Volatile Oil Profile of Prickly Ash (Zanthoxylum) Pericarps from Different Locations in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Determination of Environmental Factors
2.3. Qualitative and Quantitive Determinations of Prickly Ash Pericarp Volatile oil Profile
2.4. Data Analyses
3. Results
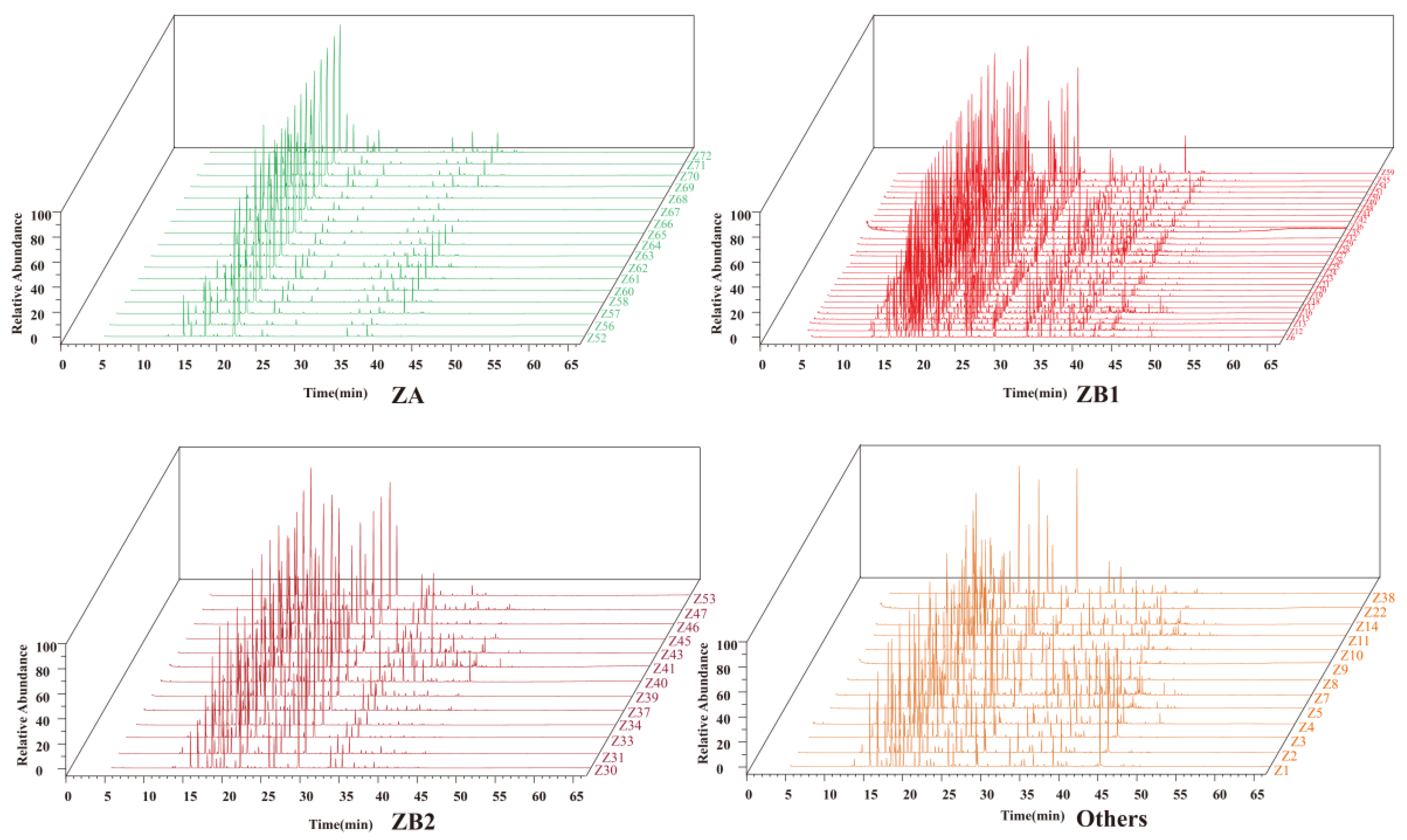
3.1. Volatile Oil Profile in Different Prickly Ash Pericarps
3.1.1. Qualitative Determination of Volatile Oil Constituents in Prickly Ash Pericarps
3.1.2. Difference of Volatile Oil Profile in Different Prickly Ash Pericarps
3.2. Chemometric Analyses for Prickly Ash Pericarps Based on Volatile Oil Profile
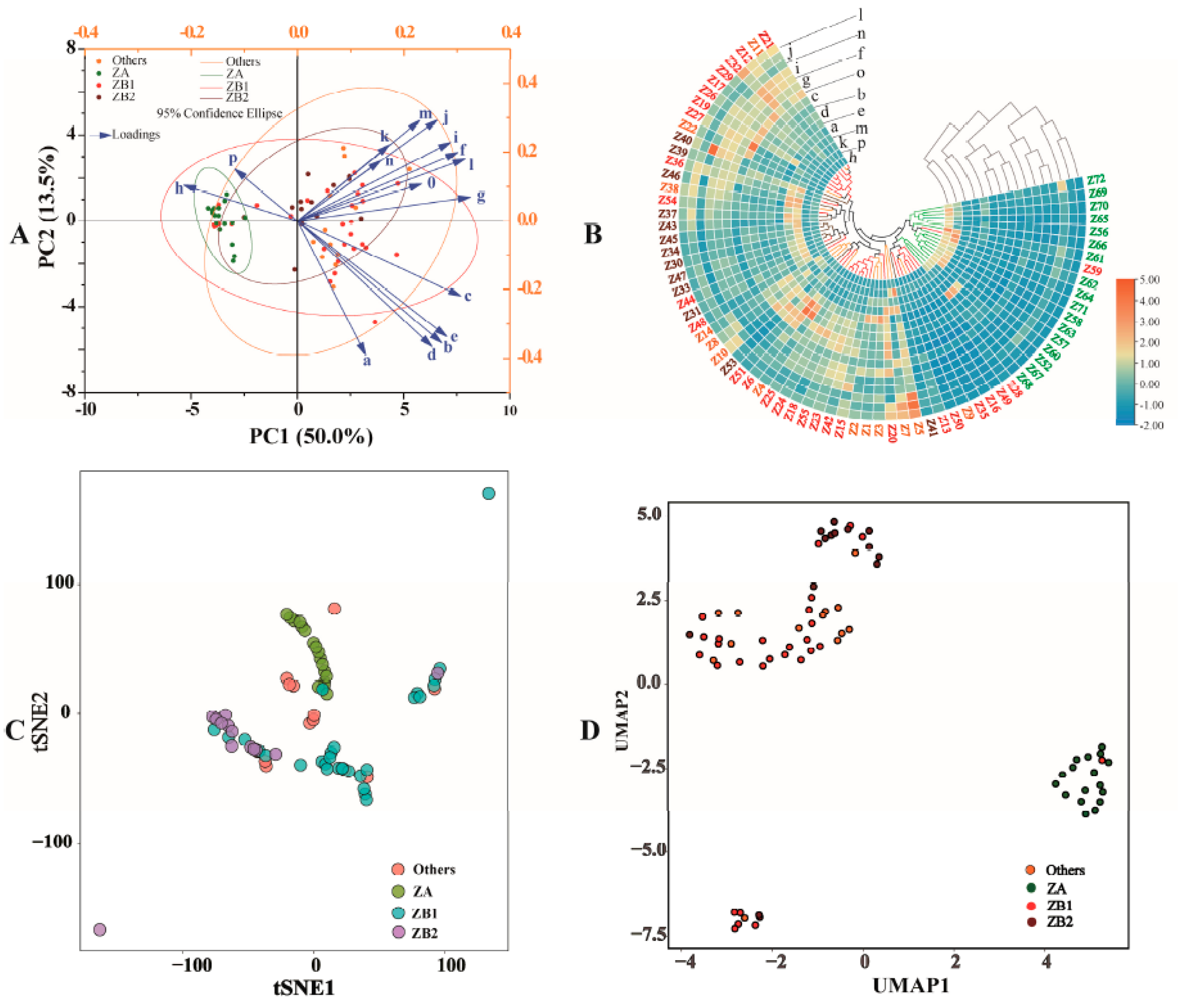
3.2.1. Principal Component Analysis (PCA)
3.2.2. Cluster Heat Map (CHM)
3.2.3. t-Distributed Stochastic Neighbor Embedding (tSNE)
3.2.4. Uniform Manifold Approximation and Projection for Dimension Reduction (UMAP)
3.2.5. Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA)
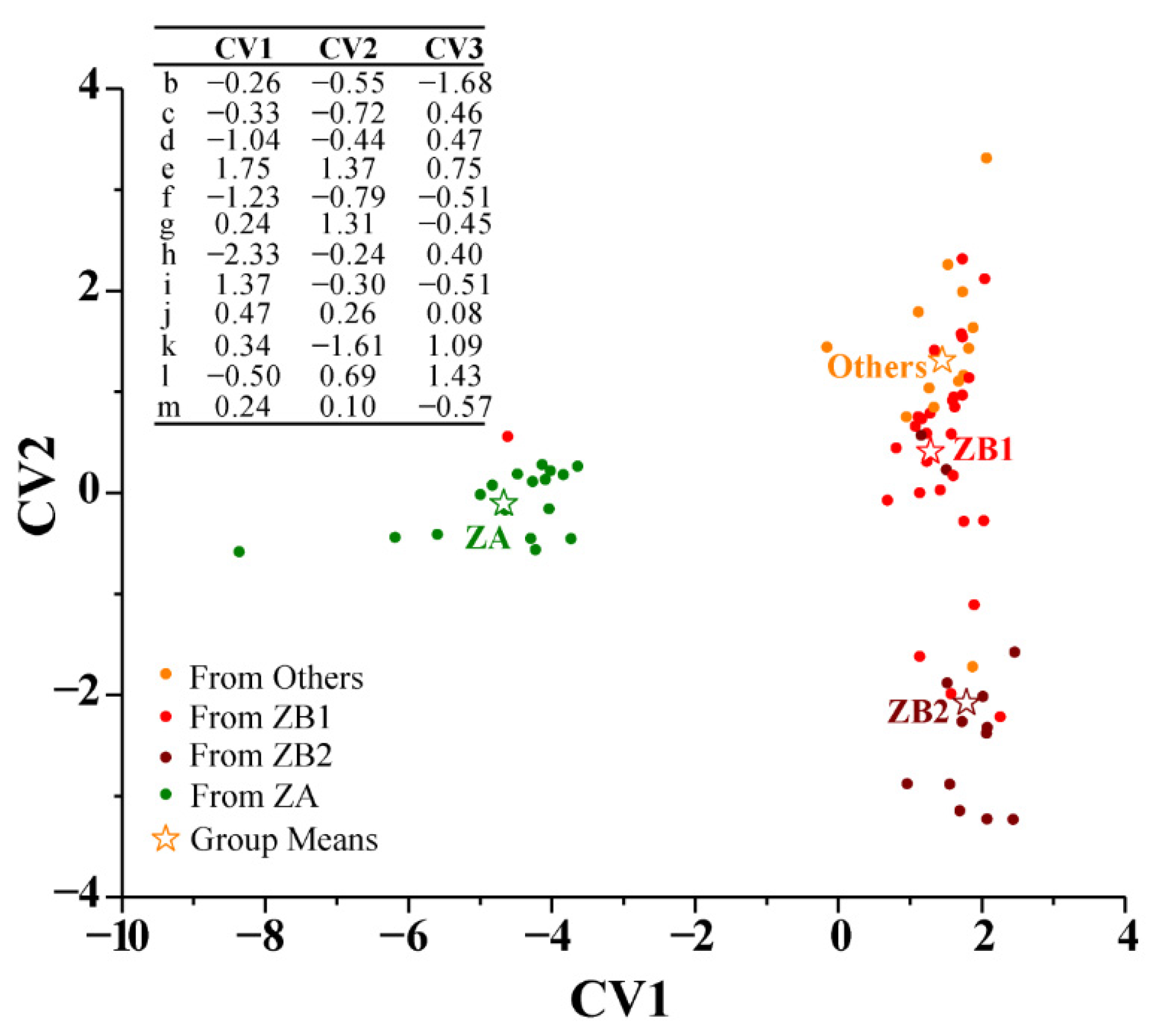
3.2.6. Discriminant Analysis (DA)
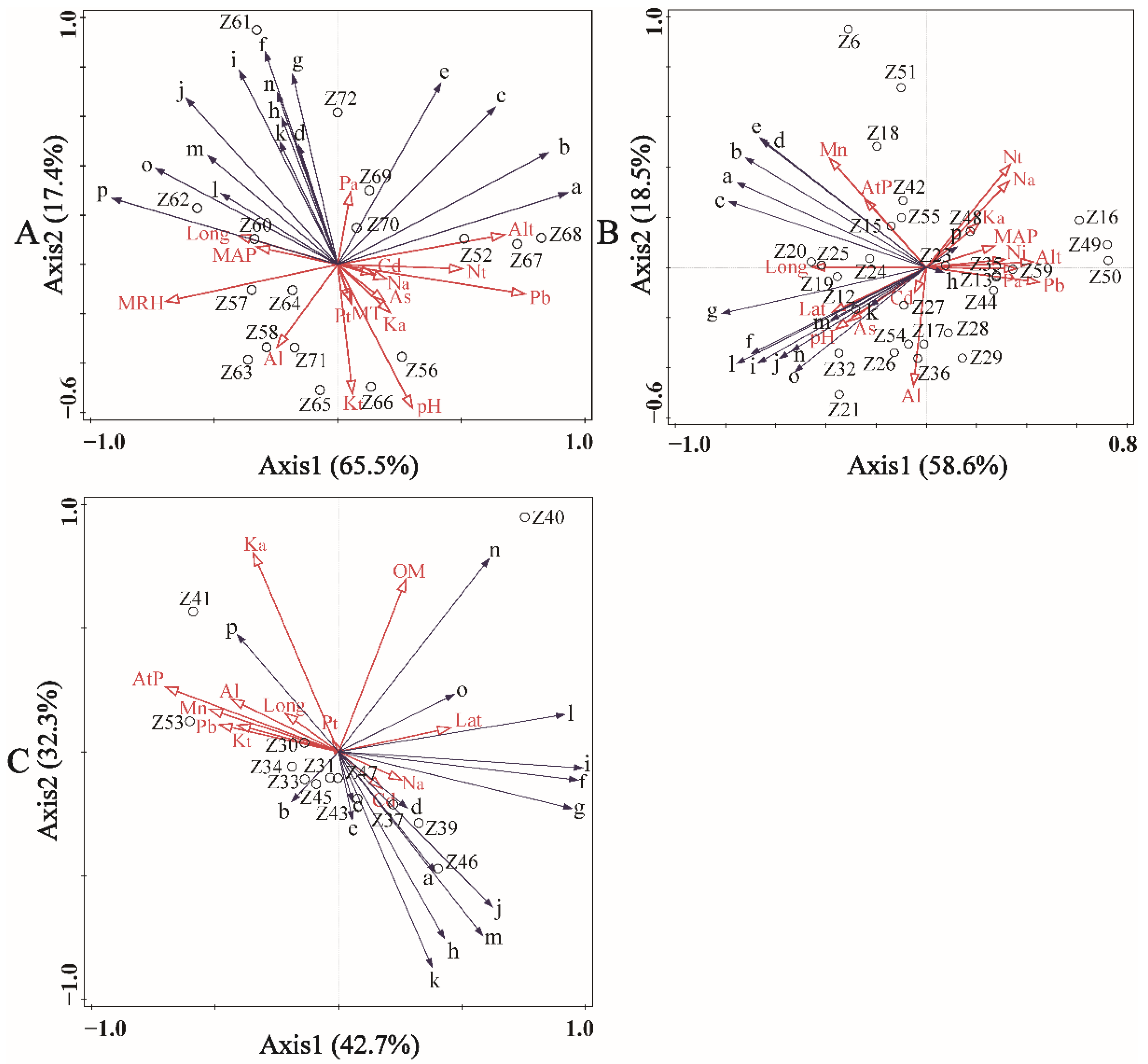
3.3. The Influences of Environmental Factors on Volatile Oil Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lee, J.; da Silva, R.R.; Jang, H.S.; Kim, H.W.; Kwon, Y.S.; Kim, J.H.; Yang, H. In silico annotation of discriminative markers of three Zanthoxylum species using molecular network derived annotation propagation. Food Chem. 2019, 295, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Sriwichai, T.; Sookwong, P.; Siddiqui, M.W.; Sommano, S.R. Aromatic profiling of Zanthoxylum myriacanthum (makwhaen) essential oils from dried fruits using different initial drying techniques. Ind. Crop. Prod. 2019, 133, 284–291. [Google Scholar] [CrossRef]
- Chen, X.; Wang, W.; Wang, C.; Liu, Z.; Sun, Q.; Wang, D. Quality evaluation and chemometric discrimination of Zanthoxylum bungeanum Maxim leaves based on flavonoids profiles, bioactivity and HPLC-fingerprint in a common garden experiment. Ind. Crop. Prod. 2019, 134, 225–233. [Google Scholar] [CrossRef]
- Xiang, L.; Liu, Y.; Xie, C.; Li, X.; Yu, Y.; Ye, M.; Chen, S. The Chemical and Genetic Characteristics of Szechuan Pepper (Zanthoxylum bungeanum and Z. armatum) Cultivars and Their Suitable Habitat. Front. Plant. Sci. 2016, 7, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, L.; Liu, Y.; Wei, A. Geographical variations in the fatty acids of Zanthoxylum seed oils: A chemometric classification based on the random forest algorithm. Ind. Crop. Prod. 2019, 134, 146–153. [Google Scholar] [CrossRef]
- Ke, J.; Qu, Y.; Li, S.; Shen, G.; Chen, A.; Luo, Q.; Liu, X.; Wu, H.; Li, M.; Pu, B.; et al. Application of HPLC fingerprint based on acid amide components in Chinese prickly ash (Zanthoxylum). Ind. Crop. Prod. 2018, 119, 267–276. [Google Scholar] [CrossRef]
- Zhuo, Z.; Xu, D.; Li, Y.; Pu, B.; Ye, M. Fingerprint analysis of Zanthoxylum armatum DC. by HPLC. J. Food Compos. Anal. 2021, 96, 103736. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Zanthoxyli Pericarpium. In Pharmacopoeia of the People’s Republic of China; China Medical And Technology Press: Beijin, China, 2020; p. 166. [Google Scholar]
- Ma, Y.; Li, J.; Tian, M.; Liu, Y.; Wei, A. Authentication of Chinese prickly ash by ITS2 sequencing and the influence of environmental factors on pericarp quality traits. Ind. Crop. Prod. 2020, 155, 112770. [Google Scholar] [CrossRef]
- Feng, S.; Liu, Z.; Hu, Y.; Tian, J.; Yang, T.; Wei, A. Genomic analysis reveals the genetic diversity, population structure, evolutionary history and relationships of Chinese pepper. Hortic. Res.-Engl. 2020, 7, 1–14. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, M.; Wei, D.; Wang, L.; Hu, M.; Zhang, Q.; He, Z.; Peng, W.; Wu, C. Hydroxy-alpha-sanshool isolated from Zanthoxylum bungeanum attenuates learning and memory impairments in scopolamine-treated mice. Food Funct. 2019, 10, 7315–7324. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Shen, P.; Cao, Y.; Lu, X.; Gao, X.; Fu, Y.; Liu, B.; Zhang, N. Zanthoxylum bungeanum pericarp extract prevents dextran sulfate sodium-induced experimental colitis in mice via the regulation of TLR4 and TLR4-related signaling pathways. Int. Immunopharmacol. 2016, 41, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhou, R.; Wang Jia, W.; Li, Z.; Li, J.; Zhang, P.; Xiao, T. Zanthoxylum bungeanum essential oil induces apoptosis of HaCaT human keratinocytes. J. Ethnopharmacol. 2016, 186, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Li, H.; Chen, Y.Y.; Zhang, Y.W.; Liu, N.; Zhang, Q.; Wu, Q. Essential oil from Zanthoxylum bungeanum Maxim. and its main components used as transdermal penetration enhancers: A comparative study. J. Zhejiang Univ.-SCI. B 2014, 15, 940–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misra, L.N.; Wouatsa, N.A.; Kumar, S.; Venkatesh Kumar, R.; Tchoumbougnang, F. Antibacterial, cytotoxic activities and chemical composition of fruits of two Cameroonian Zanthoxylum species. J. Ethnopharmacol. 2013, 148, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Zribi, I.; Bleton, J.; Moussa, F.; Abderrabba, M. GC-MS analysis of the volatile profile and the essential oil compositions of Tunisian Borago Officinalis L.: Regional locality and organ dependency. Ind. Crop. Prod. 2019, 129, 290–298. [Google Scholar] [CrossRef]
- Soltani Howyzeh, M.; Sadat Noori, S.A.; Shariati, J.V. Essential oil profiling of Ajowan (Trachyspermum ammi) industrial medicinal plant. Ind. Crop. Prod. 2018, 119, 255–259. [Google Scholar] [CrossRef]
- Tian, J.; Zeng, X.; Feng, Z.; Miao, X.; Peng, X.; Wang, Y. Zanthoxylum molle Rehd. essential oil as a potential natural preservative in management of Aspergillus flavus. Ind. Crop. Prod. 2014, 60, 151–159. [Google Scholar] [CrossRef]
- Nazem, V.; Sabzalian, M.R.; Saeidi, G.; Rahimmalek, M. Essential oil yield and composition and secondary metabolites in self- and open-pollinated populations of mint (Mentha spp.). Ind. Crop. Prod. 2019, 130, 332–340. [Google Scholar] [CrossRef]
- Hassanabadi, M.; Ebrahimi, M.; Farajpour, M.; Dejahang, A. Variation in essential oil components among Iranian Ferula assafoetida L. accessions. Ind. Crop. Prod. 2019, 140, 111598. [Google Scholar] [CrossRef]
- Fardhyanti, D.S.; Sediawan, W.B.; Hisyam, A. Kinetics of mace (Myristicae arillus) essential oil extraction using microwave assisted hydrodistillation: Effect of microwave power. Ind. Crop. Prod. 2019, 131, 315–322. [Google Scholar] [CrossRef]
- Patel, R.P.; Singh, R.; Saikia, S.K.; Rao, B.R.R.; Sastry, K.P.; Zaim, M.; Lal, R.K. Phenotypic characterization and stability analysis for biomass and essential oil yields of fifteen genotypes of five Ocimum species. Ind. Crop. Prod. 2015, 77, 21–29. [Google Scholar] [CrossRef]
- Sonmezdag, A.S.; Kelebek, H.; Selli, S. Pistachio oil (Pistacia vera L. cv. Uzun): Characterization of key odorants in a representative aromatic extract by GC-MS-olfactometry and phenolic profile by LC-ESI-MS/MS. Food Chem. 2018, 240, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Fei, X.; Li, J.; Liu, Y.; Wei, A. Effects of location, climate, soil conditions and plant species on levels of potentially toxic elements in Chinese Prickly Ash pericarps from the main cultivation regions in China. Chemosphere 2020, 244, 125501. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tian, J.; Wang, X.; Huang, C.; Tian, M.; Wei, A. Fatty Acid Profiling and Chemometric Analyses for Zanthoxylum Pericarps from Different Geographic Origin and Genotype. Foods 2020, 9, 1676. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tian, L.; Wang, X.; Huang, C.; Tian, M.; Wei, A. Alkylamide Profiling of Pericarps Coupled with Chemometric Analysis to Distinguish Prickly Ash Pericarps. Foods 2021, 10, 866. [Google Scholar] [CrossRef] [PubMed]
- Bagheri Bodaghabadi, M.; Faskhodi, A.A.; Salehi, M.H.; Hosseinifard, S.J.; Heydari, M. Soil suitability analysis and evaluation of pistachio orchard farming, using canonical multivariate analysis. Sci. Hortic. 2019, 246, 528–534. [Google Scholar] [CrossRef]
- Shi, J.; Fei, X.; Hu, Y.; Liu, Y.; Wei, A. Identification of Key Genes in the Synthesis Pathway of Volatile Terpenoids in Fruit of Zanthoxylum bungeanum Maxim. Forests 2019, 10, 328. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Jing, T.; Zhao, M.; Jin, J.; Xu, M.; Chen, Y.; Zhang, S.; Wan, X.; Schwab, W.; Song, C. Untargeted metabolomics coupled with chemometrics analysis reveals potential non-volatile markers during oolong tea shaking. Food Res. Int. 2019, 123, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Hussain, J.; Wang, X.; Sousa, L.; Ali, R.; Rittmann, B.E.; Liao, W. Using non-metric multi-dimensional scaling analysis and multi-objective optimization to evaluate green algae for production of proteins, carbohydrates, lipids, and simultaneously fix carbon dioxide. Biomass Bioenerg. 2020, 141, 105711. [Google Scholar] [CrossRef]
- Tishkoff, S.A.; Diaz-Papkovich, A.; Anderson-Trocmé, L.; Ben-Eghan, C.; Gravel, S. UMAP reveals cryptic population structure and phenotype heterogeneity in large genomic cohorts. PLoS Genet. 2019, 15, e1008432. [Google Scholar]
- Becht, E.; McInnes, L.; Healy, J.; Dutertre, C.-A.; Kwok, I.W.H.; Ng, L.G.; Ginhoux, F.; Newell, E.W. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 2018, 37, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Zhao, J.; Hu, R.; Dong, Y.; Tan, L. Differentiation of Chinese robusta coffees according to species, using a combined electronic nose and tongue, with the aid of chemometrics. Food Chem. 2017, 229, 743–751. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, W.; Sun, L.; Wei, A.; Wang, D. Accumulation and biosynthesis of hydroxyl-α-sanshool in varieties of Zanthoxylum bungeanum Maxim. by HPLC-fingerprint and transcriptome analyses. Ind. Crop. Prod. 2020, 145, 111998. [Google Scholar] [CrossRef]
- Jia, G.; Sha, K.; Feng, X.; Liu, H. Post-thawing metabolite profile and amino acid oxidation of thawed pork tenderloin by HVEF-A short communication. Food Chem. 2019, 291, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xie, H.; Shi, S. Multielement analysis of Zanthoxylum bungeanum Maxim. essential oil using ICP-MS/MS. Anal. Bioanal. Chem. 2018, 410, 3769–3778. [Google Scholar] [CrossRef]
| Component | ZA | ZB1 | ZB2 | Others |
|---|---|---|---|---|
| Alfa-myrcene | 25.3 ± 17.8 b | 147.2 ± 98.4 a | 115.7 ± 66.5 a | 118.1 ± 56.4 a |
| Alfa-ocimene | 3.5 ± 1.7 b | 38.4 ± 27.4 a | 30.9 ± 14.9 a | 44.1 ± 24.3 a |
| Linalool | 256.5 ± 54.2 a | 35.5 ± 26.1 c | 77.7 ± 26.3 b | 52.3 ± 30.4 c |
| Linalyl acetate | 0.8 ± 0.5 c | 5.4 ± 4.9 b | 15.2 ± 5.6 a | 6.5 ± 5.2 b |
| Geranyl acetate | 1.1 ± 1.0 c | 11.5 ± 9.9 b | 23.1 ± 10.8 a | 18.0 ± 13.2 ab |
| D-limonene | 55.1 ± 31.0 b | 172.9 ± 97.0 a | 158.3 ± 49.3 a | 175.4 ± 82.0 a |
| Gamma-terpinene | 10.1 ± 4.9 b | 52.5 ± 30.9 a | 47.6 ± 22.8 a | 42.2 ± 25.8 a |
| Terpinolene | 2.8 ± 1.3 b | 10.5 ± 6.0 a | 9.5 ± 4.1 a | 10.4 ± 4.9 a |
| Terpinen-4-ol | 17.1 ± 7.9 b | 78.2 ± 47.0 a | 72.1 ± 34.3 a | 63.2 ± 38.5 a |
| Terpineol | 12.3 ± 5.7 b | 46.4 ± 30.8 a | 60.6 ± 23.0 a | 72.5 ± 55.8 a |
| Alfa-terpinyl acetate | 1.7 ± 2.3 c | 35.8 ± 23.0 ab | 28.7 ± 12.0 b | 47.8 ± 25.1 a |
| Sabinene | 40.5 ± 37.5 | 40.0 ± 28.2 | 33.3 ± 17.6 | 56.0 ± 41.1 |
| Alfa-pinene | 1.2 ± 0.9 c | 28.1 ± 22.6 a | 15.2 ± 7.2 b | 35.3 ± 25.8 a |
| Nerolidol | 5.1 ± 3.8 a | 0.3 ± 0.4 c | 0.3 ± 0.4 c | 2.7 ± 3.7 b |
| Beta-elemene | 1.1 ± 0.9 b | 6.2 ± 5.0 a | 1.4 ± 1.0 b | 6.0 ± 3.7 a |
| Caryophyllene | 4.7 ± 2.3 ab | 4.9 ± 3.3 ab | 3.6 ± 6.0 b | 7.8 ± 4.6 a |
| Indicators | ZA vs. ZB1 | ZA vs. ZB2 | ZA vs. Others | ZB1 vs. ZB2 | ZB1 vs. Others | ZB2 vs. Others |
|---|---|---|---|---|---|---|
| Sabinene | 0.02 | 0.21 | 0.49 | 0.26 | 0.43 | 0.80 |
| Alfa-myrcene | 1.05 | 0.94 | 1.01 | 0.55 | 0.23 | 0.23 |
| D-limonene | 1.02 | 1.01 | 1.08 | 0.16 | 0.39 | 0.39 |
| Alfa-pinene | 0.97 | 0.80 | 1.17 | 0.86 | 0.40 | 1.39 |
| Alfa-ocimene | 1.03 | 1.02 | 1.21 | 0.43 | 0.45 | 0.85 |
| Gamma-terpinene | 1.13 | 1.04 | 0.95 | 0.17 | 0.84 | 0.46 |
| Terpinolene | 1.09 | 1.02 | 1.11 | 0.01 | 0.14 | 0.03 |
| Linalool | 1.63 | 1.31 | 1.37 | 0.94 | 0.85 | 0.87 |
| Terpinen-4-ol | 1.09 | 1.02 | 0.94 | 0.14 | 0.93 | 0.42 |
| Terpineol | 0.85 | 1.11 | 1.03 | 1.16 | 1.74 | 0.11 |
| Linalyl acetate | 0.69 | 1.52 | 0.79 | 2.39 | 0.62 | 2.05 |
| Alfa-terpinyl acetate | 1.17 | 1.01 | 1.28 | 0.16 | 0.90 | 0.89 |
| Geranyl acetate | 0.83 | 1.35 | 0.98 | 1.97 | 1.57 | 1.07 |
| Caryophyllene | 0.04 | 0.26 | 0.59 | 0.83 | 1.42 | 1.44 |
| Beta-elemene | 0.95 | 0.12 | 0.95 | 1.45 | 0.43 | 1.38 |
| Nerolidol | 1.18 | 0.99 | 0.55 | 0.21 | 2.01 | 1.09 |
| R2X | 0.70 | 0.70 | 0.69 | 0.64 | 0.57 | 0.48 |
| R2Y | 0.83 | 0.94 | 0.90 | 0.51 | 0.34 | 0.67 |
| Q2Y | 0.78 | 0.91 | 0.85 | 0.34 | −0.001 | 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Tian, J.; Chen, Y.; Chen, M.; Liu, Y.; Wei, A. Volatile Oil Profile of Prickly Ash (Zanthoxylum) Pericarps from Different Locations in China. Foods 2021, 10, 2386. https://doi.org/10.3390/foods10102386
Ma Y, Tian J, Chen Y, Chen M, Liu Y, Wei A. Volatile Oil Profile of Prickly Ash (Zanthoxylum) Pericarps from Different Locations in China. Foods. 2021; 10(10):2386. https://doi.org/10.3390/foods10102386
Chicago/Turabian StyleMa, Yao, Jieyun Tian, Yabing Chen, Ming Chen, Yulin Liu, and Anzhi Wei. 2021. "Volatile Oil Profile of Prickly Ash (Zanthoxylum) Pericarps from Different Locations in China" Foods 10, no. 10: 2386. https://doi.org/10.3390/foods10102386
APA StyleMa, Y., Tian, J., Chen, Y., Chen, M., Liu, Y., & Wei, A. (2021). Volatile Oil Profile of Prickly Ash (Zanthoxylum) Pericarps from Different Locations in China. Foods, 10(10), 2386. https://doi.org/10.3390/foods10102386





