Significantly Different Lipid Profile Analysis of Litopenaeus vannamei under Low-Temperature Storage by UPLC-Q-Exactive Orbitrap/MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Shrimp Preparation and Reagents
2.2. Physical Properties Analysis
2.3. Lipid Extraction
2.4. Instrument Conditions
2.5. Statistics
3. Results and Discussion
3.1. Quality Analysis of Texture and Color
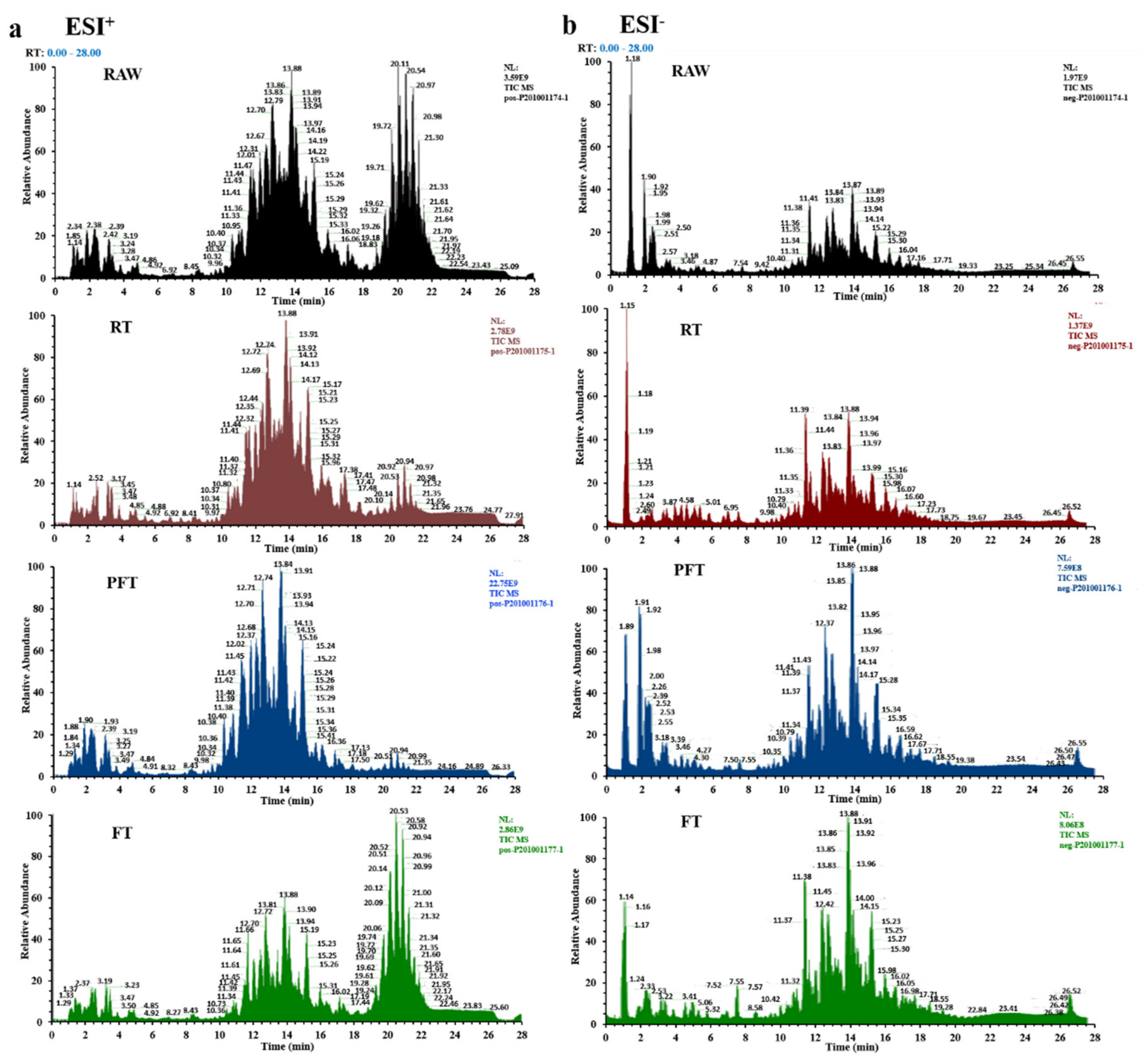
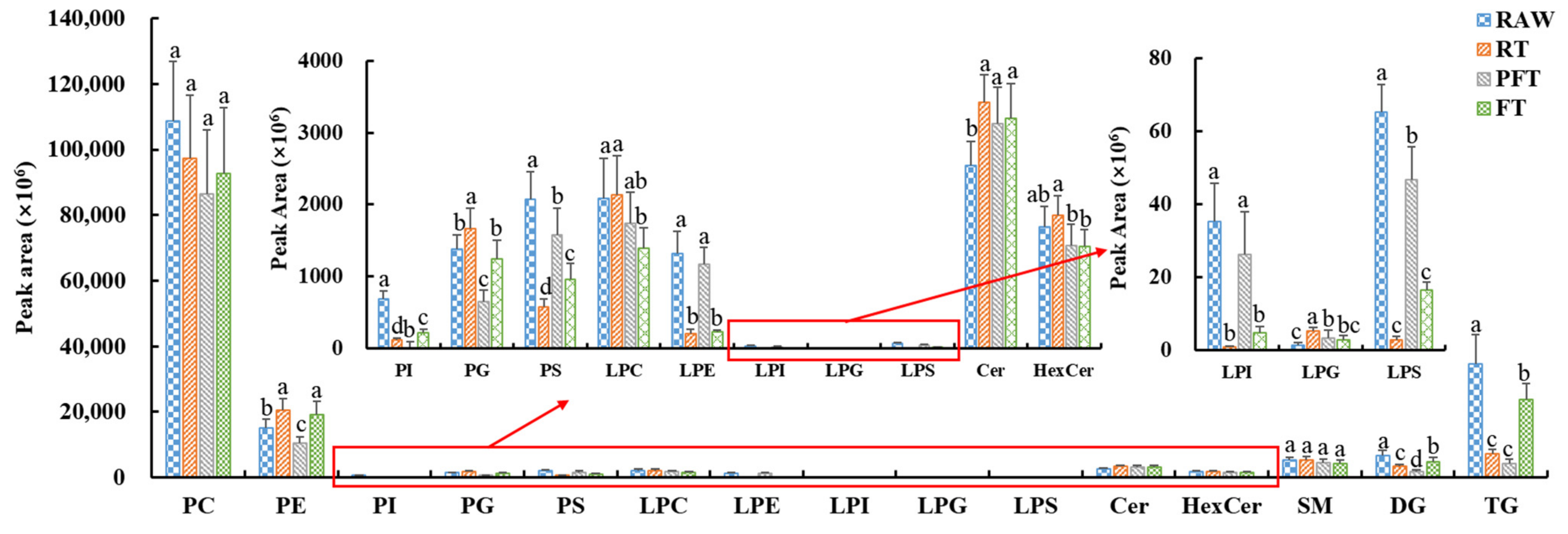
3.2. Lipid Species Differences
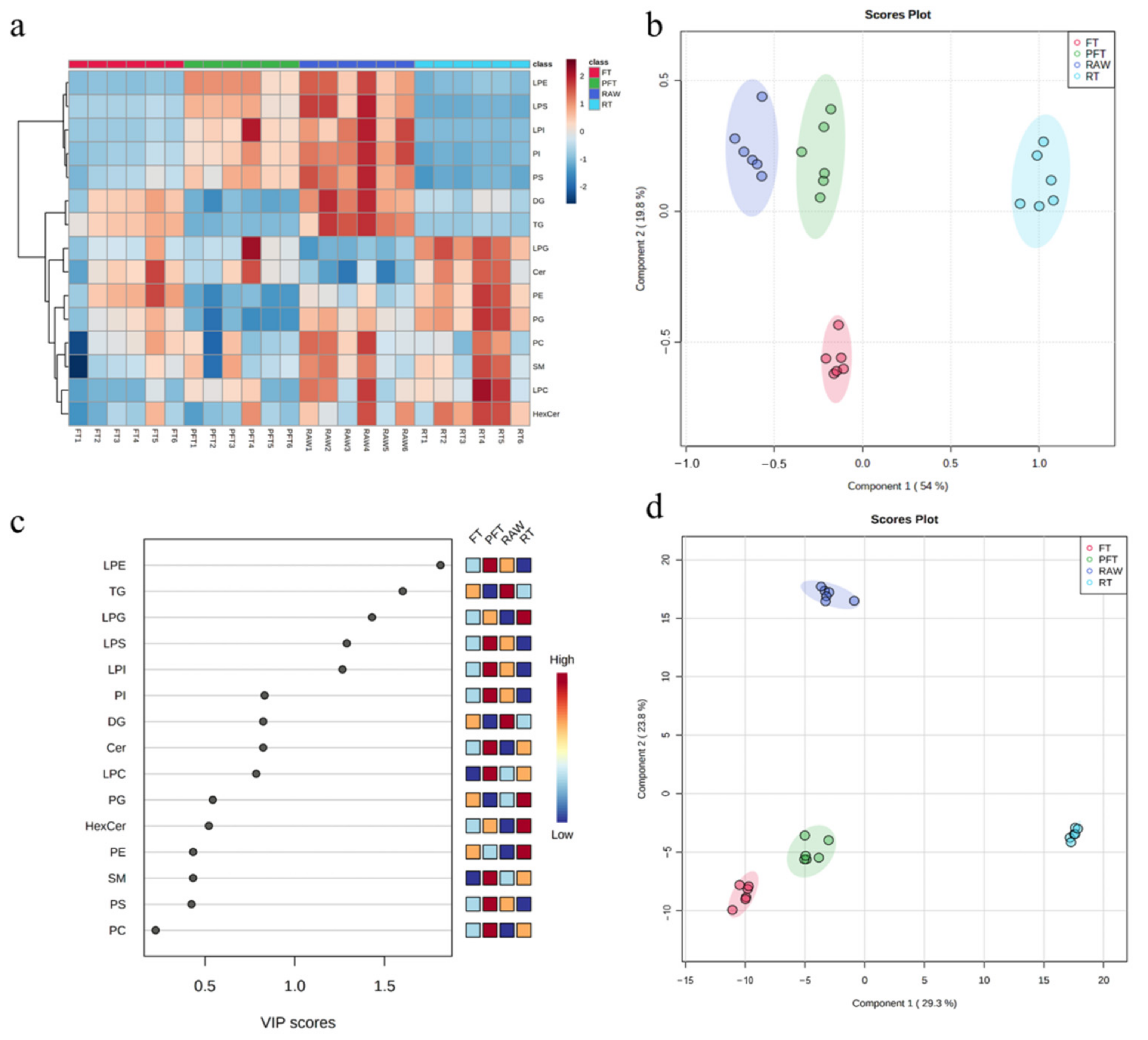
3.3. Multivariate Statistical Analysis and Lipid Metabolism Pathways
3.4. Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ekezie, F.-G.C.; Cheng, J.H.; Sun, D.W. Effects of mild oxidative and structural modifications induced by argon plasma on physicochemical properties of actomyosin from King Prawn (Litopenaeus vannamei). J. Agric. Food Chem. 2018, 66, 13285–13294. [Google Scholar] [CrossRef] [PubMed]
- Ekezie, F.G.C.; Cheng, J.H.; Sun, D.W. Effects of atmospheric pressure plasma jet on the conformation and physicochemical properties of myofibrillar proteins from king prawn (Litopenaeus vannamei). Food Chem. 2019, 276, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Udayasoorian, L.; Peter, M.; Sabina, K.; Indumathi, C.; Muthusamy, S. Comparative evaluation on shelf life extension of MAP packed Litopenaeus vannamei shrimp treated with natural extracts. LWT 2017, 77, 217–224. [Google Scholar] [CrossRef]
- Gallart-Jornet, L.; Rustad, T.; Barat, J.M.; Fito, P.; Escriche, I. Effect of superchilled storage on the freshness and salting behaviour of Atlantic salmon (Salmo salar) fillets. Food Chem. 2007, 103, 1268–1281. [Google Scholar] [CrossRef]
- Fernandez, K.; Aspe, E.; Roeckel, M. Scaling up parameters for shelf-life extension of Atlantic Salmon (Salmo salar) fillets using superchilling and modified atmosphere packaging. Food Control 2010, 21, 857–862. [Google Scholar] [CrossRef]
- Duun, A.S.; Rustad, T. Quality changes during superchilled storage of cod (Gadus morhua) fillets. Food Chem. 2007, 105, 1067–1075. [Google Scholar] [CrossRef]
- Soyer, A.; Özalp, B.; Dalmış, Ü.; Bilgin, V. Effects of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat. Food Chem. 2010, 120, 1025–1030. [Google Scholar] [CrossRef]
- Kubow, S. Routes of formation and toxic consequences of lipid oxidation products in foods. Free Radic. Biol. Med. 1992, 12, 63–81. [Google Scholar] [CrossRef]
- Lee, C.H.; Krueger, C.G.; Reed, J.D.; Richards, M.P. Inhibition of hemoglobin-mediated lipid oxidation in washed fish muscle by cranberry components. Food Chem. 2006, 99, 591–599. [Google Scholar] [CrossRef]
- Li, D.Y.; Zhou, D.Y.; Yin, F.W.; Dong, X.P.; Xie, H.K.; Liu, Z.Y.; Li, A.; Li, J.X.; Rakariyatham, K.; Shahidi, F. Impact of different drying processes on the lipid deterioration and color characteristics of Penaeus vannamei. J. Sci. Food Agric. 2020, 100, 2544–2553. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Menard, O. Human milk fat globules: Polar lipid composition and in situ structural investigations revealing the heterogeneous distribution of proteins and the lateral segregation of sphingomyelin in the biological membrane. Colloids Surf. B Biointerfaces 2011, 83, 29–41. [Google Scholar] [CrossRef]
- Sun, Y.E.; Wang, W.D.; Chen, H.W.; Li, C. Autoxidation of unsaturated lipids in food emulsion. Crit. Rev. Food Sci. Nutr. 2011, 51, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Shang, K.; Li, X.; Zhang, C.H.; Liu, J.Q.; Huang, D.Q. Characterization and discrimination of selected China’s domestic pork using an LC-MS-based lipidomics approach. Food Control. 2019, 100, 305–314. [Google Scholar] [CrossRef]
- Garcia, J.S.; Sanvido, G.B.; Saraiva, S.A.; Zacca, J.J.; Cosso, R.G.; Eberlin, M.N. Bovine milk powder adulteration with vegetable oils or fats revealed by MALDI-QTOF MS. Food Chem. 2012, 131, 722–726. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Santos, J.M.; Breitkreitz, M.C.; Santos Ferreira, J.M.; Pontes Lins, P.M.; Farias, S.C.; de Morais, D.R.; Eberlin, M.N.; Grespan Bottoli, C.B. Characterization of the lipid profile from coconut (Cocos nucifera L.) oil of different varieties by electrospray ionization mass spectrometry associated with principal component analysis and independent component analysis. Food Res. Int. 2019, 123, 189–197. [Google Scholar] [CrossRef]
- Shi, C.; Guo, H.; Wu, T.; Tao, N.; Wang, X.; Zhong, J. Effect of three types of thermal processing methods on the lipidomics profile of tilapia fillets by UPLC-Q-Extractive Orbitrap mass spectrometry. Food Chem. 2019, 298, 125029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Duan, X.; Shang, B.; Hong, Y.; Sun, H. Analysis of lipidomics profile of rice and changes during storage by UPLC-Q-extractive orbitrap mass spectrometry. Food Res. Int. 2021, 142, 110214. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Bligh, E.G. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.Q.; Zhao, Y.; Zhu, D.; Pang, X.M.; Liu, Y.; Frew, R.; Chen, G. Lipidomics profiling of goat milk, soymilk and bovine milk by UPLC-Q-Exactive Orbitrap Mass Spectrometry. Food Chem. 2017, 224, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Troy, D.J.; Kerry, J.P. Consumer perception and the role of science in the meat industry. Meat Sci. 2010, 86, 214–226. [Google Scholar] [CrossRef]
- Aussanasuwannakul, A.; Slider, S.D.; Salem, M.; Yao, J.; Kenney, P.B. Comparison of variable-blade to allo-kramer shear method in assessing rainbow trout (Oncorhynchus mykiss) fillet firmness. J. Food Sci. 2012, 77, S335–S341. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, N.P.; Benjakul, S. Effect of ferulic acid on inhibition of polyphenoloxidase and quality changes of Pacific white shrimp (Litopenaeus vannamei) during iced storage. Food Chem. 2009, 116, 323–331. [Google Scholar] [CrossRef]
- Benjakul, S.; Visessanguan, W.; Tanaka, M. Properties of phenoloxidase isolated from the cephalothorax of kuruma prawn (Penaeus japonicus). J. Food Biochem. 2005, 29, 470–485. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W.; Han, Z.; Zeng, X.A. Texture and structure measurements and analyses for evaluation of fish and fillet freshness quality: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 52–61. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, D.; Su, M.; Tan, D.; Zhang, X.; Jia, M.; Wu, H.; Chen, G. Lipidomics strategy for the identification of ultra-high temperature and reconstituted milk by UPLC-Q-Exactive Orbitrap mass spectrometry. Food Anal. Methods 2021, 14, 1064–1073. [Google Scholar] [CrossRef]
- Denny, P.W.; Field, M.C.; Smith, D.F. GPI-anchored proteins and glycoconjugates segregate into lipid rafts in Kinetoplastida. FEBS Lett. 2001, 491, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Ikezawa, H. Glycosylphosphatidylinositol (GPI)-anchored proteins. Biol. Pharm. Bull. 2002, 25, 409–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.C.; Wright, L.C.; Golding, J.C.; Sorrell, T.C. Purification and characterization of secretory phospholipase B, lysophospholipase and lysophospholipase/transacylase from a virulent strain of the pathogenic fungus Cryptococcus neoformans. Biochem. J. 2000, 347, 431–439. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, S.S.; Hellgren, L.I.; Jonsson, G.E.; Xu, X.B. Phospholipase C-catalyzed sphingomyelin hydrolysis in a membrane reactor for ceramide production. J. Membr. Sci. 2008, 325, 895–902. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, D.Y.; Liu, Z.Y.; Yin, F.W.; Liu, Z.Q.; Li, D.Y.; Shahidi, F. Hydrolysis and oxidation of lipids in mussel Mytilus edulis during cold storage. Food Chem. 2019, 272, 109–116. [Google Scholar] [CrossRef] [PubMed]
| RAW 1 | RT 2 | PFT 3 | FT 4 | |
|---|---|---|---|---|
| L* 5 | 41.04 ± 0.89 a 8 | 28.52 ± 1.43 d | 31.46 ± 0.54 c | 39.48 ± 0.31 b |
| a* 6 | 0.2 ± 0.13 c | 1.75 ± 0.43 a | 1.51 ± 0.22 a | 0.9 ± 0.12 b |
| b* 7 | 0.89 ± 0.25 b | 4.11 ± 0.58 a | 1.41 ± 0.31 b | 1.04 ± 0.36 b |
| Springiness (N) | 0.99 ± 0.01 a | 0.62 ± 0.05 c | 0.9 ± 0.02 b | 0.93 ± 0.02 b |
| Cohesiveness | 0.44 ± 0.02 a | 0.3 ± 0.02 c | 0.39 ± 0.01 b | 0.43 ± 0.01 a |
| Gumminess (g) | 816.53 ± 97.59 a | 534.17 ± 34.88 c | 615.65 ± 20.39 b | 757.2 ± 2.95 a |
| Chewiness (g·mm) | 700.66 ± 24.21 a | 421.23 ± 24.56 d | 490.18 ± 52.15 c | 552.06 ± 56.03 b |
| Hardness (g) | 1887.43 ± 85.81 a | 1009.8 ± 93.01 c | 1362.43 ± 27.81 b | 1778.55 ± 117.51 a |
| Treatments | MS Spectra Mode | PC | PE | PI | PG | PS | LPC | LPE | LPI | LPG | LPS | Cer | HexCer | SM | DG | TG | Sum |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RAW | ESI+ | 349 | 128 | 10 | 3 | 14 | 42 | 22 | 1 | 0 | 6 | 30 | 0 | 94 | 173 | 621 | 1493 |
| ESI− | 217 | 203 | 35 | 31 | 88 | 16 | 11 | 5 | 4 | 0 | 121 | 60 | 0 | 0 | 0 | 801 | |
| RT | ESI+ | 346 | 129 | 8 | 2 | 14 | 42 | 21 | 1 | 0 | 6 | 30 | 0 | 94 | 173 | 615 | 1481 |
| ESI− | 217 | 198 | 33 | 31 | 88 | 16 | 10 | 4 | 5 | 0 | 123 | 60 | 0 | 0 | 0 | 795 | |
| PFT | ESI+ | 348 | 124 | 10 | 3 | 14 | 42 | 22 | 1 | 0 | 6 | 30 | 0 | 94 | 171 | 615 | 1480 |
| ESI− | 217 | 197 | 35 | 28 | 87 | 16 | 11 | 3 | 5 | 0 | 123 | 60 | 0 | 0 | 0 | 792 | |
| FT | ESI+ | 349 | 129 | 10 | 2 | 14 | 42 | 21 | 1 | 0 | 6 | 29 | 0 | 94 | 173 | 622 | 1492 |
| ESI− | 217 | 199 | 35 | 31 | 88 | 16 | 11 | 5 | 5 | 0 | 123 | 59 | 0 | 0 | 0 | 799 |
| Molecular Species | Ion (m/z) | L* 1 | a* 2 | b* 3 | Springiness | Gumminess | Cohesiveness | Chewiness | Hardness |
|---|---|---|---|---|---|---|---|---|---|
| PC4 | |||||||||
| (11:0/16:1) | 662.48 | −0.676 | 0.589 | 0.934 | −0.902 | −0.644 | −0.861 | −0.575 | −0.741 |
| (26:5e) | 626.42 | −0.677 | 0.588 | 0.930 | −0.915 | −0.637 | −0.880 | −0.578 | −0.755 |
| (29:2) | 688.49 | −0.625 | 0.558 | 0.918 | −0.921 | −0.628 | −0.854 | −0.564 | −0.718 |
| (47:1) | 942.79 | −0.711 | 0.607 | 0.930 | −0.910 | −0.679 | −0.885 | −0.584 | −0.781 |
| PE5 | |||||||||
| (15:0/16:1) | 674.48 | −0.695 | 0.577 | 0.948 | −0.930 | −0.657 | −0.897 | −0.592 | −0.780 |
| (16:1/14:1) | 658.45 | −0.732 | 0.622 | 0.962 | −0.954 | −0.708 | −0.910 | −0.657 | −0.804 |
| (36:3e) | 750.54 | −0.656 | 0.610 | 0.919 | −0.914 | −0.639 | −0.851 | −0.630 | −0.734 |
| PG6 | |||||||||
| (16:1/14:0) | 691.46 | −0.717 | 0.608 | 0.948 | −0.942 | −0.697 | −0.697 | −0.635 | −0.798 |
| (17:1/16:1) | 731.49 | −0.707 | 0.584 | 0.956 | −0.948 | −0.685 | −0.685 | −0.623 | −0.795 |
| LPG7 | |||||||||
| (16:1) | 481.26 | −0.707 | 0.615 | 0.924 | −0.917 | −0.655 | −0.898 | −0.596 | −0.789 |
| DG8 | |||||||||
| (16:0/23:0) | 684.65 | −0.698 | 0.558 | 0.928 | −0.917 | −0.668 | −0.894 | −0.574 | −0.800 |
| (18:2e) | 355.28 | −0.696 | 0.590 | 0.939 | −0.907 | −0.650 | −0.878 | −0.579 | −0.766 |
| (36:0e) | 633.58 | 0.937 | −0.861 | −0.643 | 0.703 | 0.866 | 0.792 | 0.849 | 0.902 |
| (38:3e) | 633.58 | 0.937 | −0.861 | −0.643 | 0.703 | 0.866 | 0.792 | 0.849 | 0.902 |
| TG9 | |||||||||
| (14:0/14:0/20:3) | 801.70 | −0.909 | 0.790 | 0.936 | −0.932 | −0.847 | −0.847 | −0.749 | −0.939 |
| (16:0/10:0/16:1) | 738.66 | 0.939 | −0.805 | −0.734 | 0.746 | 0.887 | 0.887 | 0.785 | 0.921 |
| (16:0/17:0/22:6) | 910.79 | 0.943 | −0.858 | −0.641 | 0.693 | 0.877 | 0.877 | 0.832 | 0.903 |
| (16:0/18:1/22:1) | 937.82 | 0.952 | −0.820 | −0.693 | 0.735 | 0.880 | 0.880 | 0.791 | 0.920 |
| (16:0/18:1/24:1) | 965.85 | 0.935 | −0.788 | −0.660 | 0.685 | 0.860 | 0.860 | 0.756 | 0.900 |
| (16:0/19:0/22:6) | 938.82 | 0.941 | −0.821 | −0.650 | 0.688 | 0.865 | 0.865 | 0.788 | 0.902 |
| (16:1/12:0/16:1) | 764.68 | 0.946 | −0.827 | −0.689 | −0.689 | 0.882 | 0.821 | 0.800 | 0.920 |
| (17:0/18:2/18:3) | 884.77 | 0.931 | −0.881 | −0.635 | −0.635 | 0.903 | 0.757 | 0.854 | 0.890 |
| (18:1/12:0/14:0) | 766.69 | 0.949 | −0.823 | −0.691 | −0.691 | 0.887 | 0.822 | 0.800 | 0.915 |
| (18:1/12:3/18:1) | 814.69 | 0.923 | −0.845 | −0.705 | −0.705 | 0.880 | 0.826 | 0.840 | 0.914 |
| (18:1/18:1/21:1) | 949.82 | 0.942 | −0.792 | −0.658 | −0.658 | 0.859 | 0.797 | 0.755 | 0.903 |
| (18:1/18:2/22:0) | 963.84 | 0.946 | −0.799 | −0.674 | −0.674 | 0.864 | 0.816 | 0.763 | 0.909 |
| (18:1/18:2/22:1) | 961.82 | 0.957 | −0.831 | −0.721 | −0.721 | 0.902 | 0.847 | 0.812 | 0.928 |
| (18:1/20:2/22:5) | 976.83 | 0.942 | −0.835 | −0.647 | −0.647 | 0.876 | 0.795 | 0.806 | 0.906 |
| (18:1/20:5/23:0) | 994.88 | 0.945 | −0.845 | −0.646 | −0.646 | 0.867 | 0.791 | 0.813 | 0.904 |
| (18:1/22:1/22:6) | 1004.86 | 0.956 | −0.820 | −0.699 | −0.699 | 0.876 | 0.828 | 0.791 | 0.928 |
| (18:1/22:6/24:1) | 1032.90 | 0.925 | −0.746 | −0.658 | −0.658 | 0.827 | 0.796 | 0.715 | 0.903 |
| (18:1/24:1/24:1) | 1071.00 | 0.952 | −0.863 | −0.657 | −0.657 | 0.875 | 0.800 | 0.839 | 0.918 |
| (19:0/18:1/22:6) | 964.83 | 0.945 | −0.822 | −0.653 | −0.653 | 0.868 | 0.798 | 0.782 | 0.906 |
| (19:0/22:1/22:1) | 1030.97 | 0.946 | −0.872 | −0.645 | −0.645 | 0.883 | 0.790 | 0.846 | 0.905 |
| (19:1/18:1/22:6) | 962.82 | 0.949 | −0.849 | −0.645 | −0.645 | 0.884 | 0.785 | 0.813 | 0.906 |
| (20:1/18:1/22:6) | 976.83 | 0.946 | −0.796 | −0.686 | −0.686 | 0.872 | 0.823 | 0.757 | 0.910 |
| (20:1/18:1/24:0) | 1016.96 | 0.949 | −0.822 | −0.664 | −0.664 | 0.875 | 0.806 | 0.788 | 0.912 |
| (20:1e/20:5/20:5) | 956.81 | 0.946 | −0.824 | −0.675 | −0.675 | 0.895 | 0.800 | 0.784 | 0.911 |
| (22:0/18:2/20:5) | 961.82 | 0.955 | −0.841 | −0.722 | −0.722 | 0.893 | 0.845 | 0.843 | 0.935 |
| (22:1/18:2/22:6) | 1002.85 | 0.941 | −0.876 | −0.672 | −0.672 | 0.886 | 0.814 | 0.867 | 0.913 |
| (22:1/18:2/23:0) | 1028.96 | 0.951 | −0.900 | −0.644 | −0.644 | 0.887 | 0.789 | 0.869 | 0.903 |
| (22:2/18:2/22:6) | 1000.83 | 0.937 | −0.850 | −0.649 | −0.649 | 0.883 | 0.790 | 0.826 | 0.906 |
| (24:1/18:2/22:6) | 1030.88 | 0.945 | −0.800 | −0.671 | −0.671 | 0.863 | 0.803 | 0.758 | 0.906 |
| (24:1/18:2/23:0) | 1056.99 | 0.948 | −0.857 | −0.652 | −0.652 | 0.877 | 0.794 | 0.834 | 0.912 |
| (24:1/18:2/24:1) | 1068.99 | 0.948 | −0.863 | −0.651 | −0.651 | 0.884 | 0.794 | 0.835 | 0.909 |
| (25:0/18:1/18:1) | 1002.94 | 0.939 | −0.868 | −0.636 | −0.636 | 0.880 | 0.781 | 0.843 | 0.901 |
| (26:0/18:1/18:2) | 1014.94 | 0.945 | −0.820 | −0.651 | −0.651 | 0.865 | 0.799 | 0.783 | 0.907 |
| (26:0/18:2/18:2) | 1012.93 | 0.936 | −0.835 | −0.640 | −0.640 | 0.879 | 0.774 | 0.802 | 0.900 |
| (28:0/18:1/18:1) | 1044.99 | 0.943 | −0.831 | −0.647 | −0.647 | 0.879 | 0.798 | 0.795 | 0.902 |
| (28:0/18:1/18:2) | 1042.97 | 0.949 | −0.841 | −0.662 | −0.662 | 0.879 | 0.803 | 0.812 | 0.912 |
| (29:0/18:1/18:1) | 1059.00 | 0.952 | −0.835 | −0.653 | −0.653 | 0.860 | 0.800 | 0.793 | 0.903 |
| (30:0/18:1/18:1) | 1073.02 | 0.943 | −0.817 | −0.650 | −0.650 | 0.860 | 0.796 | 0.788 | 0.907 |
| (50:6e) | 831.68 | −0.867 | 0.759 | 0.959 | 0.959 | −0.817 | −0.957 | −0.734 | −0.906 |
| (59:6) | 966.85 | 0.942 | −0.823 | −0.650 | −0.650 | 0.858 | 0.800 | 0.794 | 0.908 |
| (62:6e) | 994.92 | 0.932 | −0.875 | −0.624 | −0.624 | 0.903 | 0.769 | 0.839 | 0.886 |
| (63:9) | 1016.86 | 0.949 | −0.861 | −0.648 | −0.648 | 0.876 | 0.797 | 0.826 | 0.908 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Chen, Y.; Chen, Y.; Liang, P.; Pang, J.; Zhu, B.; Dong, X. Significantly Different Lipid Profile Analysis of Litopenaeus vannamei under Low-Temperature Storage by UPLC-Q-Exactive Orbitrap/MS. Foods 2021, 10, 2624. https://doi.org/10.3390/foods10112624
Wang S, Chen Y, Chen Y, Liang P, Pang J, Zhu B, Dong X. Significantly Different Lipid Profile Analysis of Litopenaeus vannamei under Low-Temperature Storage by UPLC-Q-Exactive Orbitrap/MS. Foods. 2021; 10(11):2624. https://doi.org/10.3390/foods10112624
Chicago/Turabian StyleWang, Shengnan, Yongshi Chen, Yu Chen, Peng Liang, Jie Pang, Beiwei Zhu, and Xiuping Dong. 2021. "Significantly Different Lipid Profile Analysis of Litopenaeus vannamei under Low-Temperature Storage by UPLC-Q-Exactive Orbitrap/MS" Foods 10, no. 11: 2624. https://doi.org/10.3390/foods10112624
APA StyleWang, S., Chen, Y., Chen, Y., Liang, P., Pang, J., Zhu, B., & Dong, X. (2021). Significantly Different Lipid Profile Analysis of Litopenaeus vannamei under Low-Temperature Storage by UPLC-Q-Exactive Orbitrap/MS. Foods, 10(11), 2624. https://doi.org/10.3390/foods10112624





