Metabolic Effects of Bee Larva-Derived Protein in Mice: Assessment of an Alternative Protein Source
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Ingredients of the Diets
2.3. Oral Glucose Tolerance Test
2.4. Wire Hang Test
2.5. Analysis of mRNA Gene Expression
2.6. Metabolite Extraction and Capillary Electrophoresis Time-of-Flight Mass Spectrometry (CE-TOF/MS)-Based Metabolome Analysis
2.7. Histological Analysis and Transmission Electron Microscopy (TEM)
2.8. Statistical Analysis
3. Results
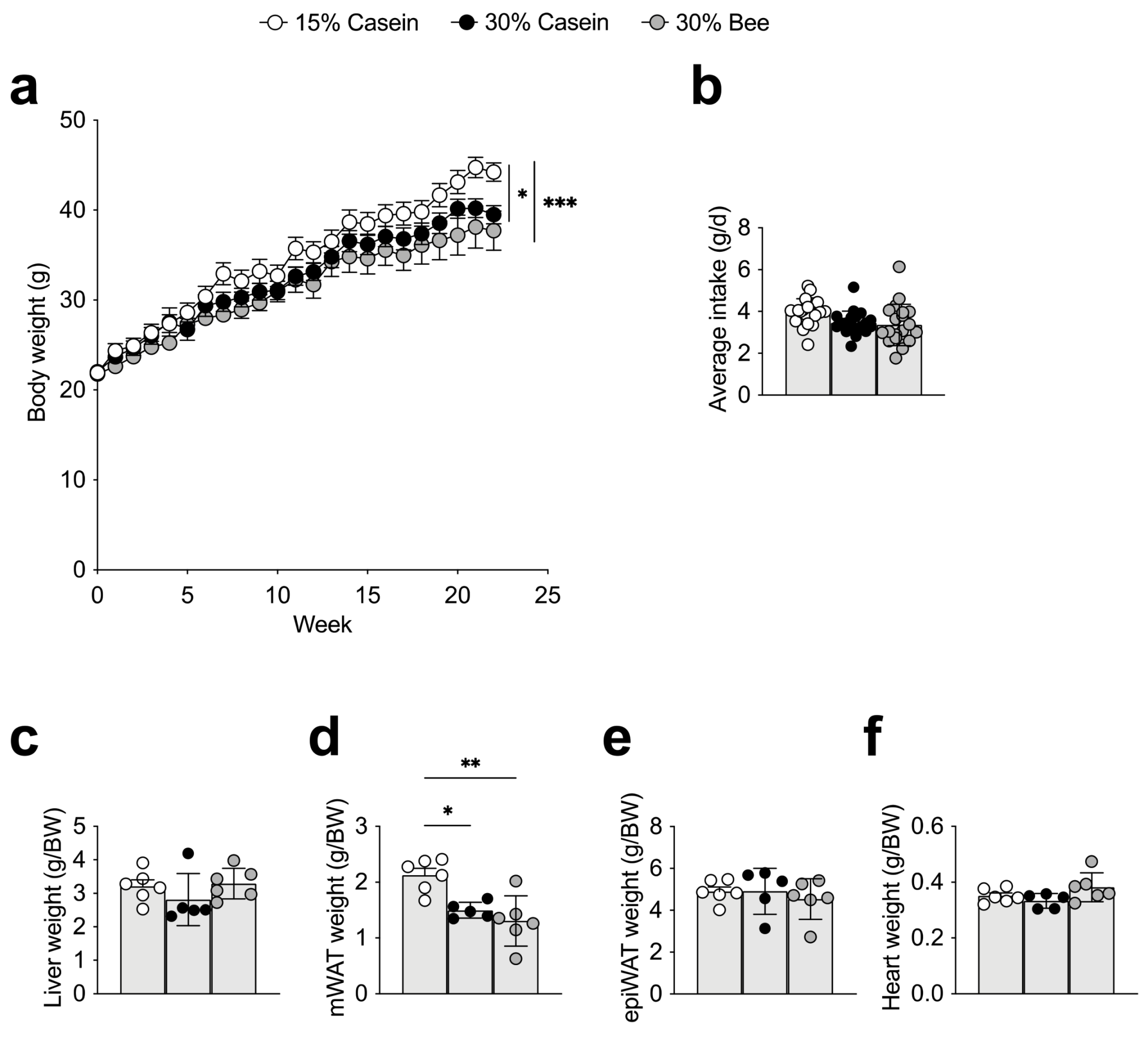
3.1. Bee Larva Protein-Fed Mice Did Not Decrease Body Weight
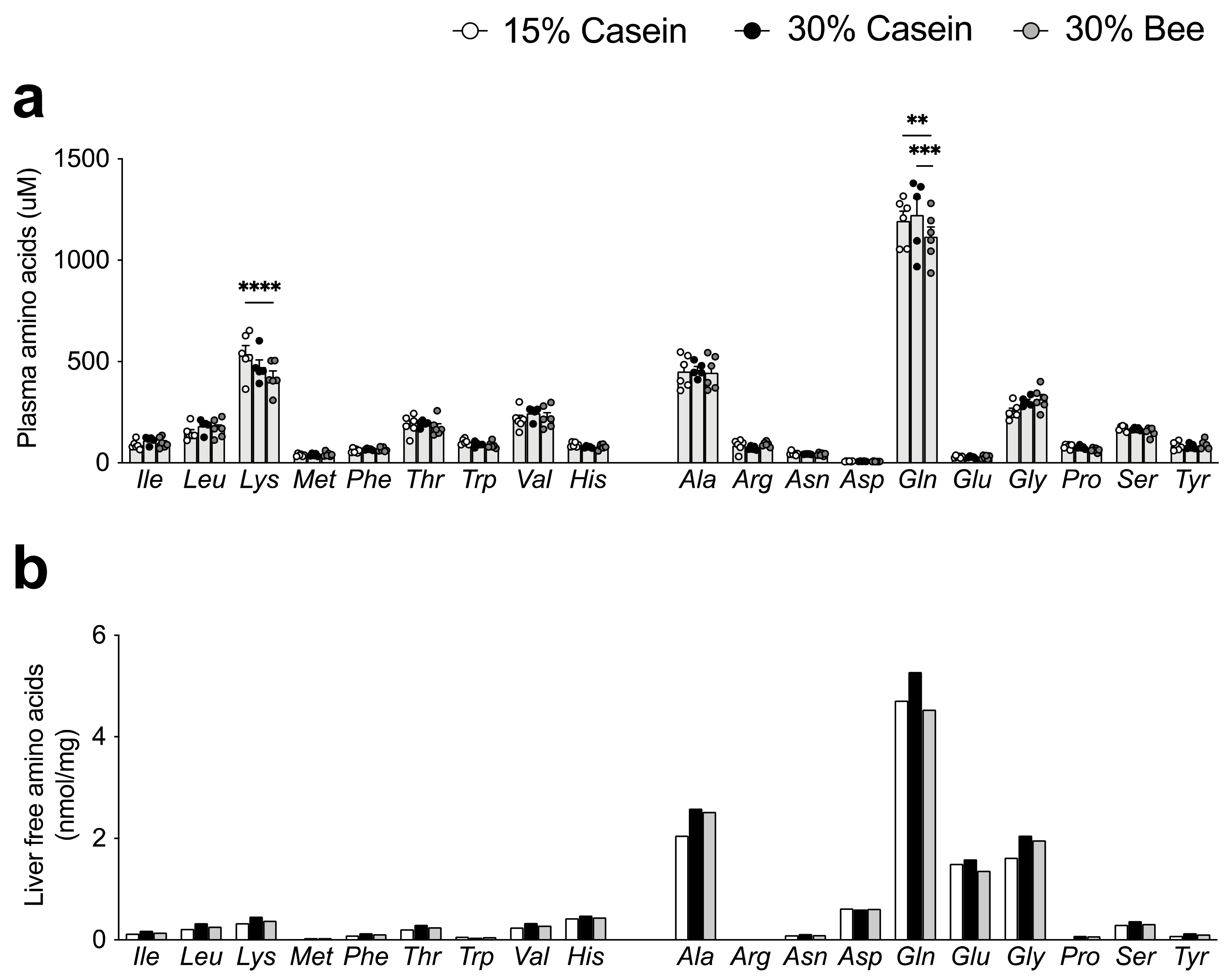
3.2. Bee Larva Protein Did Not Cause Amino Acids Deficiency in Plasma and Liver
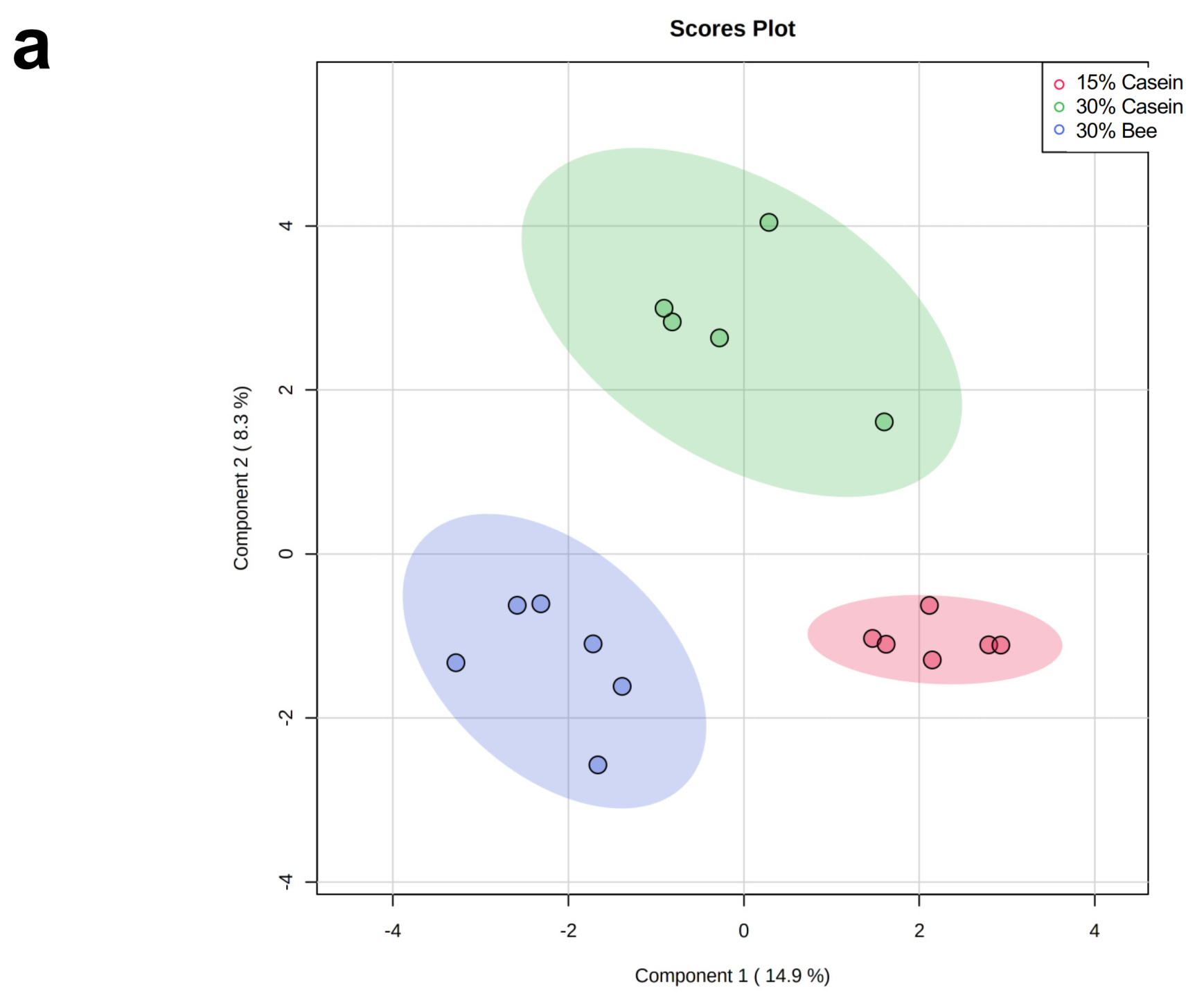
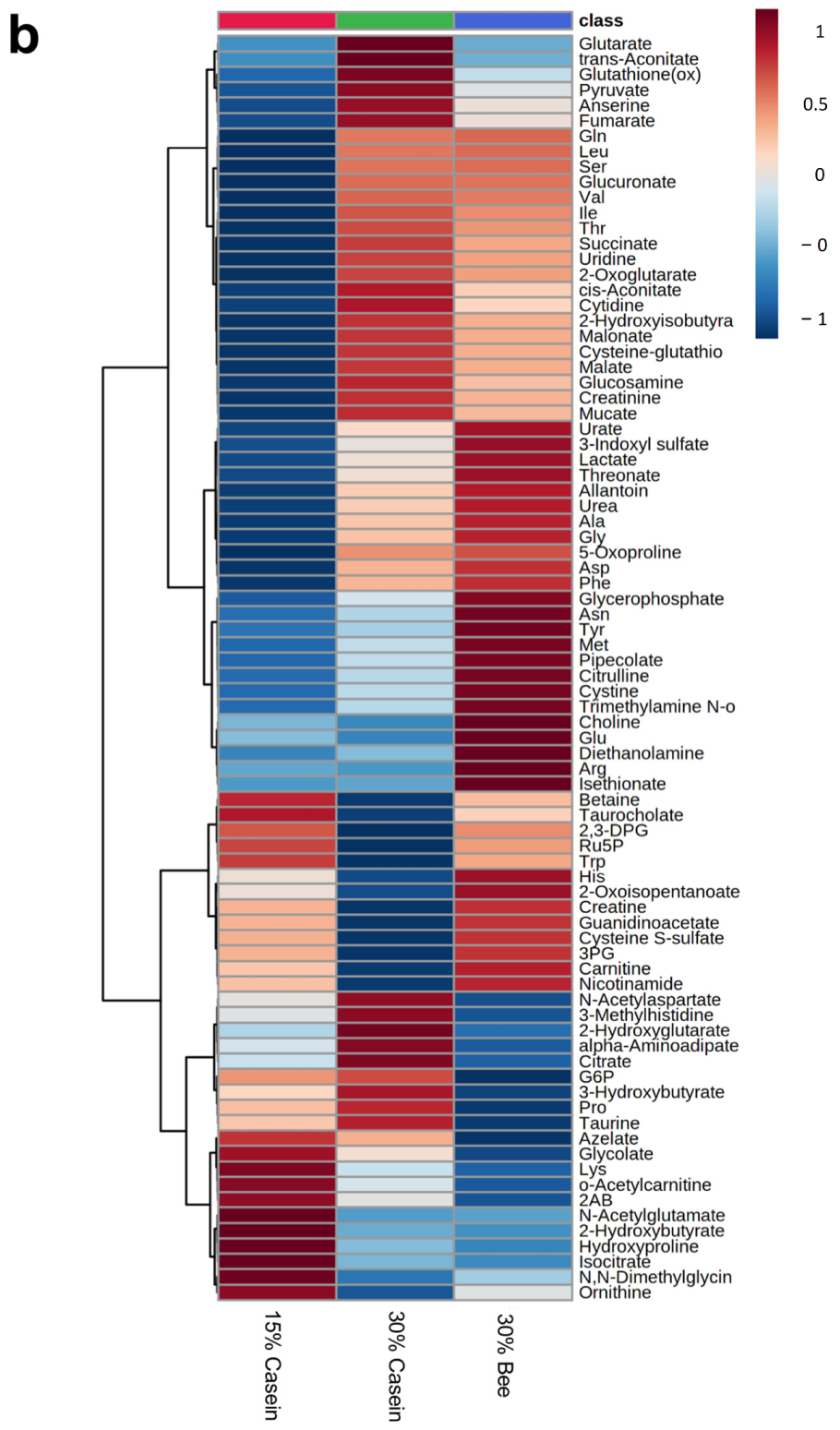
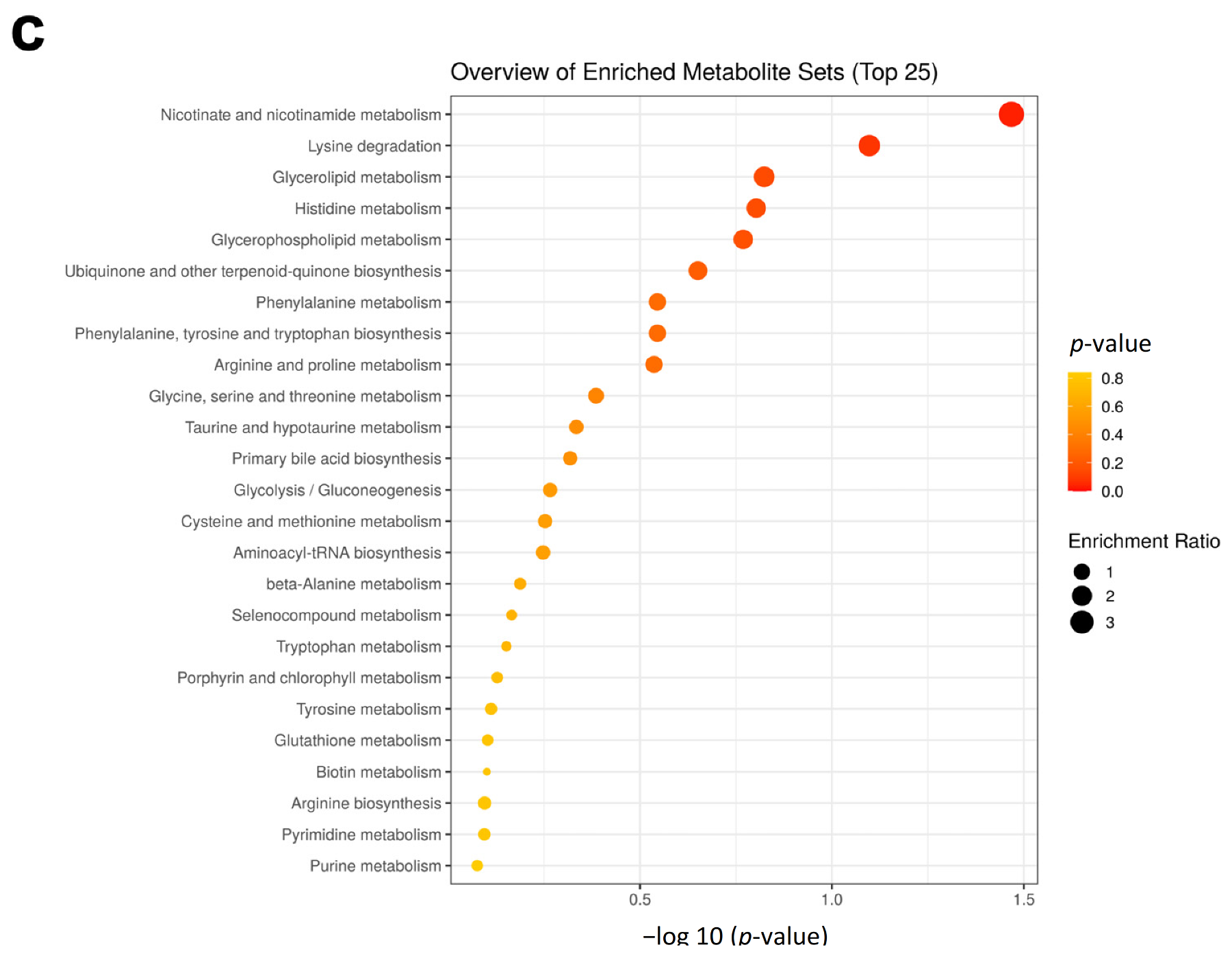
3.3. Bee Larva Protein Changed Plasma Metabolites
3.4. Plasma 3-Methylhistidine Was Decreased in Bee Larva Protein-Fed Mice
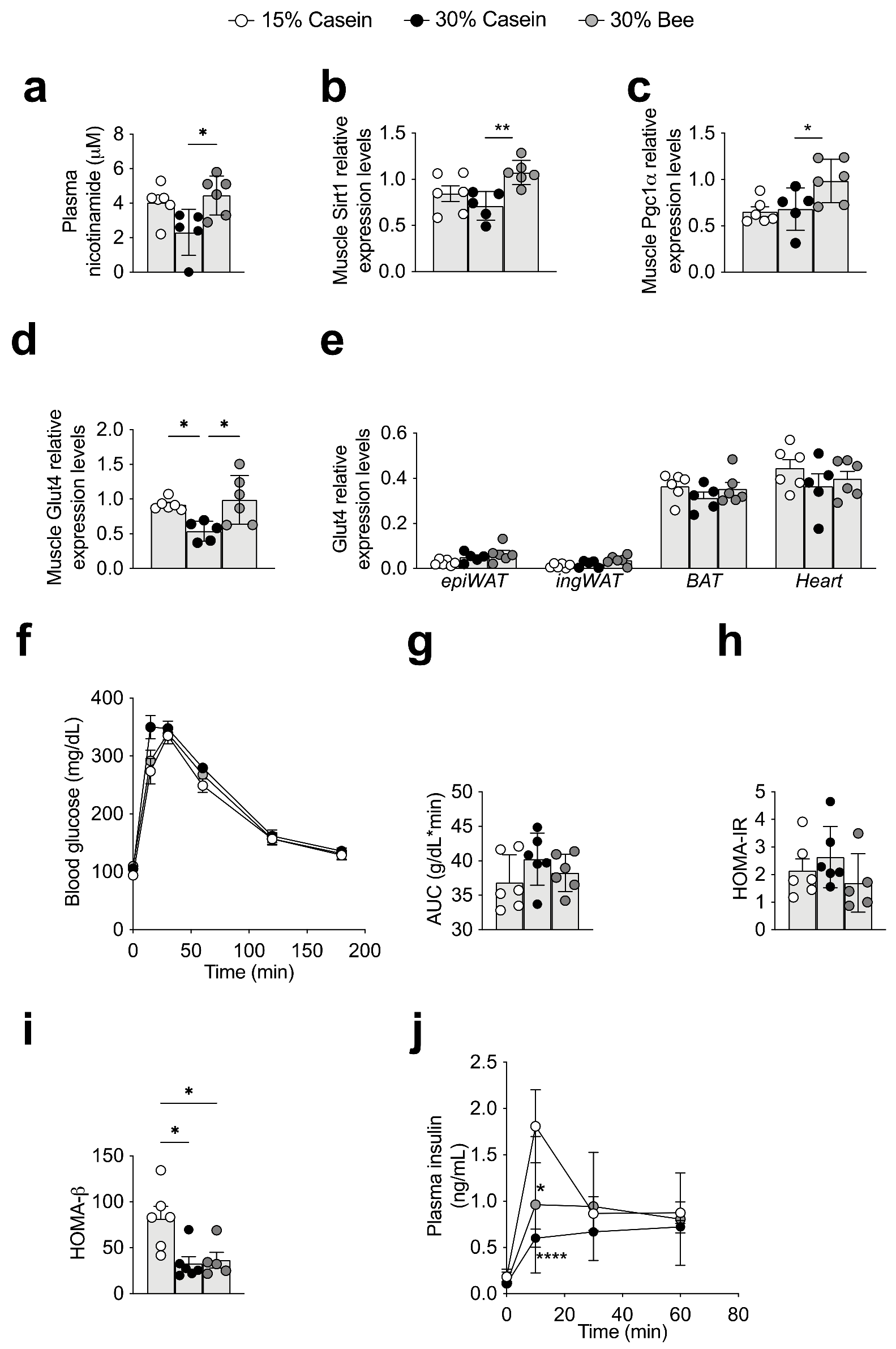
3.5. Plasma Nicotinamide Was Increased in Bee Larva-Fed Mice
3.6. Bee Larva Increased Glucose Uptake to Muscle
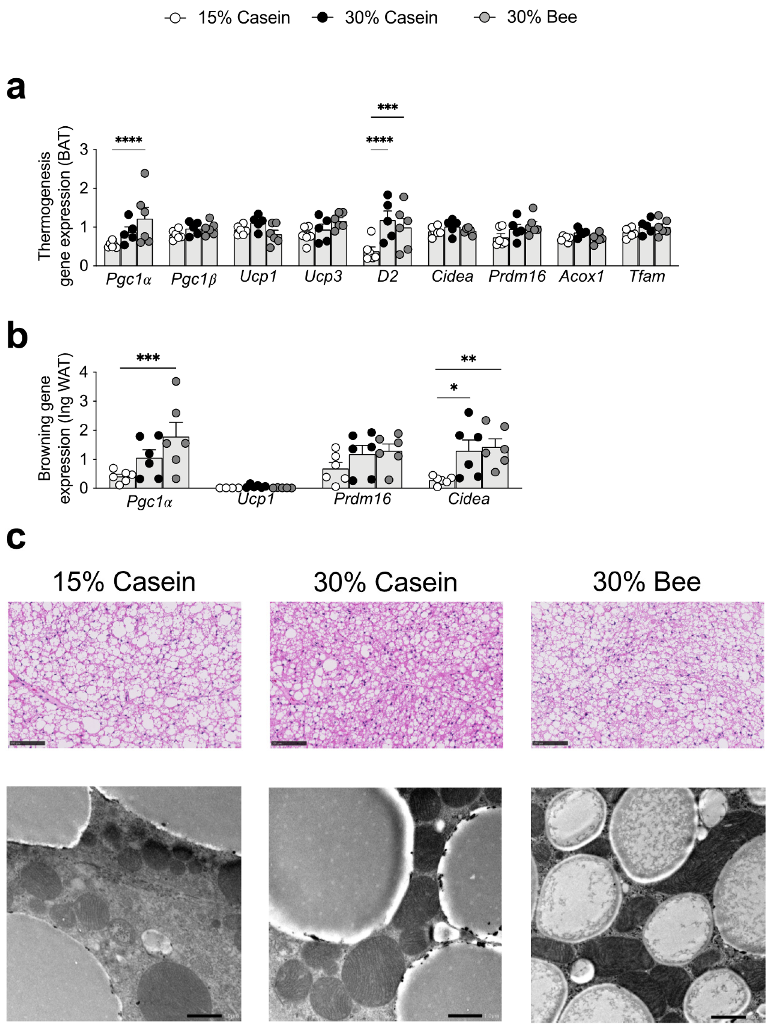
3.7. Bee Larva Stimulate Thermogenesis in Brown and White Adipose Tissue
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockstrom, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Foley, J.A.; Defries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermeulen, S.J.; Campbell, B.M.; Ingram, J.S. Climate change and food systems. Annu. Rev. Environ. Resour. 2012, 37, 195–222. [Google Scholar] [CrossRef] [Green Version]
- Steffen, W.; Richardson, K.; Rockstrom, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Sustainability. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molden, D. Comprehensive Assessment of Water Management in Agriculture. Water for Food, Water for Life: A Comprehensive Assessment of Water Management in Agriculture; International Water Management Institute: London, UK, 2007. [Google Scholar]
- Verneau, F.; Amato, M.; La Barbera, F. Edible Insects and Global Food Security. Insects 2021, 12, 472. [Google Scholar] [CrossRef]
- Parodi, A.; Leip, A.; de Boer, I.J.M.; Slegers, P.M.; Ziegler, F.; Temme, E.H.M.; Herrero, M.; Tuomisto, H.; Valin, H.; van Middelaar, C.E.; et al. The potential of future foods for sustainable and healthy diets. Nat. Sustain. 2018, 1, 782–789. [Google Scholar] [CrossRef] [Green Version]
- Huis, A.V.; Itterbeeck, J.V.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible insects: Future prospects for food and feed security; Forestry Paper 171; FAO: Rome, Italy, 2013. [Google Scholar]
- Huis, A.V. Edible insects contributing to food security? Agric. Food Secur. 2015, 4, 20. [Google Scholar] [CrossRef] [Green Version]
- OECD. Obesity Update. 2017. Available online: www.oecd.org/health/obesity-update.htm (accessed on 3 December 2019).
- United Nations Population Division. World Population Prospects: The 2019 Revision; United Nations: New York, NY, USA, 2017; Available online: https://esa.un.org/unpd/wpp (accessed on 8 September 2021).
- Cesard, N.; Komatsu, S.; Iwata, A. Processing insect abundance: Trading and fishing of zazamushi in Central Japan (Nagano Prefecture, Honshu Island). J. Ethnobiol. Ethnomed. 2015, 11, 78. [Google Scholar] [CrossRef] [Green Version]
- Nonaka, K. Feasting on insects. Entomol. Res. 2009, 39, 304–312. [Google Scholar] [CrossRef]
- Mitsuhashi, J. Insects as traditional foods in Japan. Ecol. Food Nutr. 1997, 36, 187–199. [Google Scholar] [CrossRef]
- Mitsuhashi, J. The Complete World of Entomophagy; Yasaka Shobo Inc.: Tokyo, Japan, 2008. (In Japanese) [Google Scholar]
- Nonaka, K. Cultural and commercial roles of edible wasps in Japan. In Forest Insects as Food: Humans Bite Back; Patrick, B., Durst, D.V.J., Robin, N.L., Kenichi, S., Eds.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010. [Google Scholar]
- Kim, T.K.; Yong, H.I.; Kim, Y.B.; Kim, H.W.; Choi, Y.S. Edible Insects as a Protein Source: A Review of Public Perception, Processing Technology, and Research Trends. Food Sci. Anim. Resour. 2019, 39, 521–540. [Google Scholar] [CrossRef] [Green Version]
- Kageyama, M.; Li, K.; Sun, S.; Xing, G.; Gao, R.; Lei, Z.; Zhang, Z. Anti-tumor and anti-metastasis activities of honey bee larvae powder by suppressing the expression of EZH2. Biomed. Pharmacother. 2018, 105, 690–696. [Google Scholar] [CrossRef]
- Jensen, A.B.; Evans, J.; Jonas-Levi, A.; Benjamin, O.; Martinez, I.; Dahle, B.; Roos, N.; Lecocq, A.; Foley, K. Standard methods for Apis mellifera brood as human food. J. Apic. Res. 2019, 58. [Google Scholar] [CrossRef] [Green Version]
- Dietemann, V.; Ellis, J.D.; Neumann, P. The COLOSS BEEBOOK Volume II, Standard methods for Apis mellifera pest and pathogen research: Introduction. J. Apic. Res. 2013, 52, 299. [Google Scholar] [CrossRef] [Green Version]
- Hashidume, T.; Kato, A.; Tanaka, T.; Miyoshi, S.; Itoh, N.; Nakata, R.; Inoue, H.; Oikawa, A.; Nakai, Y.; Shimizu, M.; et al. Single ingestion of soy beta-conglycinin induces increased postprandial circulating FGF21 levels exerting beneficial health effects. Sci. Rep. 2016, 6, 28183. [Google Scholar] [CrossRef] [Green Version]
- Wojcik, J.L.; Devassy, J.G.; Wu, Y.; Zahradka, P.; Taylor, C.G.; Aukema, H.M. Protein source in a high-protein diet modulates reductions in insulin resistance and hepatic steatosis in fa/fa Zucker rats. Obesity (Silver Spring) 2016, 24, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Mariotti, F. Animal and Plant Protein Sources and Cardiometabolic Health. Adv. Nutr. 2019, 10, S351–S366. [Google Scholar] [CrossRef]
- Berrazaga, I.; Micard, V.; Gueugneau, M.; Walrand, S. The Role of the Anabolic Properties of Plant- versus Animal-Based Protein Sources in Supporting Muscle Mass Maintenance: A Critical Review. Nutrients 2019, 11, 1825. [Google Scholar] [CrossRef] [Green Version]
- Levine, M.E.; Suarez, J.A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.W.; Madia, F.; Fontana, L.; Mirisola, M.G.; Guevara-Aguirre, J.; Wan, J.; et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014, 19, 407–417. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.K.; Yong, H.I.; Jang, H.W.; Kim, Y.B.; Choi, Y.S. Functional Properties of Extracted Protein from Edible Insect Larvae and Their Interaction with Transglutaminase. Foods 2020, 9, 591. [Google Scholar] [CrossRef]
- van Huis, A. Nutrition and health of edible insects. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 228–231. [Google Scholar] [CrossRef]
- Jeong, H.; Shin, K. What Is Required for Edible Insects to Become Medical Food? From a Health Professionals and Caregivers’ Perspective. Insects 2020, 11, 388. [Google Scholar] [CrossRef]
- Berryman, C.E.; Lieberman, H.R.; Fulgoni, V.L., 3rd; Pasiakos, S.M. Protein intake trends and conformity with the Dietary Reference Intakes in the United States: Analysis of the National Health and Nutrition Examination Survey, 2001–2014. Am. J. Clin. Nutr. 2018, 108, 405–413. [Google Scholar] [CrossRef]
- Austin, G.L.; Ogden, L.G.; Hill, J.O. Trends in carbohydrate, fat, and protein intakes and association with energy intake in normal-weight, overweight, and obese individuals: 1971–2006. Am. J. Clin. Nutr. 2011, 93, 836–843. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 2010, 6, 78–95. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef]
- Mitchell, S.J.; Bernier, M.; Aon, M.A.; Cortassa, S.; Kim, E.Y.; Fang, E.F.; Palacios, H.H.; Ali, A.; Navas-Enamorado, I.; Di Francesco, A.; et al. Nicotinamide Improves Aspects of Healthspan, but Not Lifespan, in Mice. Cell Metab. 2018, 27, 667–676. [Google Scholar] [CrossRef] [Green Version]
- Turner, R.C.; Holman, R.R.; Matthews, D.; Hockaday, T.D.; Peto, J. Insulin deficiency and insulin resistance interaction in diabetes: Estimation of their relative contribution by feedback analysis from basal plasma insulin and glucose concentrations. Metabolism 1979, 28, 1086–1096. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Rudenski, A.S.; Matthews, D.R.; Levy, J.C.; Turner, R.C. Understanding “insulin resistance”: Both glucose resistance and insulin resistance are required to model human diabetes. Metabolism 1991, 40, 908–917. [Google Scholar] [CrossRef]
- World Food Programme; United Nations. 2020—Global Report on Food Crises. Available online: https://www.wfp.org/publications/2020-global-report-food-crises (accessed on 5 May 2021).
- McLaren, D.S. The great protein fiasco revisited. Nutrition 2000, 16, 464–465. [Google Scholar] [CrossRef]
- McLaren, D.S. The great protein fiasco. Lancet 1974, 2, 93–96. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schluter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Ghosh, S.; Jung, C.; Meyer-Rochow, V.B. Nutritional value and chemical composition of larvae, pupae, and adults of worker honey bee, Apis mellifera ligustica as a sustainable food source. J. Asia Pac. Entomol. 2016, 19, 487–495. [Google Scholar] [CrossRef]
- Lizarazo, C.I.; Lampi, A.M.; Liu, J.W.; Sontag-Strohm, T.; Piironen, V.; Stoddard, F.L. Nutritive quality and protein production from grain legumes in a boreal climate. J. Sci. Food Agric. 2015, 95, 2053–2064. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; Lam, H.M.; Nguyen, H.T.; Siddique, K.H.M.; Varshney, R.K.; Colmer, T.D.; Cowling, W.; Bramley, H.; Mori, T.A.; Hodgson, J.M.; et al. Neglecting legumes has compromised human health and sustainable food production. Nat. Plants 2016, 2. [Google Scholar] [CrossRef]
- DeFoliart, G.R. Insects as human food: Gene DeFoliart discusses some nutritional and economic aspects. Crop Prot. 1992, 11, 395–399. [Google Scholar] [CrossRef]
- Leinonen, I.; Iannetta, P.P.M.; Rees, R.M.; Russell, W.; Watson, C.; Barnes, A.P. Lysine Supply Is a Critical Factor in Achieving Sustainable Global Protein Economy. Front. Sustain. Food Syst. 2019, 3, 27. [Google Scholar] [CrossRef]
- Liang, F.; Kume, S.; Koya, D. SIRT1 and insulin resistance. Nat. Rev. Endocrinol. 2009, 5, 367–373. [Google Scholar] [CrossRef]
- Fujita, S.; Volpi, E. Amino acids and muscle loss with aging. J. Nutr. 2006, 136, 277S–280S. [Google Scholar] [CrossRef]
- McKeran, R.O.; Halliday, D.; Purkiss, P.; Royston, P. 3-Methylhistidine excretion as an index of myofibrillar protein catabolism in neuromuscular disease. J. Neurol. Neurosurg. Psychiatry 1979, 42, 536–541. [Google Scholar] [CrossRef] [Green Version]
- Trappe, T.; Williams, R.; Carrithers, J.; Raue, U.; Esmarck, B.; Kjaer, M.; Hickner, R. Influence of age and resistance exercise on human skeletal muscle proteolysis: A microdialysis approach. J. Physiol. 2004, 554, 803–813. [Google Scholar] [CrossRef]
- Mussini, E.; Cornelio, F.; Dworzak, F.; Cotellessa, L.; Morandi, L.; Colombo, L.; De Ponte, G.; Marcucci, F. Content of methylhistidines in normal and pathological human skeletal muscles. Muscle Nerve 1983, 6, 423–429. [Google Scholar] [CrossRef]
- Soga, T.; Ohashi, Y.; Ueno, Y.; Naraoka, H.; Tomita, M.; Nishioka, T. Quantitative metabolome analysis using capillary electrophoresis mass spectrometry. J. Proteome Res. 2003, 2, 488–494. [Google Scholar] [CrossRef]
- Hirayama, A.; Ikeda, S.; Sato, A.; Soga, T. Amino Acid Analysis by Capillary Electrophoresis-Mass Spectrometry. Methods Mol. Biol. 2019, 2030, 307–313. [Google Scholar] [CrossRef]
- Wali, J.A.; Milner, A.J.; Luk, A.W.S.; Pulpitel, T.J.; Dodgson, T.; Facey, H.J.W.; Wahl, D.; Kebede, M.A.; Senior, A.M.; Sullivan, M.A.; et al. Impact of dietary carbohydrate type and protein-carbohydrate interaction on metabolic health. Nat. Metab. 2021, 3, 810–828. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokoyama, Y.; Shinohara, K.; Kitamura, N.; Nakamura, A.; Onoue, A.; Tanaka, K.; Hirayama, A.; Aw, W.; Nakamura, S.; Ogawa, Y.; et al. Metabolic Effects of Bee Larva-Derived Protein in Mice: Assessment of an Alternative Protein Source. Foods 2021, 10, 2642. https://doi.org/10.3390/foods10112642
Yokoyama Y, Shinohara K, Kitamura N, Nakamura A, Onoue A, Tanaka K, Hirayama A, Aw W, Nakamura S, Ogawa Y, et al. Metabolic Effects of Bee Larva-Derived Protein in Mice: Assessment of an Alternative Protein Source. Foods. 2021; 10(11):2642. https://doi.org/10.3390/foods10112642
Chicago/Turabian StyleYokoyama, Yoko, Kawori Shinohara, Naho Kitamura, Anna Nakamura, Ai Onoue, Kazuki Tanaka, Akiyoshi Hirayama, Wanping Aw, Shigeru Nakamura, Yoko Ogawa, and et al. 2021. "Metabolic Effects of Bee Larva-Derived Protein in Mice: Assessment of an Alternative Protein Source" Foods 10, no. 11: 2642. https://doi.org/10.3390/foods10112642
APA StyleYokoyama, Y., Shinohara, K., Kitamura, N., Nakamura, A., Onoue, A., Tanaka, K., Hirayama, A., Aw, W., Nakamura, S., Ogawa, Y., Fukuda, S., Tsubota, K., & Watanabe, M. (2021). Metabolic Effects of Bee Larva-Derived Protein in Mice: Assessment of an Alternative Protein Source. Foods, 10(11), 2642. https://doi.org/10.3390/foods10112642








