Chemical Composition and Sensory Evaluation of Saffron
Abstract
:1. Introduction
2. Materials and Methods
2.1. Saffron Samples and Preparation
2.2. HPLC/DAD Analysis
2.3. Nutritional Table
2.4. Descriptive Sensory Analysis
2.5. Statistical Analysis
3. Results
3.1. Chemical Profile of Saffron Samples
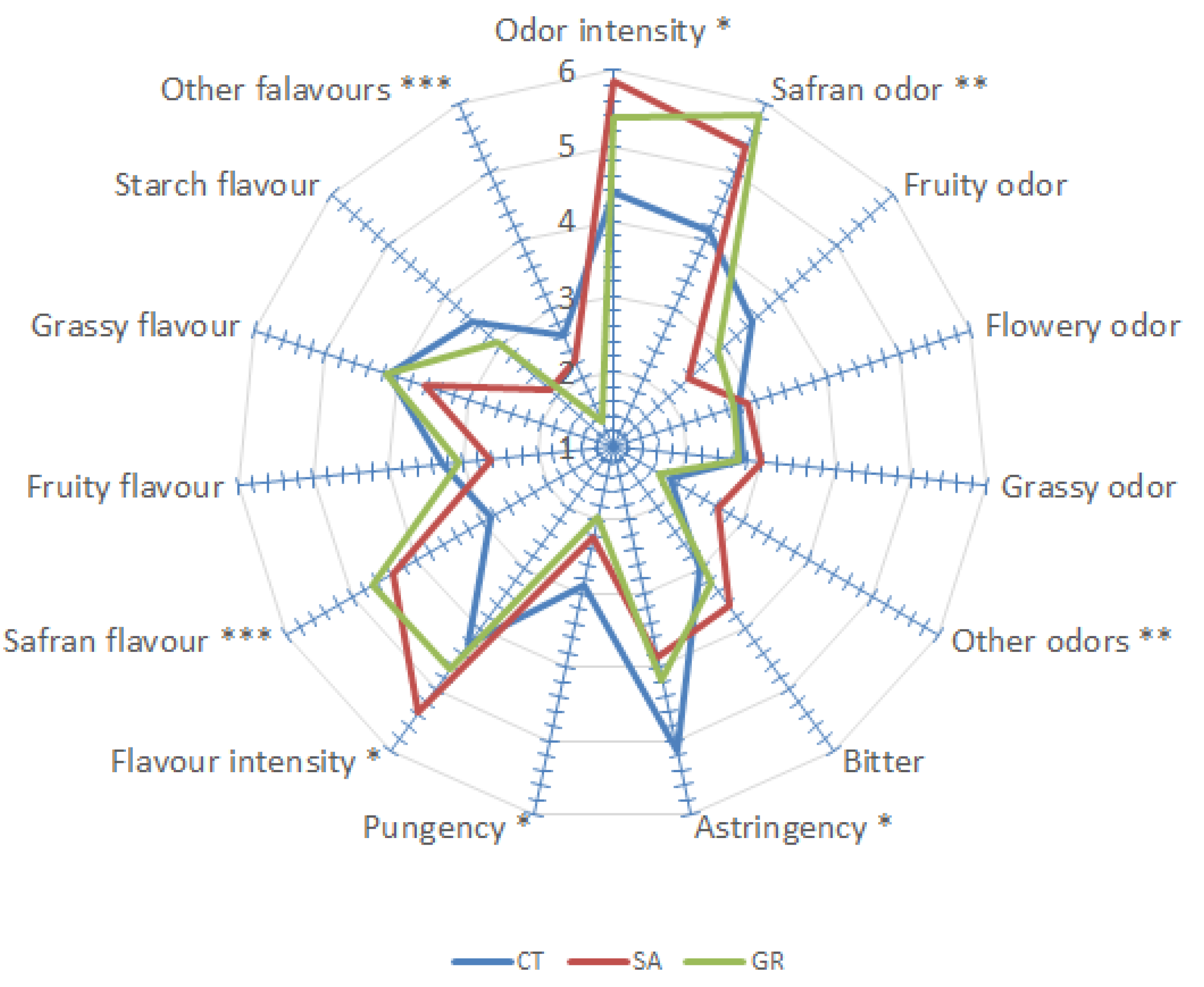
3.2. Sensory Profile of the Saffron Samples
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ríos, J.L.; Recio, M.C.; Giner, R.M.; Mhnez, S. An Update Review of Saffron and its Active Constituents. Phytother. Res. 1996, 10, 189–193. [Google Scholar] [CrossRef]
- Fernandez, J.A. Biology, biotechnology and biomedicine of saffron. Recent Res. Dev. Plant Sci. 2004, 2, 127–159. [Google Scholar]
- Gresta, F.; Lombardo, M.G.; Siracusa, L.; Ruberto, G. Saffron, an alternative crop for sustainable agricultural systems. A review. Agron. Sustain. Dev. 2008, 28, 95–112. [Google Scholar] [CrossRef] [Green Version]
- Pieroni, A. Medicinal plants and food medicines in the folk traditions of the upper Lucca Province, Italy. J. Ethnopharmacol. 2000, 70, 235–273. [Google Scholar] [CrossRef]
- Pieroni, A.; Quave, C.L.; Villanelli, M.L.; Mangino, P.; Sabbatini, G.; Santini, L.; Boccetti, T.; Profili, M.; Ciccioli, T.; Rampad, L.G.; et al. Ethnopharmacognostic survey on the natural ingredients used in folk cosmetics, cosmeceuticals and remedies for healing skin diseases in the inland Marches, Central-Eastern Italy. J. Ethnopharmacol. 2004, 91, 331–344. [Google Scholar] [CrossRef]
- Mosaddegh, M.; Naghibi, F.; Moazzeni, H.; Pirani, A.; Esmaeili, S. Ethnobotanical survey of herbal remedies traditionally used in Kohghiluyeh va Boyer Ahmad province of Iran. J. Ethnopharmacol. 2012, 141, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Nassiri-Asl, M. Avicenna’s (Ibn Sina) the Canon of Medicine and Saffron (Crocus sativus): A review. Phytother. Res. 2013, 27, 475–483. [Google Scholar] [CrossRef]
- Joukar, S.; Najafipour, H.; Khaksari, M.; Sepehri, G.; Shahrokhi, N.; Dabiri, S.; Gholamhoseinian, A.; Hasanzadeh, S. The Effect of Saffron Consumption on Biochemical and Histopathological Heart Indices of Rats with Myocardial Infarction. Cardiovasc. Toxicol. 2010, 10, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Sadeghnia, R.; Kamkar, M.; Assadpour, E.; Boroushaki, M.T.; Ghorbani, A. Protective Effect of Safranal, a Constituent of Crocus sativus, on Quinolinic Acid-induced Oxidative Damage in Rat Hippocampus. Hamid Iran. J. Basic Med. Sci. 2013, 16, 73–82. [Google Scholar]
- Bisti, S.; Maccarone, R.; Falsini, B. Saffron and retina: Neuroprotection and pharmacokinetics. Visual Neurosci. 2014, 31, 355–361. [Google Scholar] [CrossRef]
- Saki, K.; Bahmani, M.; Rafieian-Kopaei, M. The effect of most important medicinal plants on two important psychiatric disorders (anxiety and depression)—A review. Asian Pac. J. Trop. Med. 2014, 7, S34–S42. [Google Scholar] [CrossRef] [Green Version]
- Khazdair, M.R.; Boskabady, M.H.; Rezaee, R.; Tsatsakis, A.M. The effects of Crocus sativus (saffron) and its constituents on nervous system: A review. Avicenna J. Phytomed 2015, 5, 376–391. [Google Scholar] [PubMed]
- Kyriakoudi, A.; Ordoudi, S.A.; Roldán-Medina, M.; Tsimidou, M.Z. Saffron, A Functional Spice. Austin J. Nutr. Food Sci. 2015, 3, 32–38. [Google Scholar]
- Bukhari, S.I.; Syed, I.; Manzoor, M.; Dhar, M.k. A comprehensive review of the pharmacological potential of Crocus sativus and its bioactive apocarotenoids. Biomed. Pharmacother. 2018, 98, 733–745. [Google Scholar] [CrossRef]
- Finley, J.W.; Gao, S.A. Perspective on Crocus sativus L. (Saffron) Constituent Crocin: A Potent Water-Soluble Antioxidant and Potential Therapy for Alzheimer’s Disease. J. Agric. Food Chem. 2017, 65, 1005–1020. [Google Scholar] [CrossRef]
- Shaterzadeh-Yazdi, H.; Samarghandian, S.; Farkhondeh, T. Effects of Crocins in the Management of Neurodegenerative Pathologies: A Review. Neurophysiology 2018, 50, 302–308. [Google Scholar] [CrossRef]
- Alonso, G.L.; Salinas, M.R.; Garijo, J.; Sanchez-Fernandez, M.A. Composition of crocins and picocrocin from Spanish saffron (Crocus sativus L.). J. Food Qual. 2001, 24, 219–233. [Google Scholar] [CrossRef]
- Anastasakis, E.; Kanakis, C.; Pappas, C.; Maggi, L.; Del Campo, C.P.; Carmona, M.; Alonso, G.L.; Polissiou, M.G. Geographical differentiation of saffron by GC–MS/FID and chemometrics. Eur. Food Res. Technol. 2009, 229, 899–905. [Google Scholar] [CrossRef]
- Anastasakis, E.; Kanakis, C.; Pappas, C.; Maggi, L.; Del Campo, C.P.; Carmona, M.; Alonso, G.L.; Polissiou, M.G. Differentiation of saffron from four countries by mid-infrared spectroscopy and multivariate analysis. Eur. Food Res. Technol. 2010, 230, 571–577. [Google Scholar] [CrossRef]
- Masi, E.; Taiti, C.; Heimler, D.; Vignolini, P.; Romani, A.; Mancuso, S. PTR-TOF-MS and HPLC analysis in the characterization of saffron (Crocus sativus L.) from Italy and Iran. Food Chem. 2016, 192, 75–81. [Google Scholar] [CrossRef]
- Chrysanthou, A.; Pouliou, E.; Kyriakoudi, A.; Tsimidou, M.Z. Sensory Threshold Studies of Picrocrocin, the Major Bitter Compound of Saffron. J. Food Sci. 2016, 81, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.M.; Carmona, M.; Jarén-Galán, M.; Mínguez Mosquera, M.; Alonso, G.L. Picrocrocin. Kinetics in Aqueous Saffron Spice Extracts (Crocus sativus L.) upon Thermal Treatment. J. Agric. Food Chem. 2011, 59, 249–255. [Google Scholar] [CrossRef]
- Council Regulation (EEC). Council Regulation (EEC) No. 2081/92 of 14 July 1992 on the Protection of Geographical Indications and Designations of Origin for Agricultural Products and Foodstuffs; Council Regulation (EEC): Brussels, Belgium, 1992. [Google Scholar]
- Sonnino, R. The power of place: Embeddedness and local food systems in Italy and the UK. 2007 Anthropology of food, Special issue on local food products and systems. Special issue 2. 2007. Available online: https://journals.openedition.org/aof/454 (accessed on 18 September 2020). [CrossRef]
- Rulli, M.C.; Veroni, A.; Rosso, R. The Water Footprint and Environmental Sustainability of Italian DOP, DOC and DOCG Food Product; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 9783319163925/9783319163932. [Google Scholar]
- Belletti, G.; Marescotti, A.; Brazzini, A. Old World Case Study: The Role of Protected Geographical Indications to Foster Rural Development Dynamics: The Case of Sorana Bean PGI. In The Importance of Place: Geographical Indications as a Tool for Local and Regional Development. Ius Gentium: Comparative Perspectives on Law and Justice; van Caenegem, W., Cleary, J., Eds.; Springer: Cham, Switzerland, 2017; Volume 58. [Google Scholar] [CrossRef]
- Vignolini, P.; Heimler, D.; Pinelli, P.; Ieri, F.; Sciullo, A.; Romani, A. Characterization of by-products of saffron (Crocus sativus L.) production. Nat. Prod. Commun. 2008, 3, 1959–1962. [Google Scholar] [CrossRef] [Green Version]
- Carmona, M.; Sanchez, A.M.; Ferreres, F.; Zalacain, A.; Tomas-Barberan, F.; Alonso, G.L. Identification of the flavonoids fraction in saffron spice by LC/DAD/MS/MS: Comparative study of samples from different geographic origins. Food Chem. 2007, 100, 445–450. [Google Scholar] [CrossRef]
- Delarue, J. Flash Profile, its evolution and uses in sensory and consumer science. In Rapid Sensory Profiling Techniques. Applications in New Product Development and Consumer Research; Delarue, J., Lawlor, B., Rogeaux, M., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 121–151. [Google Scholar]
- Semion, B.; Králb, M.; Pospiechb, M.; Reitznerováa, A.; Maľováa, J.; Tremlováb, B.; Dudrikováa, E. Application of multiple factor analysis for the descriptive sensory evaluation and instrumental measurements of bryndza cheese as affected by vacuum packaging. Int. J. Food Prop. 2018, 21, 1508–1522. [Google Scholar]
- Akbari, G.; Mard, S.A.; Veisi, A. A comprehensive review on regulatory effects of crocin on ischemia/reperfusion injury in multiple organs. Biomed. Pharmacoter. 2018, 99, 664–670. [Google Scholar] [CrossRef]

| Saffron Samples | |||
|---|---|---|---|
| COMPOUNDS | SA | CT | GR |
| Crocins | |||
| Trans crocin 5 | 5.95 ± 0.22 | 3.46 ± 0.11 | 1.43 ± 0.03 |
| crocin derivative | 2.37 ± 0.06 | 3.19 ± 0.10 | 1.74 ± 0.04 |
| crocin derivative | 2.71 ± 0.07 | 2.55 ± 0.05 | 1.33 + 0.03 |
| trans crocin 4 | 169.97 ± 2.18 | 405.19 ± 3.95 | 249.82 ± 2.92 |
| crocin derivative | 2.07 ± 0.06 | 3.67 ± 0.12 | 2.06 ± 0.08 |
| trans crocin 3 | 61.22 ± 1.80 | 132.07 ± 1.98 | 93.90 ± 2.15 |
| crocin derivative | 3.70 ± 0.16 | 4.15 ± 0.13 | 1.44 ± 0.05 |
| trans crocin 2’ | - | - | 3.74 ± 0.15 |
| crocin derivative | 1.18 ± 0.04 | 0.96 ± 0.02 | 0.48 ± 0.1 |
| crocin derivative | 1.04 ± 0.03 | 1.12 ± 0.02 | - |
| cis crocin 4 | 25.91 ± 0.92 | 24.13 ± 0.09 | 11.84 ± 0.47 |
| crocin derivative | - | - | 0.27 ± 0.1 |
| trans crocin 2 | 16.26 ± 0.55 | 26.59 ± 0.93 | 21.48 ± 0.89 |
| crocin derivative | 3.85 ± 0.11 | 3.51 ± 0.10 | 1.63 ± 0.06 |
| cis crocin 3 | 1.96 ± 0.05 | 1.77 ± 0.07 | 3.61 ± 1.02 |
| crocin derivative | 2.07 ± 0.06 | 1.44 ± 0.06 | 1.26 ± 0.05 |
| crocin derivative | - | 1.12 ± 0.05 | 0.27 ± 0.01 |
| crocin derivative | 1.18 ± 0.02 | 1.12 ± 0.05 | 0.32 ± 0.01 |
| crocin derivative | 1.04 ± 0.02 | 1.12 ± 0.05 | 0.46 ± 0.01 |
| TOTAL | 302.49 | 617.14 | 397.1 |
| safranal | 3.11 ± 0.10 | 0.48 ± 0.01 | 2.66 ± 0.08 |
| picrocrocin | 74.33 ± 1.92 | 112.62 ± 2.01 | 29.13 ± 1.01 |
| Flavonoids | |||
| K-3-sophoriside -7-glucoside | 2.86 ± 0.09 | 3.08 ± 0.10 | 5.95 ± 0.21 |
| k der | 0.89 ± 0.04 | - | - |
| K-3-sophoroside | 6.05 ± 0.23 | 8.91 ± 0.033 | 10.54 ± 0.42 |
| TOTAL | 9.79 | 11.99 | 16.49 |
| Sample | Moisture | Ash | Fibres | Proteins | Fat | Sugars | Na | K | Mg | Ca | Fe | Cu | Zn | Mn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SA | 7.6 | 8.7 | 3.8 | 14.5 | 4.9 | 59.2 | 15.5 | 13,098 | 417 | 811 | 121.8 | 10.3 | 104.2 | 31.7 |
| CT | 9.4 | 11.3 | 3.8 | 17.3 | 5.6 | 51.3 | 212.5 | 14,680 | 252 | 99.7 | 64.1 | 2.4 | 25.2 | 24.3 |
| GR | 10.8 | 6.4 | 3.7 | 15.2 | 5.1 | 57.3 | 6.25 | 13,752 | 1315 | 1005 | 57.9 | 8.1 | 87.8 | 28.4 |
| Perception Threshold | ||
|---|---|---|
| Crocins | Bitterness Scores | Astringency Scores |
| 0.94 | 1.9 | 2.6 |
| 1.87 | 2.4 | 2.6 |
| 3.75 | 2.2 | 3 |
| 7.5 | 3 | 3.2 |
| 15 | 2.8 | 3.1 |
| 30 | 2.8 | 3.2 |
| 60 | 3.2 | 3.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Predieri, S.; Magli, M.; Gatti, E.; Camilli, F.; Vignolini, P.; Romani, A. Chemical Composition and Sensory Evaluation of Saffron. Foods 2021, 10, 2604. https://doi.org/10.3390/foods10112604
Predieri S, Magli M, Gatti E, Camilli F, Vignolini P, Romani A. Chemical Composition and Sensory Evaluation of Saffron. Foods. 2021; 10(11):2604. https://doi.org/10.3390/foods10112604
Chicago/Turabian StylePredieri, Stefano, Massimiliano Magli, Edoardo Gatti, Francesca Camilli, Pamela Vignolini, and Annalisa Romani. 2021. "Chemical Composition and Sensory Evaluation of Saffron" Foods 10, no. 11: 2604. https://doi.org/10.3390/foods10112604
APA StylePredieri, S., Magli, M., Gatti, E., Camilli, F., Vignolini, P., & Romani, A. (2021). Chemical Composition and Sensory Evaluation of Saffron. Foods, 10(11), 2604. https://doi.org/10.3390/foods10112604






