Novel Histomorphometrical Approach to Evaluate the Integration Pattern and Functionality of Barrier Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Barrier Membranes
2.1.1. Test Membrane
2.1.2. Commercially Available Membranes (Control Membranes)
2.2. In Vivo Study Design, Im- and Ex-Plantation
2.3. Histological Workup and Staining
2.4. Histopathological and Histomorphometrical Analysis
2.5. Statistical Analysis
3. Results
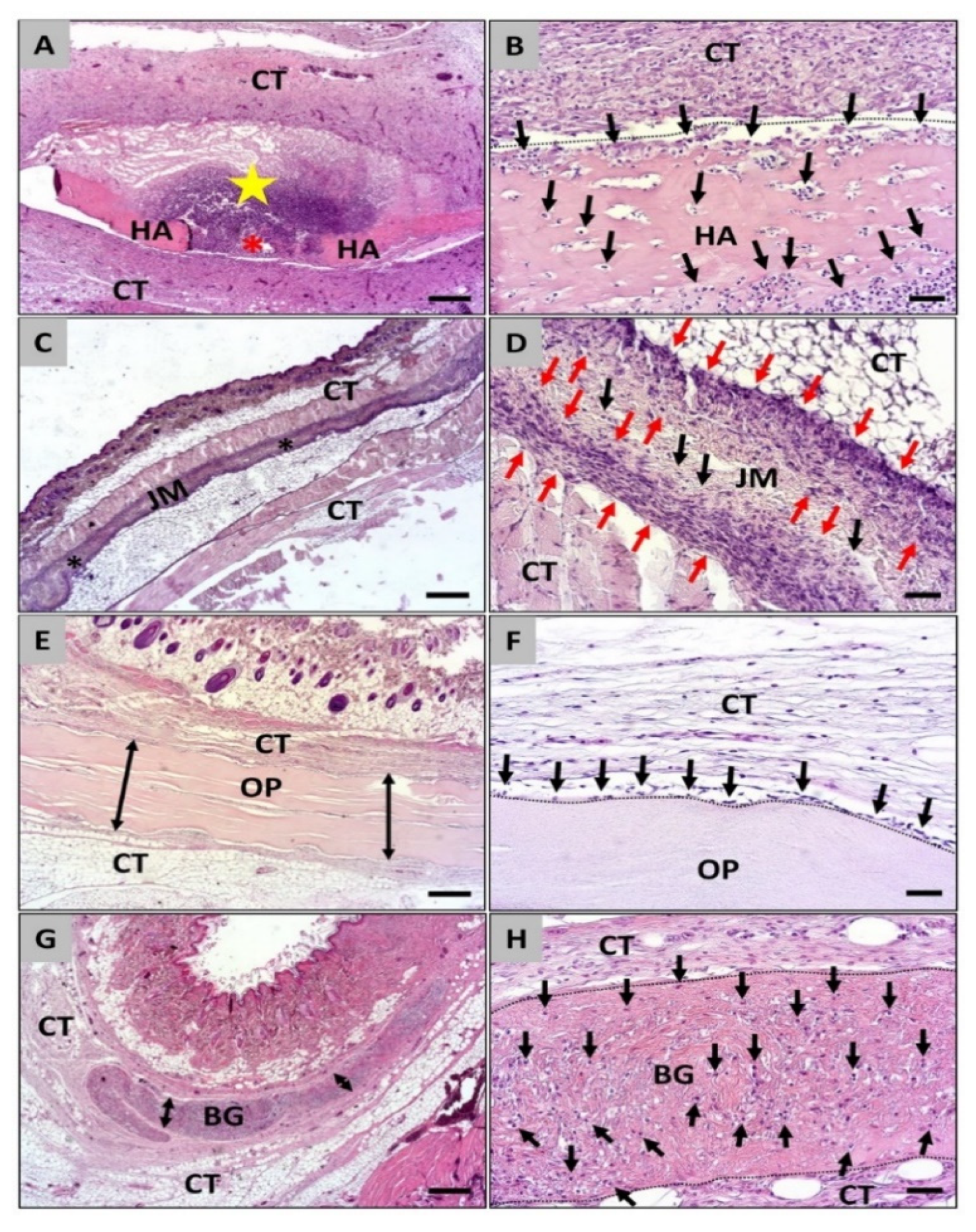
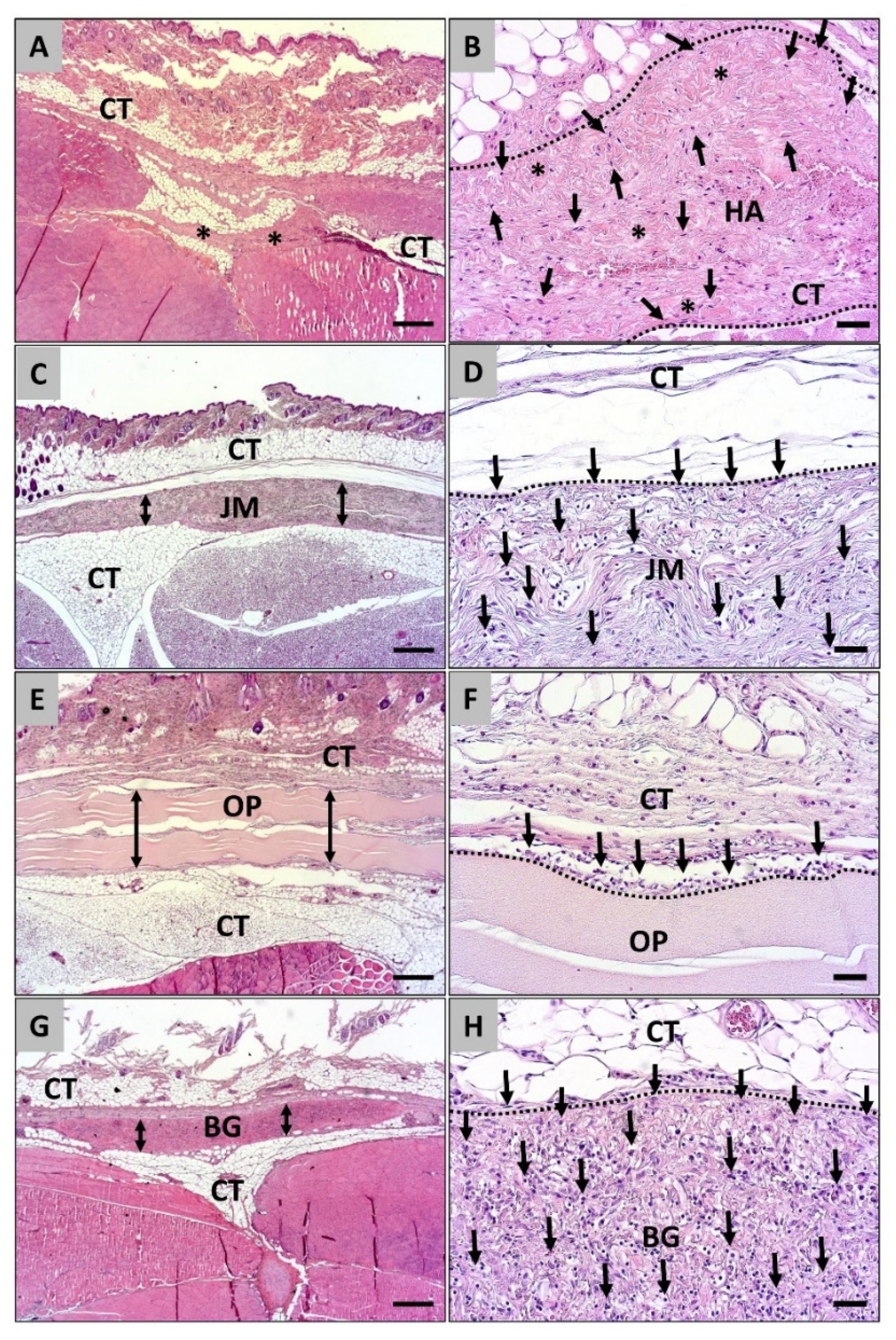
3.1. Histopathological Analysis of the Cellular Migration
3.2. Histomorphometrical Analysis of the Cellular Migration
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gruber, R.; Stadlinger, B.; Terheyden, H. Cell-to-cell communication in guided bone regeneration: Molecular and cellular mechanisms. Clin. Oral Implant. Res. 2017, 28, 1139–1146. [Google Scholar] [CrossRef]
- Troiano, G.; Zhurakivska, K.; Muzio, L.L.; Laino, L.; Cicciù, M.; Russo, L.L. Combination of Bone Graft and Resorbable Membrane for Alveolar Ridge Preservation: A Systematic Review, Meta-analysis and Trial Sequential Analysis. J. Periodontol. 2018, 89, 46–57. [Google Scholar] [CrossRef]
- Behring, J.; Junker, R.; Walboomers, X.F.; Chessnut, B.; Jansen, J.A. Toward guided tissue and bone regeneration: Morphology, attachment, proliferation, and migration of cells cultured on collagen barrier membranes. A systematic review. Odontology 2008, 96, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implant. Res. 2010, 21, 567–576. [Google Scholar] [CrossRef]
- Caballe-Serrano, J.; Munar-Frau, A.; Ortiz-Puigpelat, O.; Soto-Penaloza, D.; Penarrocha, M.; Hernandez-Alfaro, F. On the search of the ideal barrier membrane for guided bone regeneration. J. Clin. Exp. Dent. 2018, 10, e477–e483. [Google Scholar] [CrossRef]
- Radenković, M.; Alkildani, S.; Stoewe, I.; Bielenstein, J.; Sundag, B.; Bellmann, O.; Jung, O.; Najman, S.; Stojanović, S.; Barbeck, M. Comparative In Vivo Analysis of the Integration Behavior and Immune Response of Collagen-Based Dental Barrier Membranes for Guided Bone Regeneration (GBR). Membranes 2021, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Mataliotakis, G.I.; Calori, G.M.; Giannoudis, P.V. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Cucchi, A.; Sartori, M.; Parrilli, A.; Aldini, N.N.; Vignudelli, E.; Corinaldesi, G. Histological and histomorphometric analysis of bone tissue after guided bone regeneration with non-resorbable membranes vs. resorbable membranes and titanium mesh. Clin. Implant. Dent. Relat. Res. 2019, 21, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Hämmerle, C.H.F.; Jung, R.E. Bone augmentation by means of barrier membranes. Periodontology 2003, 33, 36–53. [Google Scholar] [CrossRef]
- McAllister, B.S.; Haghighat, K. Bone Augmentation Techniques. J. Periodontol. 2007, 78, 377–396. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bubalo, M.; Lazic, Z.; Tatic, Z.; Milovic, R.; Magic, M. The use of collagen membranes in guided tissue regeneration. Vojn. Pregl. 2017, 74, 767–772. [Google Scholar] [CrossRef][Green Version]
- Gelse, K. Collagens—structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Zubery, Y.; Nir, E.; Goldlust, A. Ossification of a Collagen Membrane Cross-Linked by Sugar: A Human Case Series. J. Periodontol. 2008, 79, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Atef, M.; Tarek, A.; Shaheen, M.; AlArawi, R.M.; Askar, N. Horizontal ridge augmentation using native collagen membrane vs. titanium mesh in atrophic maxillary ridges: Randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2020, 22, 156–166. [Google Scholar] [CrossRef]
- Nilius, M.; Kohlhase, J.; Lorenzen, J.; Lauer, G.; Schulz, M.C. Multidisciplinary oral rehabilitation of an adolescent suffering from juvenile Gorlin-Goltz syndrome—A case report. Head Face Med. 2019, 15, 5. [Google Scholar] [CrossRef]
- Barbeck, M.; Lorenz, J.; Kubesch, A.; Böhm, N.; Booms, P.; Choukroun, J.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Porcine Dermis-Derived Collagen Membranes Induce Implantation Bed Vascularization Via Multinucleated Giant Cells: A Physiological Reaction? J. Oral Implant. 2015, 41, e238–e251. [Google Scholar] [CrossRef] [PubMed]
- Zubery, Y.; Goldlust, A.; Alves, A.; Nir, E. Ossification of a Novel Cross-Linked Porcine Collagen Barrier in Guided Bone Regeneration in Dogs. J. Periodontol. 2007, 78, 112–121. [Google Scholar] [CrossRef]
- Kapogianni, E.; Alkildani, S.; Radenkovic, M.; Xiong, X.; Krastev, R.; Stöwe, I.; Bielenstein, J.; Jung, O.; Najman, S.; Barbeck, M.; et al. The Early Fragmentation of a Bovine Dermis-Derived Collagen Barrier Membrane Contributes to Transmembraneous Vascularization—A Possible Paradigm Shift for Guided Bone Regeneration. Membranes 2021, 11, 185. [Google Scholar] [CrossRef]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of Collagen Membranes for Bone Regeneration: A Literature Review. Materials 2020, 13, 786. [Google Scholar] [CrossRef] [PubMed]
- Daghighi, S.; Sjollema, J.; van der Mei, H.C.; Busscher, H.J.; Rochford, E.T. Infection resistance of degradable versus non-degradable biomaterials: An assessment of the potential mechanisms. Biomaterials 2013, 34, 8013–8017. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, Y.; Yu, X.; Fu, W.; Wang, W.; Huang, H. Rapid vascularization of tissue-engineered vascular grafts in vivo by endothelial cells in co-culture with smooth muscle cells. J. Mater. Sci. Mater. Electron. 2012, 23, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Chia-Lai, P.-J.; Orlowska, A.; Al-Maawi, S.; Dias, A.; Zhang, Y.; Wang, X.; Zender, N.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Sugar-based collagen membrane cross-linking increases barrier capacity of membranes. Clin. Oral Investig. 2017, 22, 1851–1863. [Google Scholar] [CrossRef]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef]
- Pröhl, A.; Batinic, M.; Alkildani, S.; Hahn, M.; Radenkovic, M.; Najman, S.; Jung, O.; Barbeck, M. In Vivo Analysis of the Biocompatibility and Bone Healing Capacity of a Novel Bone Grafting Material Combined with Hyaluronic Acid. Int. J. Mol. Sci. 2021, 22, 4818. [Google Scholar] [CrossRef]
- Sieger, D.; Korzinskas, T.; Jung, O.; Stojanovic, S.; Wenisch, S.; Smeets, R.; Gosau, M.; Schnettler, R.; Najman, S.; Barbeck, M. The Addition of High Doses of Hyaluronic Acid to a Biphasic Bone Substitute Decreases the Proinflammatory Tissue Response. Int. J. Mol. Sci. 2019, 20, 1969. [Google Scholar] [CrossRef]
- Ghanaati, S.; Barbeck, M.; Hilbig, U.; Hoffmann, C.; Unger, R.; Sader, R.; Peters, F.; Kirkpatrick, C. An injectable bone substitute composed of beta-tricalcium phosphate granules, methylcellulose and hyaluronic acid inhibits connective tissue influx into its implantation bed in vivo. Acta Biomater. 2011, 7, 4018–4028. [Google Scholar] [CrossRef]
- Xing, F.; Zhou, C.; Hui, D.; Du, C.; Wu, L.; Wang, L.; Wang, W.; Pu, X.; Gu, L.; Liu, L.; et al. Hyaluronic acid as a bioactive component for bone tissue regeneration: Fabrication, modification, properties, and biological functions. Nanotechnol. Rev. 2020, 9, 1059–1079. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Lo, Y.-J.; Feng, S.-W.; Huang, Y.-C.; Tsai, H.-Y.; Lin, C.-T.; Fan, K.-H.; Huang, H.-M. Bone Healing Improvements Using Hyaluronic Acid and Hydroxyapatite/Beta-Tricalcium Phosphate in Combination: An Animal Study. BioMed Res. Int. 2016, 2016, 8301624. [Google Scholar] [CrossRef]
- Dunn, R.M. Cross-Linking in Biomaterials: A primer for clinicians. Plast. Reconstr. Surg. 2012, 130, 18S–26S. [Google Scholar] [CrossRef]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 24. [Google Scholar] [CrossRef]
- Schwarz, F.; Rothamel, D.; Herten, M.; Sager, M.; Becker, J. Angiogenesis pattern of native and cross-linked collagen membranes: An immunohistochemical study in the rat. Clin. Oral Implant. Res. 2006, 17, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Rothamel, D.; Schwarz, F.; Sager, M.; Herten, M.; Sculean, A.; Becker, J. Biodegradation of differently cross-linked collagen membranes: An experimental study in the rat. Clin. Oral Implant. Res. 2005, 16, 369–378. [Google Scholar] [CrossRef]
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier membranes: More than the barrier effect? J. Clin. Periodontol. 2019, 46, 103–123. [Google Scholar] [CrossRef] [PubMed]
- Jung, O.; Radenkovic, M.; Stojanović, S.; Lindner, C.; Batinic, M.; Görke, O.; Pissarek, J.; Pröhl, A.; Najman, S.; Barbeck, M. In Vitro and In Vivo Biocompatibility Analysis of a New Transparent Collagen-based Wound Membrane for Tissue Regeneration in Different Clinical Indications. In Vivo 2020, 34, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Lorenz, J.; Holthaus, M.G.; Raetscho, N.; Kubesch, A.; Booms, P.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Porcine Dermis and Pericardium-Based, Non–Cross-Linked Materials Induce Multinucleated Giant Cells after Their In Vivo Implantation: A Physiological Reaction? J. Oral Implant. 2015, 41, e267–e281. [Google Scholar] [CrossRef]
- BSI. Biological Evaluation of Medical Devices; DIN EN ISO 10993; BSI: London, UK, 2003. [Google Scholar]
- Clark, J.M. The 3Rs in research: A contemporary approach to replacement, reduction and refinement. Br. J. Nutr. 2018, 120, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Rothamel, D.; Schwarz, F.; Sculean, A.; Herten, M.; Scherbaum, W.; Becker, J. Biocompatibility of various collagen membranes in cultures of human PDL fibroblasts and human osteoblast-like cells. Clin. Oral Implant. Res. 2004, 15, 443–449. [Google Scholar] [CrossRef]
- Datum Dental Ltd. Ossix Plus. Available online: https://www.datumdental.com/en-us/wp-content/uploads/sites/13/2018/11/OSSIX-Plus-brochure-MKT0037-03_web.pdf (accessed on 1 July 2021).
- Kim, H.; Cha, J.; Jang, M.; Kim, P. Hyaluronic acid-based extracellular matrix triggers spontaneous M2-like polarity of monocyte/macrophage. Biomater. Sci. 2019, 7, 2264–2271. [Google Scholar] [CrossRef]
- Imber, J.-C.; Kasaj, A. Treatment of Gingival Recession: When and How? Int. Dent. J. 2020, 71, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, R.; Testarelli, L.; Stefanelli, L.; De Angelis, F.; Mencio, F.; Pompa, G.; Carlo, S.D. Bone Healing in Extraction Sockets Covered with Collagen Membrane Alone or Associated with Porcine-Derived Bone Graft: A Comparative Histological and Histomorphometric Analysis. J. Oral Maxillofac. Res. 2017, 8, e4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barbu, H.M.; Iancu, S.A.; Mirea, I.J.; Mignogna, M.D.; Samet, N.; Calvo-Guirado, J.L. Management of Schneiderian Membrane Perforations during Sinus Augmentation Procedures: A Preliminary Comparison of Two Different Approaches. J. Clin. Med. 2019, 8, 1491. [Google Scholar] [CrossRef] [PubMed]
- Brochhausen, C.; Schmitt, V.H.; Planck, C.N.E.; Rajab, T.K.; Hollemann, D.; Tapprich, C.; Krämer, B.; Wallwiener, C.W.; Hierlemann, H.; Zehbe, R.; et al. Current Strategies and Future Perspectives for Intraperitoneal Adhesion Prevention. J. Gastrointest. Surg. 2012, 16, 1256–1274. [Google Scholar] [CrossRef] [PubMed]



| Coll-HA Membrane | Jason® Membrane | Ossix® Plus Membrane | BioGide® Membrane | |||||
|---|---|---|---|---|---|---|---|---|
| µm | % | µm | % | µm | % | µm | % | |
| 10 days | 42.7 ± 27.6 | 26.2 ± 18.6 | 26.0 ± 21.9 | 18.5 ± 11.9 | 0 | 0 | 13.6 ± 8.3 | 8.0 ± 4.8 |
| 30 days | 92.4 ± 48.9 | 50.8 ± 28.1 | 38.2 ± 25.5 | 25.2 ± 17.9 | 11.9 ± 20.8 | 5.8 ± 6.6 | 25.3 ± 17.8 | 18.4 ± 12.9 |
| 60 days | 138.5 ± 130.2 | 58.26 ± 38.2 | 68.2 ± 54.3 | 42.6 ± 24.8 | 26.5 ± 36.5 | 8.9 ± 12.3 | 60.2 ± 42.1 | 41.6 ± 24.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ottenbacher, N.; Alkildani, S.; Korzinskas, T.; Pissarek, J.; Ulm, C.; Jung, O.; Sundag, B.; Bellmann, O.; Stojanovic, S.; Najman, S.; et al. Novel Histomorphometrical Approach to Evaluate the Integration Pattern and Functionality of Barrier Membranes. Dent. J. 2021, 9, 127. https://doi.org/10.3390/dj9110127
Ottenbacher N, Alkildani S, Korzinskas T, Pissarek J, Ulm C, Jung O, Sundag B, Bellmann O, Stojanovic S, Najman S, et al. Novel Histomorphometrical Approach to Evaluate the Integration Pattern and Functionality of Barrier Membranes. Dentistry Journal. 2021; 9(11):127. https://doi.org/10.3390/dj9110127
Chicago/Turabian StyleOttenbacher, Nicola, Said Alkildani, Tadas Korzinskas, Jens Pissarek, Christian Ulm, Ole Jung, Bernd Sundag, Olaf Bellmann, Sanja Stojanovic, Stevo Najman, and et al. 2021. "Novel Histomorphometrical Approach to Evaluate the Integration Pattern and Functionality of Barrier Membranes" Dentistry Journal 9, no. 11: 127. https://doi.org/10.3390/dj9110127
APA StyleOttenbacher, N., Alkildani, S., Korzinskas, T., Pissarek, J., Ulm, C., Jung, O., Sundag, B., Bellmann, O., Stojanovic, S., Najman, S., Zechner, W., & Barbeck, M. (2021). Novel Histomorphometrical Approach to Evaluate the Integration Pattern and Functionality of Barrier Membranes. Dentistry Journal, 9(11), 127. https://doi.org/10.3390/dj9110127











