Bacillus subtilis Potentially through Promoting Gut–Brain Axis in Artery-Occluded Gerbils. Foods, 10(11), 2697. https://doi.org/10.3390/foods10112697