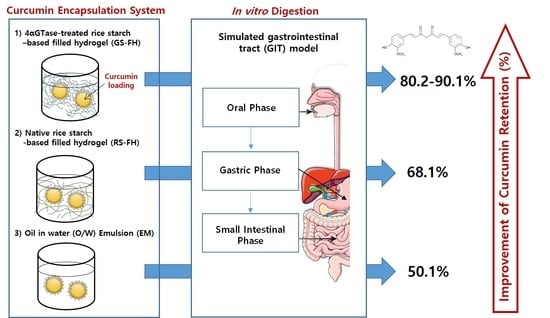
Improving the Stability and Curcumin Retention Rate of Curcumin-Loaded Filled Hydrogel Prepared Using 4αGTase-Treated Rice Starch †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of 4αGTase-Treated Rice Starch
2.3. Physicochemical Properties of 4αGTase-Treated Rice Starch
2.3.1. Molecular Weight Distribution (HPSEC)
2.3.2. Distribution of Branch Chain Length (HPAEC)
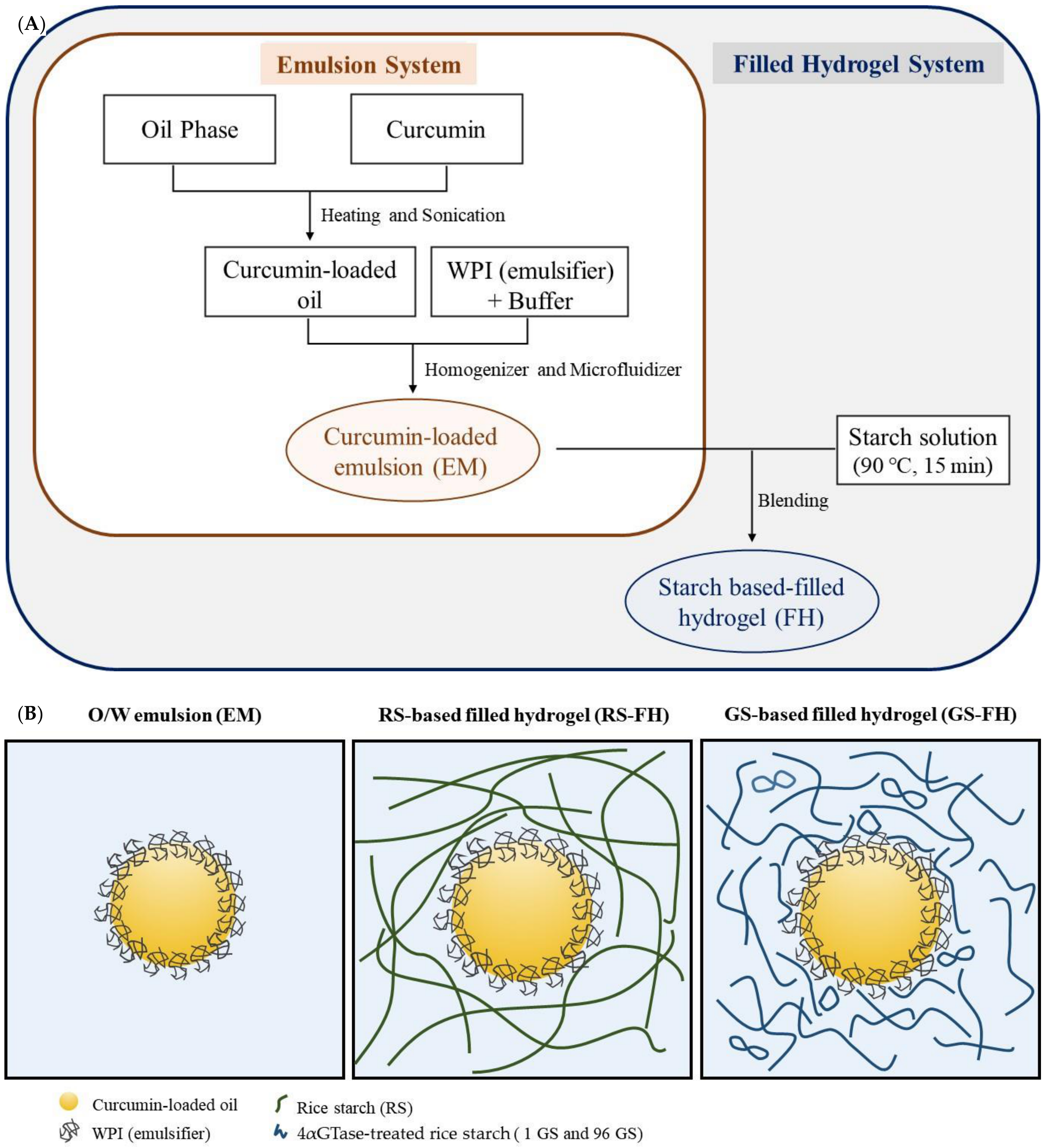
2.4. Encapsulation of the Curcumin in Filled Hydrogel
2.5. Rheological Measurements
2.6. Texture Profile Analysis
2.7. Ultraviolet Stability
2.8. In vitro Digestion Test
2.9. Measurement of Curcumin Retention Rate and Released Free Fatty Acids in the In Vitro Digestion System
2.10. Confocal Laser Scanning Microscopy
2.11. Statistical Analysis
3. Results and Discussion
3.1. Molecular Structural Properties of 4αGTase-Treated Rice Starches
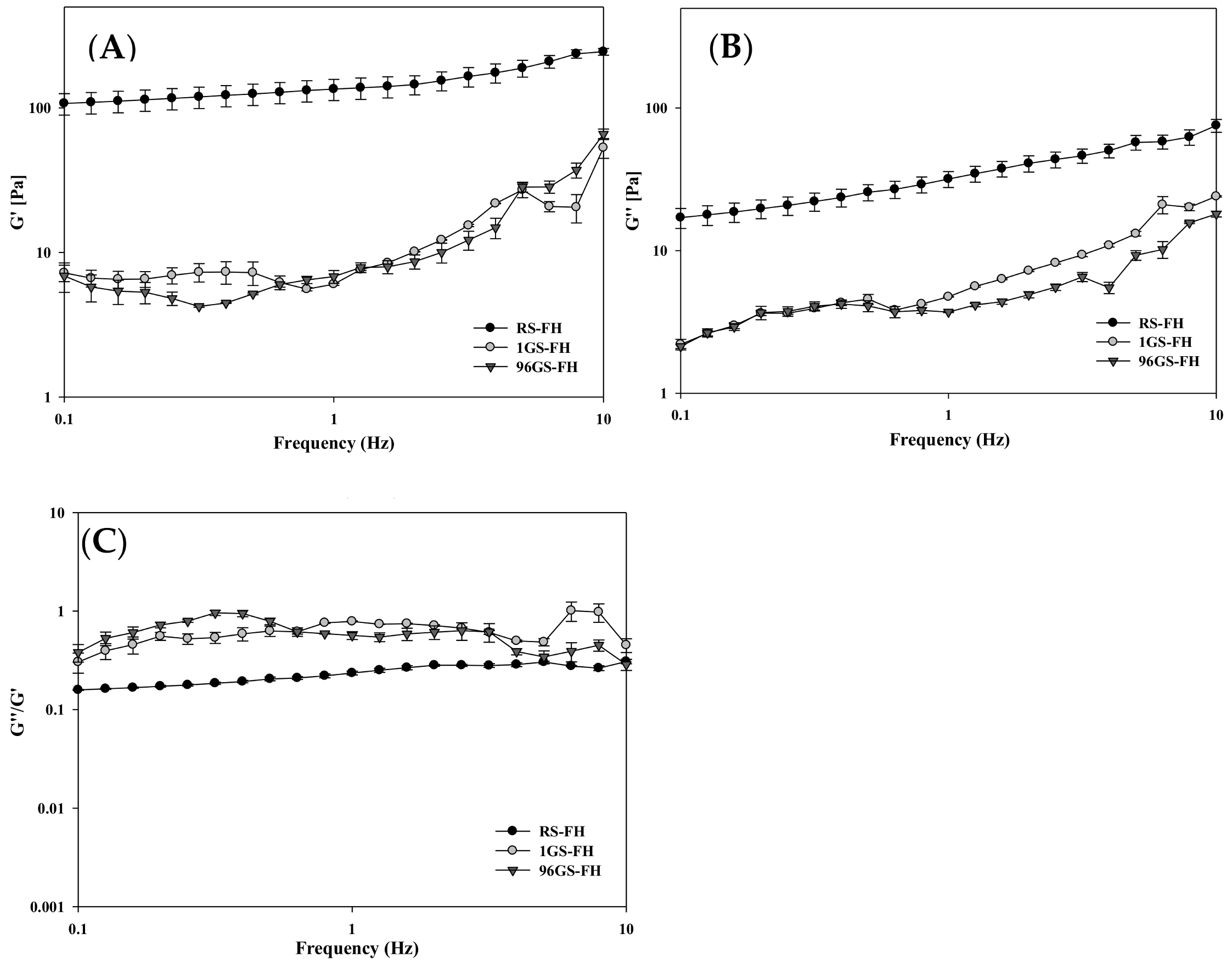
3.2. Rheological Properties of Filled Hydrogel
3.3. Textural Properties of Filled Hydrogel
3.4. Ultraviolet Stability of the Curcumin Encapsulation System
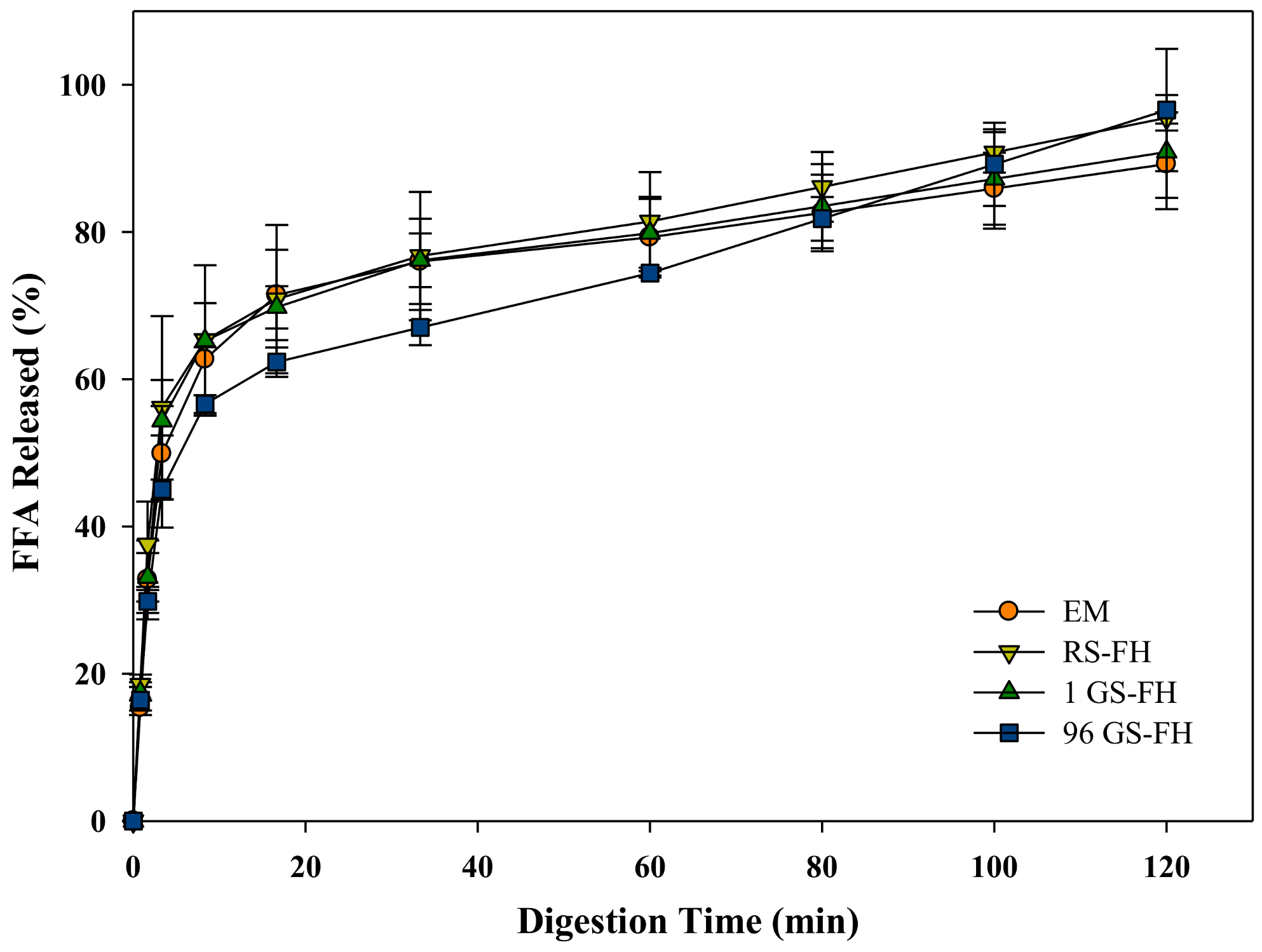
3.5. Lipid Digestibility of the Curcumin Encapsulation System
3.6. Curcumin Retention Rate in the Curcumin Encapsulation System after In Vitro Digestion
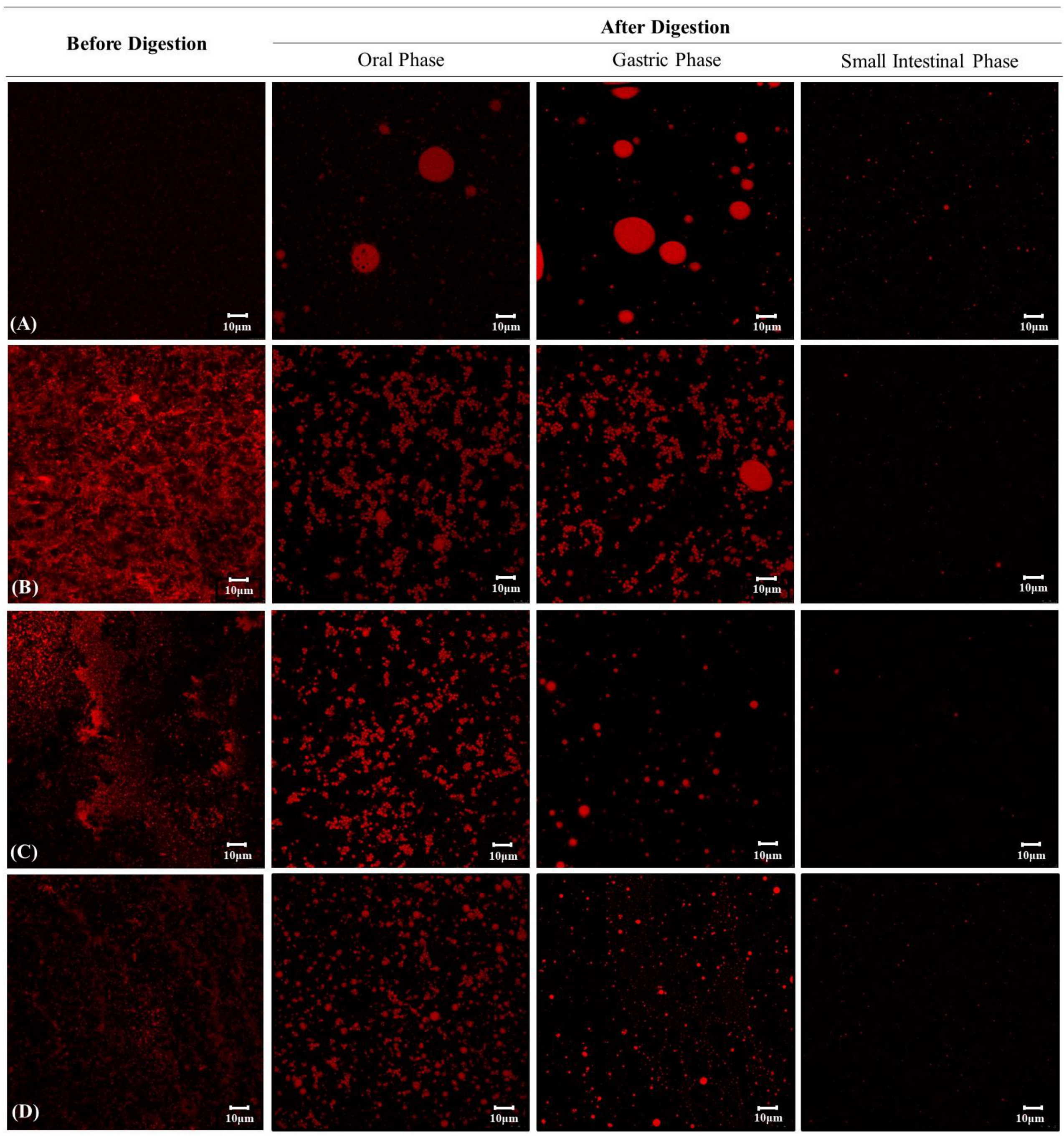
3.7. Changes in the Microstructure of Curcumin Encapsulation Systems during In Vitro Digestion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aditya, N.; Aditya, S.; Yang, H.; Kim, H.W.; Park, S.O.; Ko, S. Co-delivery of hydrophobic curcumin and hydrophilic catechin by a water-in-oil-in-water double emulsion. Food Chem. 2015, 173, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.H.; Karlsen, J.; van Henegouwen, G.B. Studies on curcumin and curcuminoids VIII. Photochemical stability of curcumin. Z. Lebensm. Unters. Forsch. 1986, 183, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Jakubek, M.; Kejík, Z.; Kaplánek, R.; Hromádka, R.; Šandriková, V.; Sýkora, D.; Antonyová, B.; Urban, M.; Dytrych, P.; Mikula, I.; et al. Strategy for improved therapeutic efficiency of curcumin in the treatment of gastric cancer. Biomed. Pharmacother. 2019, 118, 109278. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Li, Y.; McClements, D.J.; Xiao, H. Nanoemulsion-and emulsion-based delivery systems for curcumin: Encapsulation and release properties. Food Chem. 2012, 132, 799–807. [Google Scholar] [CrossRef]
- Lee, B.H.; Choi, H.A.; Kim, M.R.; Hong, J. Changes in chemical stability and bioactivities of curcumin by ultraviolet radiation. Food Sci. Biotechnol. 2013, 22, 279–282. [Google Scholar] [CrossRef]
- Nimiya, Y.; Wang, W.; Du, Z.; Sukamtoh, E.; Zhu, J.; Decker, E.; Zhang, G. Redox modulation of curcumin stability: Redox active antioxidants increase chemical stability of curcumin. Mol. Nutr. Food Res. 2016, 60, 487–494. [Google Scholar] [CrossRef]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and chemical stability of curcumin in aqueous solutions and emulsions: Impact of pH, temperature, and molecular environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef]
- Pan, K.; Zhong, Q.; Baek, S.J. Enhanced dispersibility and bioactivity of curcumin by encapsulation in casein nanocapsules. J. Agric. Food Chem. 2013, 61, 6036–6043. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Studies on Curcumin and Curcuminoids VI. Kinetics of Curcumin Degradation in Aqueous Solution. Z. Lebensm. Unters. Forsch. 1985, 180, 402–404. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Studies on curcumin and curcuminoids. V. Alkaline degradation of curcumin. Z. Lebensm. Unters. Forsch. 1985, 180, 132–134. [Google Scholar] [CrossRef]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharmaceut. Biomed. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Canamares, M.V.; Garcia-Ramos, J.V.; Sanchez-Cortes, S. Degradation of curcumin dye in aqueous solution and on Ag nanoparticles studied by ultraviolet–visible absorption and surface-enhanced Raman spectroscopy. Appl. Spectrosc. 2006, 60, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Huang, T.M.; Lin, J.K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern Med. 2006, 6, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Zheng, B.; Zhang, R.; Zhang, Z.; Liu, W.; Liu, C.; Zhang, G.; Xiao, H.; McClements, D.J. Influence of lipid phase composition of excipient emulsions on curcumin solubility, stability, and bioaccessibility. Food Biophys. 2016, 11, 213–225. [Google Scholar] [CrossRef]
- Jiang, T.; Liao, W.; Charcosset, C. Recent advances in encapsulation of curcumin in nanoemulsions: A review of encapsulation technologies, bioaccessibility and applications. Food Res. Int. 2020, 132, 109035. [Google Scholar] [CrossRef]
- Naksuriya, O.; Okonogi, S.; Schiffelers, R.M.; Hennink, W.E. Curcumin nanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials 2014, 35, 3365–3383. [Google Scholar] [CrossRef]
- Li, Z.; Shi, M.; Li, N.; Xu, R. Application of Functional Biocompatible Nanomaterials to Improve Curcumin Bioavailability. Front. Chem. 2020, 8, 929. [Google Scholar] [CrossRef]
- Li, X.; Chen, T.; Xu, L.; Zhang, Z.; Li, L.; Chen, H. Preparation of curcumin micelles and the in vitro and in vivo evaluation for cancer therapy. J. Biomed. Nanotechnol. 2014, 10, 1458–1468. [Google Scholar] [CrossRef]
- Joung, H.J.; Choi, M.J.; Kim, J.T.; Park, S.H.; Park, H.J.; Shin, G.H. Development of food-grade curcumin nanoemulsion and its potential application to food beverage system: Antioxidant property and in vitro digestion. J. Food Sci. 2016, 81, N745–N753. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.; Weiss, J. Emulsion-based delivery systems for lipophilic bioactive components. J. Food Sci. 2007, 72, R109–R124. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Zhang, Z.; Chen, F.; Luo, X.; McClements, D.J. Impact of delivery system type on curcumin stability: Comparison of curcumin degradation in aqueous solutions, emulsions, and hydrogel beads. Food Hydrocoll. 2017, 71, 187–197. [Google Scholar] [CrossRef]
- Zou, L.; Zheng, B.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Enhancing nutraceutical bioavailability using excipient emulsions: Influence of lipid droplet size on solubility and bioaccessibility of powdered curcumin. J. Funct. Foods 2015, 15, 72–83. [Google Scholar] [CrossRef]
- Calero, N.; Muñoz, J.; Cox, P.W.; Heuer, A.; Guerrero, A. Influence of chitosan concentration on the stability, microstructure and rheological properties of O/W emulsions formulated with high-oleic sunflower oil and potato protein. Food Hydrocoll. 2013, 30, 152–162. [Google Scholar] [CrossRef]
- Jung, W.J.; Han, J.A.; Lim, S.T. Thermal and rheological properties of hydrogels prepared with retrograded waxy rice starch powders. Food Sci. Biotechnol. 2010, 19, 1649–1654. [Google Scholar] [CrossRef]
- Peppas, N.; Slaughter, B.; Kanzelberger, M.; Matyjaszewski, K.; Möller, M. 9.20–Hydrogels. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2012; pp. 385–395. [Google Scholar]
- Do, H.V.; Lee, E.J.; Park, J.H.; Park, K.H.; Shim, J.Y.; Mun, S.; Kim, Y.R. Structural and physicochemical properties of starch gels prepared from partially modified starches using Thermus aquaticus 4-α-glucanotransferase. Carbohydr. Polym. 2012, 87, 2455–2463. [Google Scholar] [CrossRef]
- Polesi, L.F.; Sarmento, S.B.S.; de Moraes, J.; Franco, C.M.L.; Canniatti-Brazaca, S.G. Physicochemical and structural characteristics of rice starch modified by irradiation. Food Chem. 2016, 191, 59–66. [Google Scholar] [CrossRef]
- Mun, S.; Kim, Y.L.; Kang, C.G.; Park, K.H.; Shim, J.Y.; Kim, Y.R. Development of reduced-fat mayonnaise using 4αGTase-modified rice starch and xanthan gum. Int. J. Biol. Macromol. 2009, 44, 400–407. [Google Scholar] [CrossRef]
- Lee, K.Y.; Kim, Y.R.; Park, K.H.; Lee, H.G. Effects of α-glucanotransferase treatment on the thermo-reversibility and freeze-thaw stability of a rice starch gel. Carbohydr. Polym. 2006, 63, 347–354. [Google Scholar] [CrossRef]
- Do, V.H.; Mun, S.; Kim, Y.L.; Rho, S.J.; Park, K.H.; Kim, Y.R. Novel formulation of low-fat spread using rice starch modified by 4-α-glucanotransferase. Food Chem. 2016, 208, 132–141. [Google Scholar] [CrossRef]
- Binnema, D.J.; Euverink, G.J.W. Use of Modified Starch as an Agent for Forming a Thermoreversible Gel. Patent Application WO9815347, 14 April 1998. [Google Scholar]
- Cho, K.H.; Auh, J.H.; Ryu, J.H.; Kim, J.H.; Park, K.H.; Park, C.S.; Yoo, S.H. Structural modification and characterization of rice starch treated by Thermus aquaticus 4-α-glucanotransferase. Food Hydrocoll. 2009, 23, 2403–2409. [Google Scholar] [CrossRef]
- Park, H.R.; Rho, S.J.; Kim, Y.R. Solubility, stability, and bioaccessibility improvement of curcumin encapsulated using 4-α-glucanotransferase-modified rice starch with reversible pH-induced aggregation property. Food Hydrocoll. 2019, 95, 19–32. [Google Scholar] [CrossRef]
- Mun, S.; Choi, Y.; Shim, J.Y.; Park, K.H.; Kim, Y.R. Effects of enzymatically modified starch on the encapsulation efficiency and stability of water-in-oil-in-water emulsions. Food Chem. 2011, 128, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.J. Comparison of protease digestion at neutral pH with alkaline steeping method for rice starch isolation. Cereal Chem. 2001, 78, 690–692. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, H.J.; Kim, Y.H.; Cha, H.; Kim, Y.W.; Kim, T.J.; Kim, Y.R.; Park, K.H. The action mode of Thermus aquaticus YT-1 4-α-glucanotransferase and its chimeric enzymes introduced with starch-binding domain on amylose and amylopectin. Carbohydr. Polym. 2007, 67, 164–173. [Google Scholar] [CrossRef]
- Li, J.; Hwang, I.C.; Chen, X.; Park, H.J. Effects of chitosan coating on curcumin loaded nano-emulsion: Study on stability and in vitro digestibility. Food Hydrocoll. 2016, 60, 138–147. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, B.; Li, M.N.; Xie, Y.; Chen, H.Q. Effects of glutenin and gliadin modified by protein-glutaminase on pasting, rheological properties and microstructure of potato starch. Food Chem. 2018, 253, 148–155. [Google Scholar] [CrossRef]
- Sarkar, A.; Goh, K.K.; Singh, R.P.; Singh, H. Behaviour of an oil-in-water emulsion stabilized by β-lactoglobulin in an in vitro gastric model. Food Hydrocoll. 2009, 23, 1563–1569. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Zhang, H.; Decker, E.A.; McClements, D.J. Influence of emulsifier type on gastrointestinal fate of oil-in-water emulsions containing anionic dietary fiber (pectin). Food Hydrocoll. 2015, 45, 175–185. [Google Scholar] [CrossRef]
- Lopez-Pena, C.L.; Zheng, B.; Sela, D.A.; Decker, E.A.; Xiao, H.; McClements, D.J. Impact of ε-polylysine and pectin on the potential gastrointestinal fate of emulsified lipids: In vitro mouth, stomach and small intestine model. Food Chem. 2016, 192, 857–864. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Qian, C.; Martín-Belloso, O.; McClements, D.J. Influence of particle size on lipid digestion and β-carotene bioaccessibility in emulsions and nanoemulsions. Food Chem. 2013, 141, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.L.; Mun, S.; Rho, S.J.; Do, H.V.; Kim, Y.R. Influence of physicochemical properties of enzymatically modified starch gel on the encapsulation efficiency of W/O/W emulsion containing NaCl. Food Bioprocess Tech. 2017, 10, 77–88. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, Y.L.; Trinh, K.S.; Kim, Y.R.; Moon, T.W. Texture properties of rice cakes made of rice flours treated with 4-α-glucanotransferase and their relationship with structural characteristics. Food Sci. Biotechnol. 2012, 21, 1707–1714. [Google Scholar] [CrossRef]
- van der Maarel, M.J.; Leemhuis, H. Starch modification with microbial alpha-glucanotransferase enzymes. Carbohydr. Polym. 2013, 93, 116–121. [Google Scholar] [CrossRef]
- Takaha, T.; Yanase, M.; Takata, H.; Okada, S.; Smith, S.M. Cyclic glucans produced by the intramolecular transglycosylation activity of potato D-enzyme on amylopectin. Biochem. Biophys. Res. Commun. 1998, 247, 493–497. [Google Scholar] [CrossRef]
- BeMiller, J.N.; Whistler, R.L. (Eds.) Starch: Chemistry and Technology; Academic Press: New York, NY, USA, 2009; pp. 293–372. [Google Scholar]
- Jiménez-Avalos, H.A.; Ramos-Ramírez, E.G.; Salazar-Montoya, J.A. Viscoelastic characterization of gum arabic and maize starch mixture using the Maxwell model. Carbohydr. Polym. 2005, 62, 11–18. [Google Scholar] [CrossRef]
- Song, J.C.; Park, H.J. Physical, Functional, Textural and Rheological Properties of Foods; University of Ulsan: Ulsan, Korea, 1995. [Google Scholar]
- Li, Y.; Li, C.; Gu, Z.; Hong, Y.; Cheng, L.; Li, Z. Effect of modification with 1,4-α-glucan branching enzyme on the rheological properties of cassava starch. Int. J. Biol. Macromol. 2017, 103, 630–639. [Google Scholar] [CrossRef]
- Stephen, A.M.; Phillips, G.O. Food Polysaccharides and Their Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 544–550. [Google Scholar] [CrossRef]
- Yang, H.; Irudayaraj, J.; Otgonchimeg, S.; Walsh, M. Rheological study of starch and dairy ingredient-based food systems. Food Chem. 2004, 86, 571–578. [Google Scholar] [CrossRef]
- Lee, K.Y.; Kim, Y.R.; Park, K.H.; Lee, H.G. Rheological and gelation properties of rice starch modified with 4-α-glucanotransferase. Int. J. Biol. Macromol. 2008, 42, 298–304. [Google Scholar] [CrossRef]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. Chemistry, structure, functionality and applications of rice starch. J. Cereal Sci. 2016, 70, 291–300. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, P.; Sui, Z.; Bao, J. Physicochemical properties of starches from diverse rice cultivars varying in apparent amylose content and gelatinisation temperature combinations. Food Chem. 2015, 172, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Li, R.; Wei, Y.; Sun, C.; Mao, L.; Gao, Y. Fabrication of zein and rhamnolipid complex nanoparticles to enhance the stability and in vitro release of curcumin. Food Hydrocoll. 2018, 77, 617–628. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Nanoemulsion delivery systems: Influence of carrier oil on β-carotene bioaccessibility. Food Chem. 2012, 135, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Li, Y. Review of in vitro digestion models for rapid screening of emulsion-based systems. Food Funct. 2010, 1, 32–59. [Google Scholar] [CrossRef]
- McClements, D.J.; Zou, L.; Zhang, R.; Salvia-Trujillo, L.; Kumosani, T.; Xiao, H. Enhancing nutraceutical performance using excipient foods: Designing food structures and compositions to increase bioavailability. Compr. Rev. Food Sci. Food Saf. 2015, 14, 824–847. [Google Scholar] [CrossRef]
- Park, S.; Mun, S.; Kim, Y.R. Effect of xanthan gum on lipid digestion and bioaccessibility of β-carotene-loaded rice starch-based filled hydrogels. Food Res. Int. 2018, 105, 440–445. [Google Scholar] [CrossRef]
- Cheong, A.M.; Tan, C.P.; Nyam, K.L. In vitro evaluation of the structural and bioaccessibility of kenaf seed oil nanoemulsions stabilised by binary emulsifiers and β-cyclodextrin complexes. J. Food Eng. 2016, 189, 90–98. [Google Scholar] [CrossRef]
- Liu, F.; Ma, D.; Luo, X.; Zhang, Z.; He, L.; Gao, Y.; McClements, D.J. Fabrication and characterization of protein-phenolic conjugate nanoparticles for co-delivery of curcumin and resveratrol. Food Hydrocoll. 2018, 79, 450–461. [Google Scholar] [CrossRef]
- Porter, C.J.; Kaukonen, A.M.; Boyd, B.J.; Edwards, G.A.; Charman, W.N. Susceptibility to lipase-mediated digestion reduces the oral bioavailability of danazol after administration as a medium-chain lipid-based microemulsion formulation. Pharm. Res. 2004, 21, 1405–1412. [Google Scholar] [CrossRef]
- Porter, C.J.; Pouton, C.W.; Cuine, J.F.; Charman, W.N. Enhancing intestinal drug solubilisation using lipid-based delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 673–691. [Google Scholar] [CrossRef]
- Mun, S.; Park, S.; Kim, Y.R.; McClements, D.J. Influence of methylcellulose on attributes of β-carotene fortified starch-based filled hydrogels: Optical, rheological, structural, digestibility, and bioaccessibility properties. Food Res. Int. 2016, 87, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Vingerhoeds, M.H.; Blijdenstein, T.B.; Zoet, F.D.; van Aken, G.A. Emulsion flocculation induced by saliva and mucin. Food Hydrocoll. 2005, 19, 915–922. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; Zou, L.; Chen, L.; Ahmed, Y.; Al Bishri, W.; Balamash, K.; McClements, D.J. Encapsulation of curcumin in polysaccharide-based hydrogel beads: Impact of bead type on lipid digestion and curcumin bioaccessibility. Food Hydrocoll. 2016, 58, 160–170. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.R. Clean label starch: Production, physicochemical characteristics, and industrial applications. Food Sci. Biotechnol. 2020, 1–17. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Yuan, R. Applications of natural polymer-based hydrogels in the food industry. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 357–410. [Google Scholar] [CrossRef]
- Farris, S.; Schaich, K.M.; Liu, L.; Piergiovanni, L.; Yam, K.L. Development of polyion-complex hydrogels as an alternative approach for the production of bio-based polymers for food packaging applications: A review. Trends Food Sci. Technol. 2009, 20, 316–332. [Google Scholar] [CrossRef]


| FH Samples 1 | Gel Characteristics | |||||
|---|---|---|---|---|---|---|
| Hardness [N] | Adhesiveness | Cohesiveness | Springiness (%) | Gumminess [N] | Chewiness [N] | |
| RS-FH | 5.17 ± 0.10 c2 | 1.56 ± 0.07 NS3 | 0.68 ± 0.02 b | 93.53 ± 0.27 NS | 3.52 ± 0.17 c | 329.45± 14.91 c |
| 1 GS-FH | 4.10 ± 0.05 b | 1.80 ± 0.28 | 0.35 ± 0.01 a | 94.66 ± 11.34 | 1.45 ± 0.08 b | 136.90 ± 9.21 b |
| 96 GS-FH | 1.18 ± 0.13 a | 1.75 ± 0.20 | 0.43 ± 0.04 a | 86.91 ± 2.47 | 0.50 ± 0.00 a | 43.56 ± 1.06 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Kim, Y.-H.; Choi, S.-J.; Rho, S.-J.; Kim, Y.-R. Improving the Stability and Curcumin Retention Rate of Curcumin-Loaded Filled Hydrogel Prepared Using 4αGTase-Treated Rice Starch. Foods 2021, 10, 150. https://doi.org/10.3390/foods10010150
Kang J, Kim Y-H, Choi S-J, Rho S-J, Kim Y-R. Improving the Stability and Curcumin Retention Rate of Curcumin-Loaded Filled Hydrogel Prepared Using 4αGTase-Treated Rice Starch. Foods. 2021; 10(1):150. https://doi.org/10.3390/foods10010150
Chicago/Turabian StyleKang, Jihyun, Ye-Hyun Kim, Soo-Jin Choi, Shin-Joung Rho, and Yong-Ro Kim. 2021. "Improving the Stability and Curcumin Retention Rate of Curcumin-Loaded Filled Hydrogel Prepared Using 4αGTase-Treated Rice Starch" Foods 10, no. 1: 150. https://doi.org/10.3390/foods10010150
APA StyleKang, J., Kim, Y.-H., Choi, S.-J., Rho, S.-J., & Kim, Y.-R. (2021). Improving the Stability and Curcumin Retention Rate of Curcumin-Loaded Filled Hydrogel Prepared Using 4αGTase-Treated Rice Starch. Foods, 10(1), 150. https://doi.org/10.3390/foods10010150