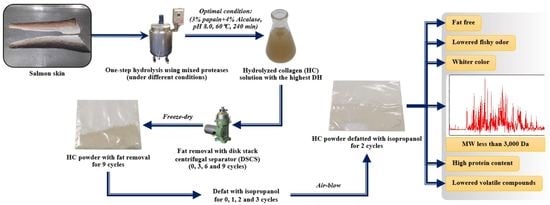
Development of Hydrolysis and Defatting Processes for Production of Lowered Fishy Odor Hydrolyzed Collagen from Fatty Skin of Sockeye Salmon (Oncorhynchus nerka)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enzymes and Chemicals
2.2. Preparation of Salmon Skin
2.3. Effect of Hydrolysis Conditions on Degree of Hydrolysis of HC
2.3.1. Effect of Mixed Proteases at Various Concentrations and Different pHs
2.3.2. Effect of Temperature
2.4. Effect of Centrifugal Cycles on Fat Removal of HC
2.4.1. Fat Content
2.4.2. Color
2.5. Effect of Isopropanol on Fat Removal of HC Powder
2.5.1. Fat Content and Color
2.5.2. Fishy Odor Intensity (FOI)
2.5.3. Peroxide Value (PV) and Thiobarbituric Acid Reactive Substances (TBARS)
2.6. Characterization of Selected Defatted HC
2.6.1. Size Distribution
2.6.2. Amino Acid Composition
2.6.3. Proximate Composition and Mineral Content
2.6.4. Volatile Compounds
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of Hydrolysis Conditions on Degree of Hydrolysis and Fatty Acid Composition in HC
3.1.1. Degree of Hydrolysis (DH)
3.1.2. Fatty Acid Composition of HC
3.2. Effect of Centrifugal Cycles on Fat Removal from HC
3.2.1. Fat Content
3.2.2. Color
3.3. Effect of Isopropanol on Fat Removal of HC Powder
3.3.1. Fat Content
3.3.2. Color
3.3.3. Fishy Odor Intensity (FOI)
3.3.4. Peroxide Value (PV)
3.3.5. Thiobarbituric Acid Reactive Substances (TBARS) Value
3.4. Characteristics of Selected Defatted HC
3.4.1. Size Distribution
3.4.2. Amino Acid Composition
3.4.3. Proximate Compositions and Mineral Content
3.4.4. Volatile Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjakul, S.; Karnjanapratum, S.; Visessanguan, W. Production and characterization of odorless antioxidative hydrolyzed collagen from seabass (Lates calcarifer) skin without descaling. Waste Biomass Valoriz. 2018, 9, 549–559. [Google Scholar] [CrossRef]
- Benjakul, S.; Karnjanapratum, S.; Visessanguan, W. Hydrolysed collagen from Lates calcarifer skin: Its acute toxicity and impact on cell proliferation and collagen production of fibroblasts. Int. J. Food Sci. Technol. 2018, 53, 1871–1879. [Google Scholar] [CrossRef]
- Chotphruethipong, L.; Binlateh, T.; Hutamekalin, P.; Aluko, R.E.; Tepaamorndech, S.; Zhang, B.; Benjakul, S. Impact of hydrolyzed collagen from defatted sea bass skin on proliferation and differentiation of preosteoblast MC3T3-E1 cells. Foods 2021, 10, 1476. [Google Scholar] [CrossRef]
- Sae-leaw, T.; O’Callaghan, Y.C.; Benjakul, S.; O’Brien, N.M. Antioxidant activities and selected characteristics of gelatin hydrolysates from seabass (Lates calcarifer) skin as affected by production processes. J. Food Sci. Technol. 2016, 53, 197–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, L.V.; Shakila, R.J.; Jeyasekaran, G. In vitro anti-cancer, anti-diabetic, anti-inflammation and wound healing properties of collagen peptides derived from unicorn leatherjacket (Aluterus monoceros) at different hydrolysis. Turk. J. Fish. Aquat. Sci. 2019, 19, 551–560. [Google Scholar]
- Chotphruethipong, L.; Binlateh, T.; Hutamekalin, P.; Sukketsiri, W.; Aluko, R.E.; Benjakul, S. In vitro antioxidant and wound-healing activities of hydrolyzed collagen from defatted Asian sea bass skin as influenced by different enzyme types and hydrolysis processes. RSC Adv. 2021, 11, 18144–18151. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Hydrolysis of salmon muscle proteins by an enzyme mixture extracted from Atlantic salmon (Salmo salar) pyloric caeca. J. Food Biochem. 2000, 24, 177–187. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Biochemical and functional properties of Atlantic salmon (Salmo salar) muscle proteins hydrolyzed with various alkaline proteases. J. Agric. Food Chem. 2000, 48, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Yarnpakdee, S.; Benjakul, S.; Kristinsson, H.G.; Kishimura, H. Antioxidant and sensory properties of protein hydrolysate derived from Nile tilapia (Oreochromis niloticus) by one-and two-step hydrolysis. J. Food Sci. Technol. 2015, 52, 3336–3349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klomklao, S.; Benjakul, S. Utilization of tuna processing byproducts: Protein hydrolysate from skipjack tuna (Katsuwonus pelamis) viscera. J. Food Process. Preserv. 2017, 41, e12970. [Google Scholar] [CrossRef]
- Aryee, A.N.A.; Simpson, B.K.; Phillip, L.E.; Cue, R.I. Effect of temperature and time on the stability of salmon skin oil during storage. J. Am. Oil Chem. Soc. 2012, 89, 287–292. [Google Scholar] [CrossRef]
- See, S.F.; Hoo, L.L.; Babji, A.S. Optimization of enzymatic hydrolysis of salmon (Salmo salar) skin by alcalase. Int. Food Res. J. 2011, 18, 1359–1365. [Google Scholar]
- Vázquez, J.A.; Hermida-Merino, C.; Hermida-Merino, D.; Piñeiro, M.M.; Johansen, J.; Sotelo, C.G.; Pérez-Martín, R.I.; Valcarcel, J. Characterization of gelatin and hydrolysates from valorization of farmed salmon skin by-products. Polymers 2021, 13, 2828. [Google Scholar] [CrossRef]
- Harnedy, P.A.; Parthsarathy, V.; McLaughlin, C.M.; O’Keeffe, M.B.; Allsopp, P.J.; McSorley, E.M.; O’Harte, F.P.M.; FitzGerald, R.J. Atlantic salmon (Salmo salar) co-product-derived protein hydrolysates: A source of antidiabetic peptides. Food Res. Int. 2018, 106, 598–606. [Google Scholar] [CrossRef] [Green Version]
- Haq, M.; Ahmed, R.; Cho, Y.-J.; Chun, B.-S. Quality properties and bio-potentiality of edible oils from Atlantic salmon by-products extracted by supercritial carbon dioxide and conventional methods. Waste Biomass Valori. 2017, 8, 1953–1967. [Google Scholar] [CrossRef]
- Sae-leaw, T.; Benjakul, S.; O’Brien, N.M. Effect of pretreatments and defatting of seabass skins on properties and fishy odor of gelatin. J. Food Biochem. 2016, 40, 741–753. [Google Scholar] [CrossRef]
- Chotphruethipong, L.; Aluko, R.E.; Benjakul, S. Effect of pulsed electric field-assisted process in combination with porcine lipase on defatting of seabass skin. J. Food Sci. 2019, 84, 1799–1805. [Google Scholar] [CrossRef]
- Chotphruethipong, L.; Aluko, R.E.; Benjakul, S. Enhanced Asian sea bass skin defatting using porcine lipase with the aid of pulsed electric field pretreatment and vacuum impregnation. Process Biochem. 2019, 86, 58–64. [Google Scholar] [CrossRef]
- Jukkola, A.; Hokkanen, S.; Kämäräinen, T.; Partanen, R.; Heino, A.; Rojas, O.J. Changes in milk fat globules and membrane lipids under the shear fields of microfiltration and centrifugation. J. Membr. Sci. Technol. 2019, 573, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Benjakul, S.; Morrissey, M.T. Protein hydrolysates from Pacific whiting solid wastes. J. Agric. Food Chem. 1997, 45, 3423–3430. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammed, M.A.; Domendra, D.; Muthukumar, S.P.; Sakhare, P.Z.; Bhaskar, N. Effects of fermentatively recovered fish waste lipids on the growth and composition of broiler meat. Br. Poult. Sci. 2015, 56, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Nilsuwan, K.; Benjakul, S.; Prodpran, T.; de la Caba, K. Fish gelatin monolayer and bilayer films incorporated with epigallocatechin gallate: Properties and their use as pouches for storage of chicken skin oil. Food Hydrocoll. 2019, 89, 783–791. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis; Association of Official Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Richards, M.P.; Hultin, H.O. Contributions of blood and blood components to lipid oxidation in fish muscle. J. Agric. Food Chem. 2002, 50, 555–564. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. In Methods in Enzymology; Flesicher, S., Packer, L., Eds.; Academic Press: New York, NY, USA, 1978; Volume 52, pp. 302–310. [Google Scholar]
- Benjakul, S.; Mad-Ali, S.; Sookchoo, P. Characteristics of biocalcium powders from pre-cooked tongol (Thunnus tonggol) and yellowfin (Thunnus albacores) tuna bones. Food Biophys. 2017, 12, 412–421. [Google Scholar] [CrossRef]
- Iglesias, J.; Medina, I. Solid-phase microextraction method for the determination of volatile compounds associated to oxidation of fish muscle. J. Chromatogr. A 2008, 1192, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adler-Nissen, J. Enzymic Hydrolysis of Food Proteins; Elsevier Applied Science Publishers: New York, NY, USA, 1986. [Google Scholar]
- Chotphruethipong, L.; Aluko, R.E.; Benjakul, S. Hydrolyzed collagen from porcine lipase-defatted seabass skin: Antioxidant, fibroblast cell proliferation, and collagen production activities. J. Food Biochem. 2019, 43, e12825. [Google Scholar] [CrossRef]
- Thiansilakul, Y.; Benjakul, S.; Shahidi, F. Antioxidative activity of protein hydrolysate from round scad muscle using alcalase and flavourzyme. J. Food Biochem. 2007, 31, 266–287. [Google Scholar] [CrossRef]
- Sparks, T.; Chase, G. Section 6—Other separation processes and equipment. In Filters and Filtration Handbook, 6th ed.; Sparks, T., Chase, G., Eds.; Butterworth-Heinemann: Oxford, UK, 2016; pp. 361–382. [Google Scholar]
- Leclercq, E.; Dick, J.R.; Taylor, J.F.; Bell, J.G.; Hunter, D.; Migaud, H. Seasonal variations in skin pigmentation and flesh quality of Atlantic salmon (Salmo salar L.): Implications for quality management. J. Agric. Food Chem. 2010, 58, 7036–7045. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N. Frying fats. In Lipid Oxidation, 2nd ed.; Frankel, E.N., Ed.; Elsevier Woodhead Publishing Limited: Cambridge, UK, 2012; pp. 355–390. [Google Scholar]
- Sae-leaw, T.; Benjakul, S. Fatty acid composition, lipid oxidation, and fishy odour development in seabass (Lates calcarifer) skin during iced storage. Eur. J. Lipid Sci. Technol. 2014, 116, 885–894. [Google Scholar] [CrossRef]
- Sibilla, S.; Godfrey, M.; Brewer, S.; Budh-Raja, A.; Genovese, L. An overview of the beneficial effects of hydrolysed collagen as a nutraceutical on skin properties: Scientific background and clinical studies. Open Nutraceuticals J. 2015, 8, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Poppe, J. Gelatin Thickening and Gelling Agents for Food; Blackie Academic & Professional; Imeson, A.P., Ed.; Springer: Boston, MA, USA, 1997; pp. 144–168. [Google Scholar]
- Idowu, A.T.; Benjakul, S.; Sinthusamran, S.; Sookchoo, P.; Kishimura, H. Protein hydrolysate from salmon frames: Production, characteristics and antioxidative activity. J. Food Biochem. 2019, 43, e12734. [Google Scholar] [CrossRef] [PubMed]
- Thiansilakul, Y.; Benjakul, S.; Shahidi, F. Compositions, functional properties and antioxidative activity of protein hydrolysates prepared from round scad (Decapterus maruadsi). Food Chem. 2007, 103, 1385–1394. [Google Scholar] [CrossRef]
- Chuaychan, S.; Benjakul, S.; Nuthong, P. Element distribution and morphology of spotted golden goatfish fish scales as affected by demineralisation. Food Chem. 2016, 197, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.F.; Smith, D.M. Use of volatiles as indicators of lipid oxidation in muscle foods. Compr. Rev. Food Sci. Food Saf. 2006, 5, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Insausti, K.; Murillo-Arbizu, M.T.; Urrutia, O.; Mendizabal, J.A.; Beriain, M.J.; Colle, M.J.; Bass, P.D.; Arana, A. Volatile compounds, odour and flavour attributes of lamb meat from the navarra breed as affected by ageing. Foods 2021, 10, 493. [Google Scholar] [CrossRef] [PubMed]
- Varlet, V.; Knockaert, C.; Prost, C.; Serot, T. Comparison of odor-active volatile compounds of fresh and smoked salmon. J. Agric. Food Chem. 2006, 54, 3391–3401. [Google Scholar] [CrossRef]
- Chang, C.; Stone, A.K.; Green, R.; Nickerson, M.T. Reduction of off-flavours and the impact on the functionalities of lentil protein isolate by acetone, ethanol, and isopropanol treatments. Food Chem. 2019, 277, 84–95. [Google Scholar] [CrossRef]



| Fatty Acids | Content (g/100 g Lipid) |
|---|---|
| C14:0 | 4.30 ± 0.06 |
| C15:0 | 0.49 ± 0.02 |
| C16:0 | 18.70 ± 0.07 |
| C16:1 | 7.83 ± 0.20 |
| C18:0 | 4.34 ± 0.01 |
| C18:1 n-9 | 16.76 ± 0.05 |
| C18:2 n-6 | 11.69 ± 0.02 |
| C20:1 n-9 | 2.45 ± 0.01 |
| C18:3 n-3 (ALA) | 0.71 ± 0.01 |
| C20:2 n-6 | 1.97 ± 0.01 |
| C22:1 n-9 | 1.94 ± 0.01 |
| C23:0 | 0.98 ± 0.00 |
| C22:2 n-6 | 2.20 ± 0.02 |
| C20:5 n-3 (EPA) | 13.07 ± 0.03 |
| C24:1 n-9 | 1.46 ± 0.02 |
| C22:6 n-3 (DHA) | 11.10 ± 0.05 |
| Saturated fatty acids (SFA) | 28.81 ± 0.11 |
| Monounsaturated fatty acid (MUFA) | 30.45 ± 0.16 |
| Polyunsaturated fatty acid (PUFA) | 40.74 ± 0.06 |
| Parameters | Cycles | |||
|---|---|---|---|---|
| 0 | 3 | 6 | 9 | |
| Fat content (%, dry weight basis) | 20.87 ± 0.45 a | 8.53 ± 0.17 b | 8.44 ± 0.08 b | 7.80 ± 0.14 c |
| L* | 55.07 ± 0.44 d | 71.81 ± 0.30 c | 76.73 ± 0.49 b | 79.05 ± 0.31 a |
| a* | 3.88 ± 0.10 a | 3.17 ± 0.08 b | 1.54 ± 0.02 c | 1.44 ± 0.03 c |
| b* | 25.36 ± 0.38 a | 20.99 ± 0.66 b | 16.30 ± 0.07 c | 15.50 ± 0.08 d |
| ∆Ε* | - | 17.33 ± 0.15 c | 23.60 ± 0.43 b | 26.04 ± 0.25 a |
| Parameters | Cycles | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Fat content (%, dry weight basis) | 7.78 ± 0.19 a | 1.23 ± 0.13 b | ND | ND |
| L* | 78.98 ± 0.17 d | 80.06 ± 0.60 c | 84.52 ± 0.35 b | 86.51 ± 0.33 a |
| a* | 1.44 ± 0.02 a | 1.38 ± 0.08 ab | 1.29 ± 0.08 b | 1.17 ± 0.09 c |
| b* | 15.47 ± 0.04 a | 14.78 ± 0.46 b | 13.37 ± 0.11 c | 12.11 ± 0.56 d |
| ΔE* | - | 1.41 ± 0.38 c | 5.93 ± 0.31 b | 8.26 ± 0.45 a |
| Fishy odor intensity | 2.37 ± 0.21 a | 1.82 ± 0.24 b | 1.49 ± 0.24 c | 1.41 ± 0.29 c |
| PV (mg cumene hydroperoxide equivalents/100 g dry HC) | 4.38 ± 0.10 a | 2.71 ± 0.01 b | 1.05 ± 0.04 c | 1.03 ± 0.04 c |
| TBARS (mg MDA equivalents/100 g dry HC) | 7.30 ± 0.20 a | 5.85 ± 0.07 b | 5.40 ± 0.06 c | 5.22 ± 0.09 c |
| Amino Acids | Content (Residues/1000 Residues) |
|---|---|
| Alanine | 80 |
| Arginine | 52 |
| Aspartic acid/asparagine | 55 |
| Glutamic acid/Glutamine | 65 |
| Glycine | 359 |
| Histidine | 14 |
| Hydroxylysine | 5 |
| Hydroxyproline | 44 |
| Isoleucine | 14 |
| Leucine | 32 |
| Lysine | 43 |
| Methionine | 15 |
| Phenylalanine | 14 |
| Proline | 93 |
| Serine | 50 |
| Threonine | 32 |
| Tryptophan | 1 |
| Tyrosine | 8 |
| Valine | 24 |
| Total amino acids | 1000 |
| Imino acids (Hyp + Pro) | 137 |
| Proximate Compositions | Content (%, Dry Weight Basis) |
|---|---|
| Protein | 94.72 ± 0.06 |
| Ash | 5.08 ± 0.07 |
| Sodium (Na) | 2.50 ± 0.05 |
| Calcium (Ca) | 0.32 ± 0.01 |
| Phosphorous (P) | 0.31 ± 0.00 |
| Compounds | HC | HC-C9 | HC-C9/ISP2 |
|---|---|---|---|
| Aldehyde | |||
| Hexanal | 0.19 | 0.19 | 0.08 |
| 2-Pentenal | 0.07 | ND | ND |
| Heptanal | 0.50 | 0.35 | 0.18 |
| 4-Heptenal | 0.11 | 0.02 | ND |
| Octanal | 0.64 | 1.09 | 0.47 |
| 2-Methyl-2-heptenal | 0.11 | 0.10 | ND |
| Nonanal | 5.11 | 7.42 | 4.53 |
| 2-Octenal | 0.18 | 0.29 | ND |
| 2-Propyl-2-heptenal | 0.16 | 0.10 | ND |
| 2,4-Heptadienal | 0.43 | 0.35 | ND |
| 2-Nonenal | 0.27 | 0.37 | 0.35 |
| 2,6-Nonadienal | 0.83 | 0.82 | 0.70 |
| Undecanal | 3.65 | 5.02 | 2.38 |
| 2-Decenal | 0.17 | 0.54 | 0.44 |
| Alcohol | |||
| 2,4-Octadien-1-ol | ND | 0.01 | ND |
| 1-Octen-3-ol | ND | 0.19 | ND |
| 1-Dodecen-3-ol | 0.24 | ND | ND |
| 1,5-Octadien-3-ol | 0.09 | 0.09 | ND |
| 1-Nonanol | ND | 0.14 | ND |
| 2-Ethylnon-1-en-3-ol | ND | 0.64 | ND |
| 7-Hexadecyn-1-ol | 0.27 | ND | ND |
| Ketone | |||
| 3-Octanone | 0.21 | 0.11 | ND |
| 2-Methyl-3-octanone | 0.53 | ND | ND |
| 2,3-Octanedione | ND | 0.56 | 0.28 |
| 3,5-Octadien-2-one | 0.82 | 0.78 | ND |
| 3-Undecen-2-one | 0.78 | 2.59 | 1.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nilsuwan, K.; Chantakun, K.; Chotphruethipong, L.; Benjakul, S. Development of Hydrolysis and Defatting Processes for Production of Lowered Fishy Odor Hydrolyzed Collagen from Fatty Skin of Sockeye Salmon (Oncorhynchus nerka). Foods 2021, 10, 2257. https://doi.org/10.3390/foods10102257
Nilsuwan K, Chantakun K, Chotphruethipong L, Benjakul S. Development of Hydrolysis and Defatting Processes for Production of Lowered Fishy Odor Hydrolyzed Collagen from Fatty Skin of Sockeye Salmon (Oncorhynchus nerka). Foods. 2021; 10(10):2257. https://doi.org/10.3390/foods10102257
Chicago/Turabian StyleNilsuwan, Krisana, Kasidate Chantakun, Lalita Chotphruethipong, and Soottawat Benjakul. 2021. "Development of Hydrolysis and Defatting Processes for Production of Lowered Fishy Odor Hydrolyzed Collagen from Fatty Skin of Sockeye Salmon (Oncorhynchus nerka)" Foods 10, no. 10: 2257. https://doi.org/10.3390/foods10102257
APA StyleNilsuwan, K., Chantakun, K., Chotphruethipong, L., & Benjakul, S. (2021). Development of Hydrolysis and Defatting Processes for Production of Lowered Fishy Odor Hydrolyzed Collagen from Fatty Skin of Sockeye Salmon (Oncorhynchus nerka). Foods, 10(10), 2257. https://doi.org/10.3390/foods10102257