Sol–Gel Synthesis and Characterization of YSZ Nanofillers for Dental Cements at Different Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Nanoparticles
2.2. Differential Thermal and Thermogravimetric Analysis (TG-DSC)
2.3. Fourier Transform Infrared Analysis (FTIR)
2.4. X-Ray Diffraction Analysis (XRD)
2.5. Scanning Electron Microscopy-Energy-Dispersive X-ray Spectroscopy (SEM/EDX)
2.6. Transmission Electron Microscopy (TEM)
2.7. Dynamic Light Scattering Analysis (DLS)
2.8. Establishment of Primary Cell Cultures
2.9. Evaluation of Cell Viability by the MTT Assay
2.10. Fluorescence Analysis for the Detection of ROS Levels
2.11. Statistical Analysis
3. Results
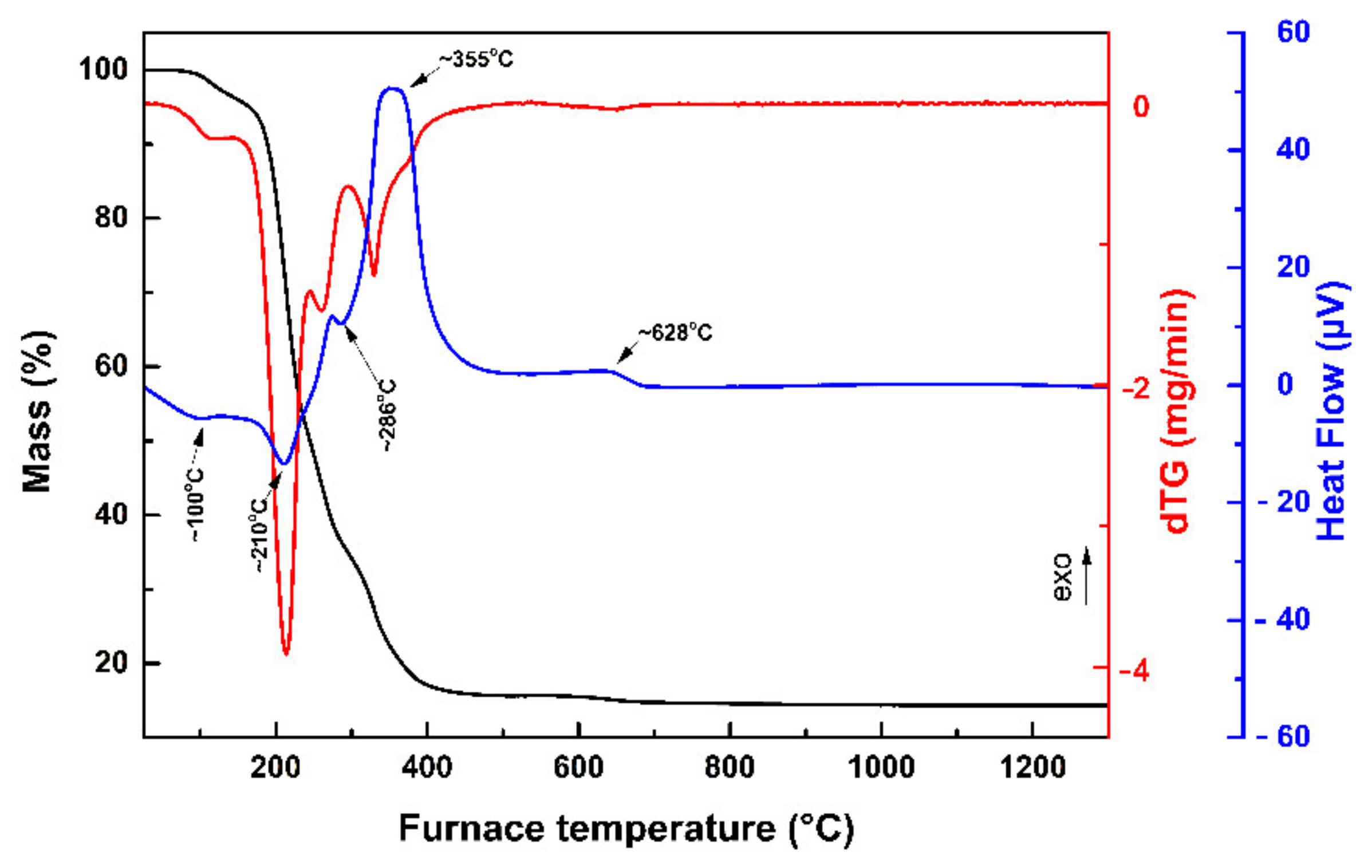
3.1. TG-DSC Analysis
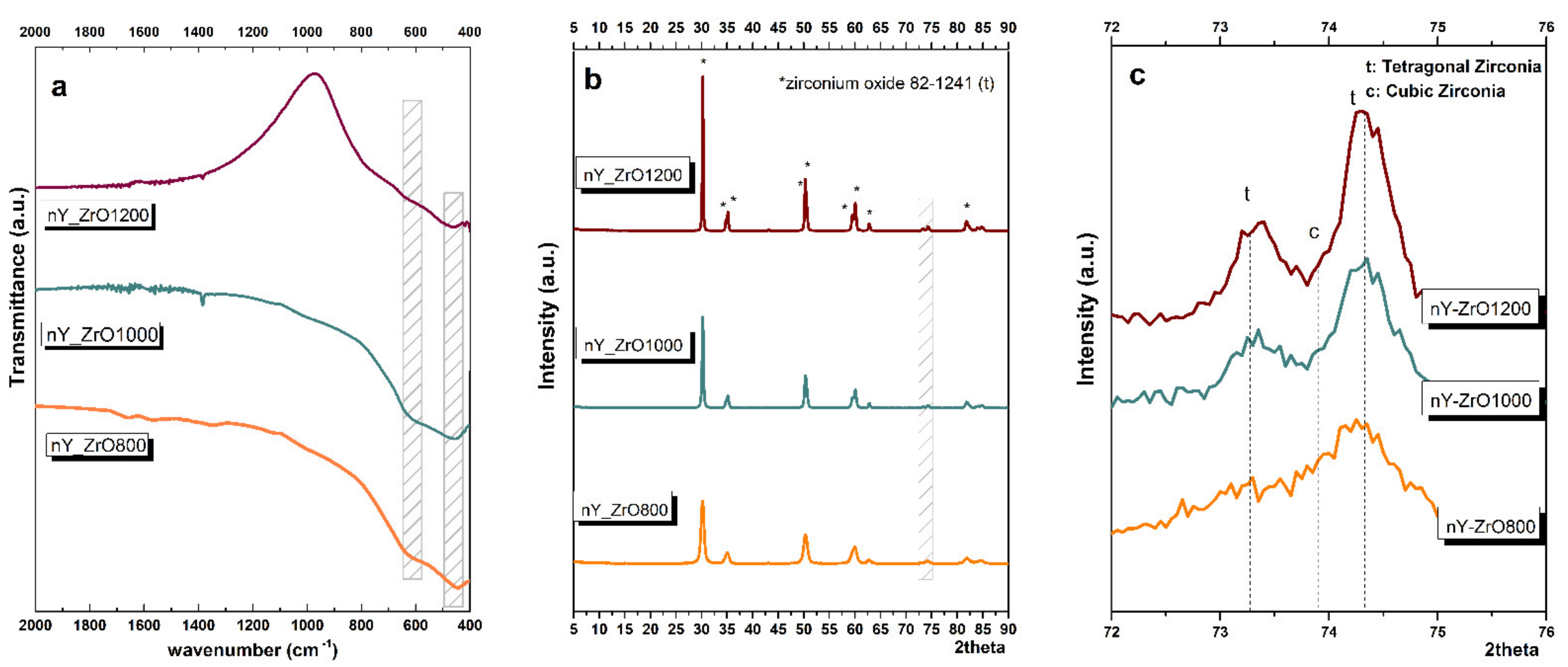
3.2. FTIR Analysis
3.3. XRD Analysis
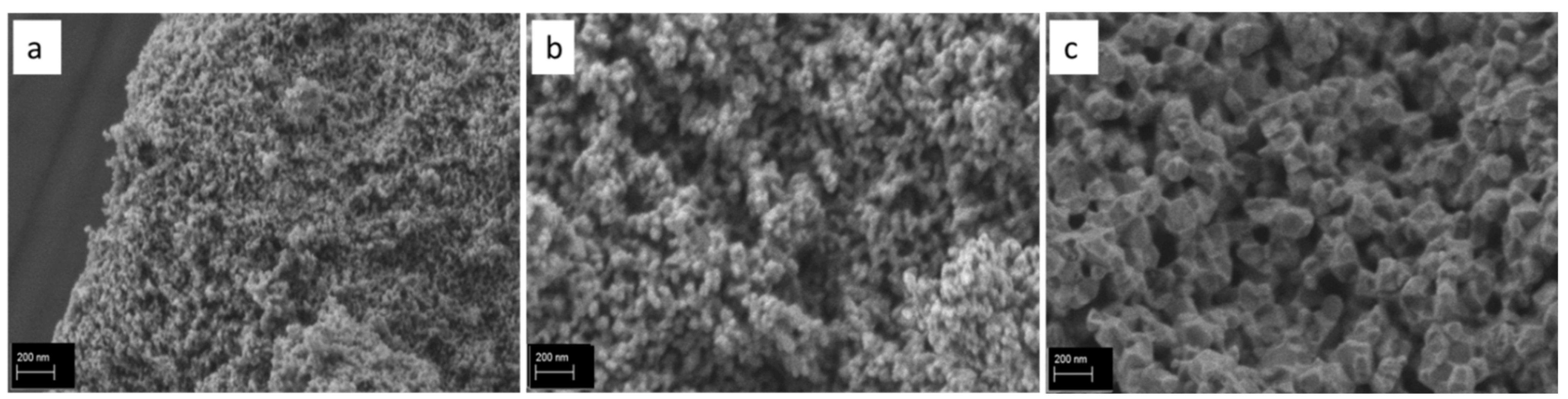
3.4. SEM/EDX
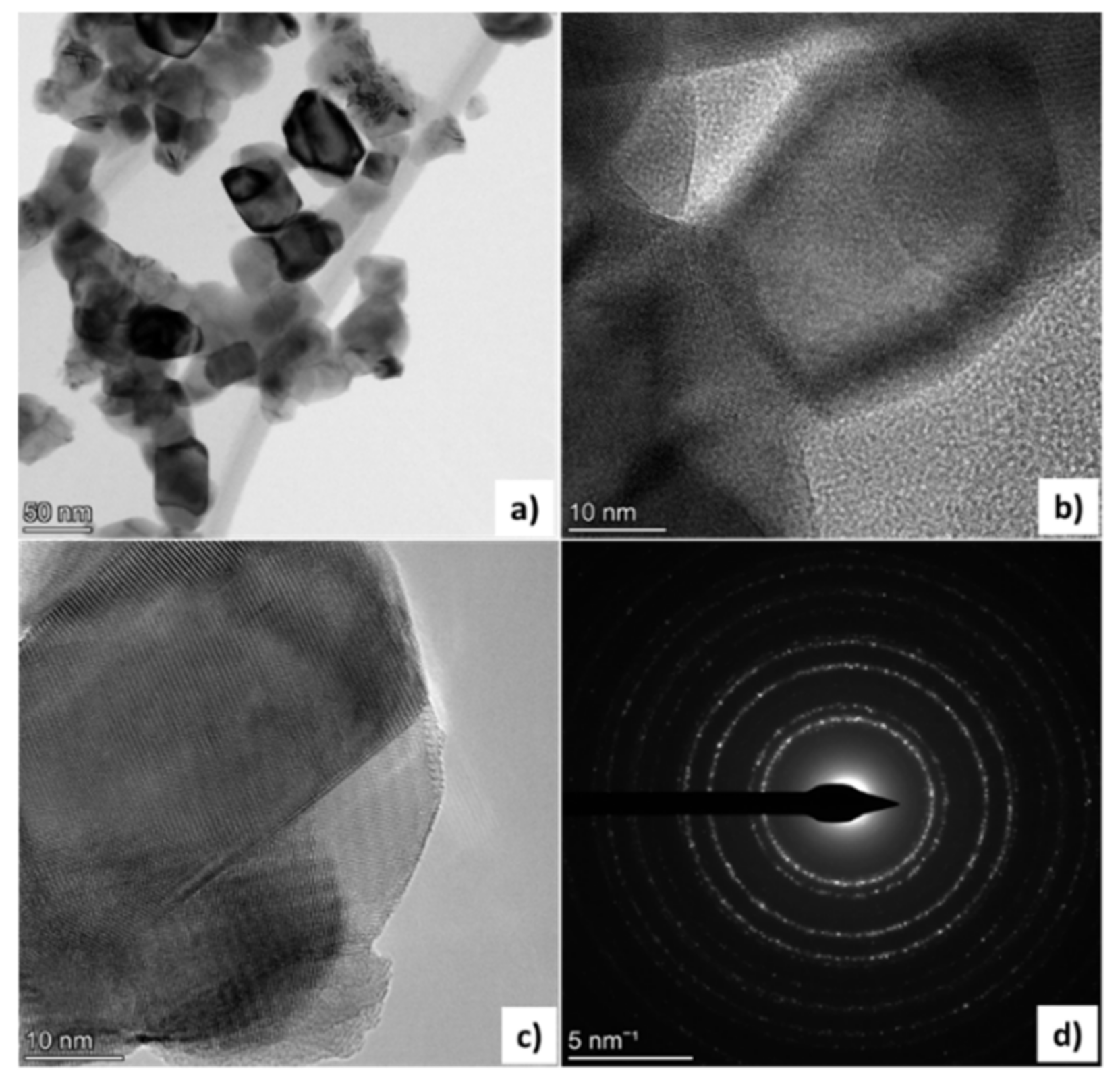
3.5. TEM
3.6. DLS
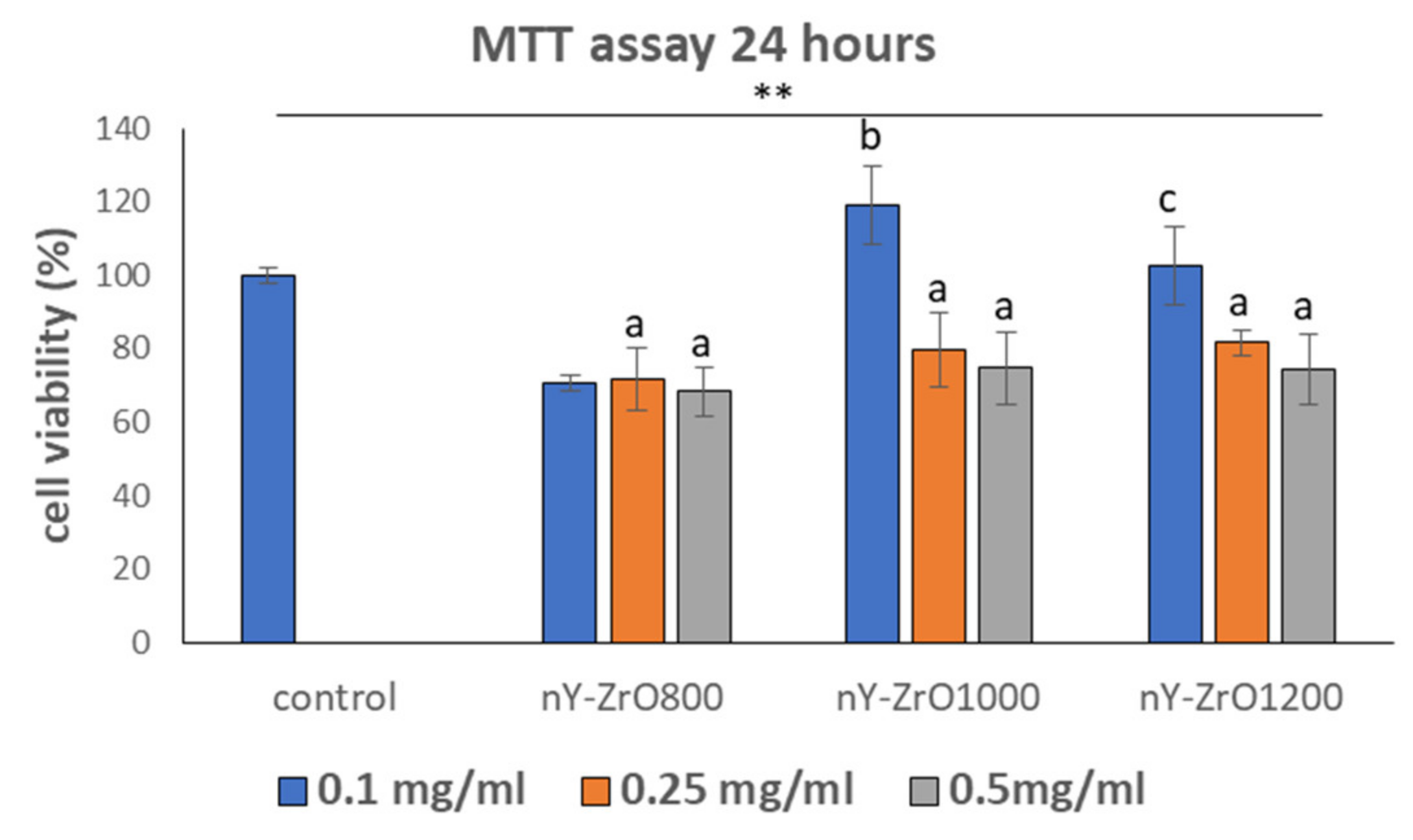
3.7. Evaluation of Cell Viability
3.8. Fluorescence Analysis for the Detection of Reactive Oxygen Species Levels
4. Discussion
5. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gautam, C.; Joyner, J.; Gautam, A.; Rao, J.; Vajtai, R. Zirconia based dental ceramics: Structure, mechanical properties, biocompatibility and applications. Dalt. Trans. 2016, 45, 19194–19215. [Google Scholar] [CrossRef] [PubMed]
- Vagkopoulou, T.; Koutayas, S.O.; Koidis, P.; Strub, J.R. Zirconia in dentistry: Part 1. Discovering the nature of an upcoming bioceramic. Eur. J. Esthet. Dent. 2009, 4, 130–151. [Google Scholar] [PubMed]
- Koutayas, S.O.; Vagkopoulou, T.; Pelekanos, S.; Koidis, P.; Strub, J.R. Zirconia in dentistry: Part 2. Evidence-based clinical breakthrough. Eur. J. Esthet. Dent. 2009, 4, 348–380. [Google Scholar] [PubMed]
- Habib, E.; Wang, R.; Wang, Y.; Zhu, M.; Zhu, X.X. Inorganic Fillers for Dental Resin Composites: Present and Future. ACS Biomater. Sci. Eng. 2016, 2, 1–11. [Google Scholar] [CrossRef]
- Wang, T.; Tsoi, J.K.H.; Matinlinna, J.P. A novel zirconia fibre-reinforced resin composite for dental use. J. Mech. Behav. Biomed. Mater. 2016, 53, 151–160. [Google Scholar] [CrossRef]
- Das, I.; Chattopadhyay, S.; Mahato, A.; Kundu, B.; De, G. Fabrication of a cubic zirconia nanocoating on a titanium dental implant with excellent adhesion, hardness and biocompatibility. RSC Adv. 2016, 6, 59030–59038. [Google Scholar] [CrossRef]
- Hu, C.; Sun, J.; Long, C.; Wu, L.; Zhou, C.; Zhang, X. Synthesis of nano zirconium oxide and its application in dentistry. Nanotechnol. Rev. 2019, 8, 396–404. [Google Scholar] [CrossRef]
- Guerreiro Tanomaru, J.M.; Storto, I.; da Silva, G.F.; Bosso, R.; Costa, B.C.; Bernardi, M.I.B.; Tanomaru-Filho, M. Radiopacity, pH and antimicrobial activity of Portland cement associated with micro- and nanoparticles of zirconium oxide and niobium oxide. Dent. Mater. J. 2014, 33, 466–470. [Google Scholar] [CrossRef]
- Bortoluzzi, E.A.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M.; Duarte, M.A.H. Radiographic effect of different radiopacifiers on a potential retrograde filling material. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2009, 108, 628–632. [Google Scholar] [CrossRef]
- Gad, M.M.; Rahoma, A.; Al-Thobity, A.M.; ArRejaie, A.S. Influence of incorporation of ZrO2 nanoparticles on the repair strength of polymethyl methacrylate denture bases. Int. J. Nanomed. 2016, 11, 5633–5643. [Google Scholar] [CrossRef]
- Yang, J.; Shen, J.; Wu, X.; He, F.; Xie, H.; Chen, C. Effects of nano-zirconia fillers conditioned with phosphate ester monomers on the conversion and mechanical properties of Bis-GMA- and UDMA-based resin composites. J. Dent. 2020, 94, 103306. [Google Scholar] [CrossRef]
- Fathima, J.B.; Pugazhendhi, A.; Venis, R. Synthesis and characterization of ZrO2 nanoparticles-antimicrobial activity and their prospective role in dental care. Microb. Pathog. 2017, 110, 245–251. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Silva, J.B.; Aladim, A.; Carvalho, O.; Nascimento, R.M.; Silva, F.S.; Martinelli, A.E.; Henriques, B. Effect of Zirconia and Alumina Fillers on the Microstructure and Mechanical Strength of Dental Glass Ionomer Cements. Open Dent. J. 2016, 10, 58–68. [Google Scholar] [CrossRef]
- Lyon, D.; Chevalier, J.; Gremillard, L.; Cam, C.a.D. Zirconia as a Biomaterial. Compr. Biomater. 2011, 20, 95–108. [Google Scholar] [CrossRef]
- Chevalier, J. What future for zirconia as a biomaterial? Biomaterials 2006, 27, 535–543. [Google Scholar] [CrossRef]
- Chevalier, J.J.; Gremillard, L.; Virkar, A.V.; Clarke, D.R. The tetragonal-monoclinic transformation in zirconia: Lessons learned and future trends. J. Am. Ceram. Soc. 2009, 92, 1901–1920. [Google Scholar] [CrossRef]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L.; Deville, S. Low-Temperature Degradation of Zirconia and Implications for Biomedical Implants. Annu. Rev. Mater. Res. 2007, 37, 1–32. [Google Scholar] [CrossRef]
- Sheu, T.-S.; Tien, T.-Y.; Chen, I.-W. Cubic-to-Tetragonal (t’) Transformation in Zirconia-Containing Systems. J. Am. Ceram. Soc. 1992, 75, 1108–1116. [Google Scholar] [CrossRef]
- Bona, A.D.; Pecho, O.E.; Alessandretti, R. Zirconia as a Dental Biomaterial. Materials 2015, 8, 4978–4991. [Google Scholar] [CrossRef]
- Kontonasaki, E.; Giasimakopoulos, P.; Rigos, A.E. Strength and aging resistance of monolithic zirconia: An update to current knowledge. Jpn. Dent. Sci. Rev. 2020, 56, 1–23. [Google Scholar] [CrossRef]
- Vasylkiv, O.; Sakka, Y. Synthesis and colloidal processing of zirconia nanopowder. J. Am. Ceram. Soc. 2001, 84, 2489–2494. [Google Scholar] [CrossRef]
- Tok, A.I.Y.; Boey, F.Y.C.; Du, S.W.; Wong, B.K. Flame spray synthesis of ZrO2 nano-particles using liquid precursors. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2006, 130, 114–119. [Google Scholar] [CrossRef]
- Hajizadeh-Oghaz, M.; Razavi, R.S.; Estarki, M.L. Large-scale synthesis of YSZ nanopowder by Pechini method. Bull. Mater. Sci. 2014, 37, 969–973. [Google Scholar] [CrossRef]
- Sagadevan, S.; Podder, J.; Das, I. Hydrothermal synthesis of zirconium oxide nanoparticles and its characterization. J. Mater. Sci. Mater. Electron. 2016, 27, 5622–5627. [Google Scholar] [CrossRef]
- Heshmatpour, F.; Khodaiy, Z.; Aghakhanpour, R.B. Synthesis and characterization of pure tetragonal nanocrystalline sulfated 8YSZ powder by sol-gel route. Powder Technol. 2012, 224, 12–18. [Google Scholar] [CrossRef]
- Hsu, Y.W.; Yang, K.H.; Chang, K.M.; Yeh, S.W.; Wang, M.C. Synthesis and crystallization behavior of 3 mol% yttria stabilized tetragonal zirconia polycrystals (3Y-TZP) nanosized powders prepared using a simple co-precipitation process. J. Alloys Compd. 2011, 509, 6864–6870. [Google Scholar] [CrossRef]
- Sato, K.; Horiguchi, K.; Nishikawa, T.; Yagishita, S.; Kuruma, K.; Murakami, T.; Abe, H. Hydrothermal Synthesis of Yttria-Stabilized Zirconia Nanocrystals with Controlled Yttria Content. Inorg. Chem. 2015, 54, 7976–7984. [Google Scholar] [CrossRef]
- Gonzalo-Juan, I.; Ferrari, B.; Colomer, M.T. Influence of the urea content on the YSZ hydrothermal synthesis under dilute conditions and its role as dispersant agent in the post-reaction medium. J. Eur. Ceram. Soc. 2009, 29, 3185–3195. [Google Scholar] [CrossRef]
- Dai, S.; Chen, Y.; Yang, J.; He, F.; Chen, C.; Xie, H. Surface treatment of nanozirconia fillers to strengthen dental bisphenol a–glycidyl methacrylate–based resin composites. Int. J. Nanomed. 2019, 14, 9185–9197. [Google Scholar] [CrossRef]
- Alhareb, A.O.; Akil, H.M.; Ahmad, Z.A. Impact strength, fracture toughness and hardness improvement of PMMA denture base through addition of nitrile rubber/ceramic fillers. Saudi. J. Dent. Res. 2017, 8, 26–34. [Google Scholar] [CrossRef]
- Lohbauer, U.; Wagner, A.; Belli, R.; Stoetzel, C.; Hilpert, A.; Kurland, H.D.; Grabow, J.; Müller, F.A. Zirconia nanoparticles prepared by laser vaporization as fillers for dental adhesives. Acta Biomater. 2010, 6, 4539–4546. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh-Oghaz, M.; Shoja Razavi, R.; Loghman-Estarki, M.R. Synthesis and characterization of non-transformable tetragonal YSZ nanopowder by means of Pechini method for thermal barrier coatings (TBCs) applications. J. Sol.-Gel Sci. Technol. 2014, 70, 6–13. [Google Scholar] [CrossRef]
- Tailor, S.; Singh, M.; Doub, A.V. Synthesis and Characterization of Yttria-Stabilized Zirconia (YSZ) Nano-Clusters for Thermal Barrier Coatings (TBCs) Applications. J. Clust. Sci. 2016, 27, 1097–1107. [Google Scholar] [CrossRef]
- Aly, K.A.; Khalil, N.M.; Algamal, Y.; Saleem, Q.M.A. Estimation of lattice strain for zirconia nano-particles based on Williamson- Hall analysis. Mater. Chem. Phys. 2017, 193, 182–188. [Google Scholar] [CrossRef]
- Wu, X.; Landheer, D.; Graham, M.J.; Chen, H.W.; Huang, T.Y.; Chao, T.S. Structure and thermal stability of MOCVD ZrO2 films on Si (1 0 0). J. Cryst. Growth 2003, 250, 479–485. [Google Scholar] [CrossRef]
- Karunakaran, G.; Suriyaprabha, R.; Manivasakan, P.; Yuvakkumar, R.; Rajendran, V.; Kannan, N. Screening of in vitro cytotoxicity, antioxidant potential and bioactivity of nano- and micro-ZrO2 and -TiO2 particles. Ecotoxicol. Environ. Saf. 2013, 93, 191–197. [Google Scholar] [CrossRef]
- Asadpour, E.; Sadeghnia, H.R.; Ghorbani, A.; Sedaghat, M.; Boroushaki, M.T. Oxidative stress-mediated cytotoxicity of zirconia nanoparticles on PC12 and N2a cells. J. Nanoparticle Res. 2016, 18, 1–13. [Google Scholar] [CrossRef]
- Avalos, A.; Haza, A.I.; Mateo, D.; Morales, P. Interactions of manufactured silver nanoparticles of different sizes with normal human dermal fibroblasts. Int. Wound J. 2016, 13, 101–109. [Google Scholar] [CrossRef]
- Tsamesidis, I.; Reybier, K.; Marchetti, G.; Pau, M.C.; Virdis, P.; Fozza, C.; Nepveu, F.; Low, P.S.; Turrini, F.M.; Pantaleo, A. Syk kinase inhibitors synergize with artemisinins by enhancing oxidative stress in plasmodium falciparum-parasitized erythrocytes. Antioxidants 2020, 9, 753. [Google Scholar] [CrossRef]
- Halmenschlager, C.M.; Vieira, R.; Falcade, T. Characterization of Cubic Yttria-Stabilized. In Proceedings of the International Latin-American Conference on Powder Technology, Atibaia, Brazil, 8–10 November 2009; pp. 890–895. [Google Scholar]
- Courtin, E.; Boy, P.; Rouhet, C.; Bianchi, L.; Bruneton, E.; Poirot, N.; Laberty-Robert, C.; Sanchez, C. Optimized Sol-Gel Routes to Synthesize Yttria- Stabilized Zirconia Thin Films as Solid Electrolytes for Solid Oxide Fuel Cells. Chem. Mater. 2012, 24, 4540–4548. [Google Scholar] [CrossRef]
- Judes, J.; Kamaraj, V. Preparation and characterization of yttria stabilized zirconia minispheres by the sol-gel drop generation method. Mater. Sci. Pol. 2009, 27, 407–415. [Google Scholar]
- Zarkov, A.; Stanulis, A.; Sakaliuniene, J.; Butkute, S.; Abakeviciene, B.; Salkus, T.; Tautkus, S.; Orliukas, A.F.; Tamulevicius, S.; Kareiva, A. On the synthesis of yttria-stabilized zirconia: A comparative study. J. Sol.-Gel Sci. Technol. 2015, 76, 309–319. [Google Scholar] [CrossRef]
- Huang, Z.; Han, W.; Feng, Z.; Qi, J.; Wu, D.; Wei, N.; Tang, Z.; Zhang, Y.; Duan, J.; Lu, T. The effects of precipitants on co-precipitation synthesis of yttria-stabilized zirconia nanocrystalline powders. J. Sol-Gel Sci. Technol. 2019, 90, 359–368. [Google Scholar] [CrossRef]
- Fernandez Lopez, E.; Sanchez Escribano, V.; Panizza, M.; Carnasciali, M.M.; Busca, G. Vibrational and electronic spectroscopic properties of zirconia powders. J. Mater. Chem. 2001, 11, 1891–1897. [Google Scholar] [CrossRef]
- Niu, X.; Xie, M.; Zhou, F.; Mu, R.; Song, X.; An, S. Substituent influence of yttria by gadolinia on the tetragonal phase stability for Y2O3-Ta2O5-ZrO2 ceramics at 1300 °C. J. Mater. Sci. Technol. 2014, 30, 381–386. [Google Scholar] [CrossRef]
- Srinivasan, R.; De Angelis, R.J.; Ice, G.; Davis, B.H. Identification of tetragonal and cubic structures of zirconia using synchrotron x-radiation source. J. Mater. Res. 1991, 6, 1287–1292. [Google Scholar] [CrossRef]
- Zhang, F.; Reveron, H.; Spies, B.C.; Van Meerbeek, B.; Chevalier, J. Trade-off between fracture resistance and translucency of zirconia and lithium-disilicate glass ceramics for monolithic restorations. Acta Biomater. 2019, 91, 24–34. [Google Scholar] [CrossRef]
- Kumari, L.; Li, W.Z.; Xu, J.M.; Leblanc, R.M.; Wang, D.Z.; Li, Y.; Guo, H.; Zhang, J. Controlled hydrothermal synthesis of zirconium oxide nanostructures and their optical properties. Cryst. Growth Des. 2009, 9, 3874–3880. [Google Scholar] [CrossRef]
- Zong, S.; Cao, Y.; Zhou, Y.; Ju, H. Zirconia nanoparticles enhanced grafted collagen tri-helix scaffold for unmediated biosensing of hydrogen peroxide. Langmuir 2006, 22, 8915–8919. [Google Scholar] [CrossRef]
- Pérez-Tanoira, R.; Horwat, D.; Kinnari, T.J.; Pérez-Jorge, C.; Gómez-Barrena, E.; Migot, S.; Esteban, J. Bacterial adhesion on biomedical surfaces covered by yttria stabilized zirconia. J. Mater. Sci. Mater. Med. 2016, 27, 1–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lahwal, A.; Bhattacharya, S.; He, J.; Wu, D.; Peterson, A.; Poon, S.J.; Williams, L.; Dehkordi, A.M.; Tritt, T.M. Impact of yttria stabilized zirconia nanoinclusions on the thermal conductivity of n-type Si80 Ge20 alloys prepared by spark plasma sintering. J. Appl. Phys. 2015, 117, 145101. [Google Scholar] [CrossRef]
- Maridurai, T.; Balaji, D.; Sagadevan, S. Synthesis and Characterization of Yttrium Stabilized Zirconia Nanoparticles. Mater. Res. 2016, 19, 812–816. [Google Scholar] [CrossRef]
- Bahamirian, M.; Hadavi, S.M.M.; Rahimipour, M.R.; Farvizi, M.; Keyvani, A. Synthesis and Characterization of Yttria-Stabilized Zirconia Nanoparticles Doped with Ytterbium and Gadolinium: ZrO2 9.5Y2O3 5.6Yb2O3 5.2Gd2O3. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2018, 49, 2523–2532. [Google Scholar] [CrossRef]
- Khajavi, P.; Xu, Y.; Frandsen, H.L.; Chevalier, J.; Gremillard, L.; Kiebach, R.; Hendriksen, P.V. Tetragonal phase stability maps of ceria-yttria co-doped zirconia: From powders to sintered ceramics. Ceram. Int. 2020, 46, 9396–9405. [Google Scholar] [CrossRef]
- Garvie, R.C. The occurrence of metastable tetragonal zirconia as a crystallite size effect. J. Phys. Chem. 1965, 69, 1238–1243. [Google Scholar] [CrossRef]
- Chraska, T.; King, A.H.; Berndt, C.C. On the size-dependent phase transformation in nanoparticulate zirconia. Mater. Sci. Eng. A 2000, 286, 169–178. [Google Scholar] [CrossRef]
- Nitsche, R.; Winterer, M.; Hahn, H. Structure of nanocrystalline zirvonia and yttria. Nanostructured Mater. 1995, 6, 679–682. [Google Scholar] [CrossRef]
- Shukla, S.; Seal, S. Mechanisms of room temperature metastable tetragonal phase stabilisation in zirconia. Int. Mater. Rev. 2005, 50, 45–64. [Google Scholar] [CrossRef]
- Tsunekawa, S.; Ito, S.; Kawazoe, Y.; Wang, J.T. Critical size of the phase transition from cubic to tetragonal in pure zirconia nanoparticles. Nano Lett. 2003, 3, 871–875. [Google Scholar] [CrossRef]
- Chatterjee, A.; Pradhan, S.K.; Datta, A.; De, M.; Chakravorty, D. Stability of Cubic Phase in Nanocrystalline ZrO2. J. Mater. Res. 1994, 9, 263–265. [Google Scholar] [CrossRef]
- Lu, K. Sintering of nanoceramics. Int. Mater. Rev. 2008, 53, 21–38. [Google Scholar] [CrossRef]
- Maca, K.; Trunec, M.; Dobsak, P. Bulk zirconia nanoceramics prepared by cold isostatic pressing and pressureless sintering. Rev. Adv. Mater. Sci. 2005, 10, 84–88. [Google Scholar]
- Stolzenburg, P.; Hämisch, B.; Richter, S.; Huber, K.; Garnweitner, G. Secondary Particle Formation during the Nonaqueous Synthesis of Metal Oxide Nanocrystals. Langmuir 2018, 34, 12834–12844. [Google Scholar] [CrossRef]
- Tobler, D.J.; Shaw, S.; Benning, L.G. Quantification of initial steps of nucleation and growth of silica nanoparticles: An in-situ SAXS and DLS study. Geochim. Cosmochim. Acta 2009, 73, 5377–5393. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection limits of DLS and UV-Vis spectroscopy in characterization of polydisperse nanoparticles colloids. J. Nanomater. 2013, 2013, 60. [Google Scholar] [CrossRef]
- Pabisch, S.; Feichtenschlager, B.; Kickelbick, G.; Peterlik, H. Effect of interparticle interactions on size determination of zirconia and silica based systems—A comparison of SAXS, DLS, BET, XRD and TEM. Chem. Phys. Lett. 2012, 521, 91–97. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-dependent cytotoxicity of gold nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Zheng, Y.F.; Qin, L. A comprehensive biological evaluation of ceramic nanoparticles as wear debris. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 975–982. [Google Scholar] [CrossRef]
- Wang, J.; Yin, W.; He, X.; Wang, Q.; Guo, M.; Chen, S. Good Biocompatibility and Sintering Properties of Zirconia Nanoparticles Synthesized via Vapor-phase Hydrolysis. Sci. Rep. 2016, 6, 35020. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, D.; Exarhos, S.; Xu, C.; Niacaris, M.; Mariano, C.; Dayap, B.; Mangolini, L.; Liu, H. Synthesis, characterization, and cytocompatibility of yttria stabilized zirconia nanopowders for creating a window to the brain. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2020, 108, 925–938. [Google Scholar] [CrossRef] [PubMed]
- Pagano, S.; Lombardo, G.; Costanzi, E.; Balloni, S.; Bruscoli, S.; Flamini, S.; Coniglio, M.; Valenti, C.; Cianetti, S.; Marinucci, L. Morpho-functional effects of different universal dental adhesives on human gingival fibroblasts: An in vitro study. Odontology 2021, 109, 524–539. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.Y.; Oca-Cossio, J.; Lin, S.M.; Woan, K.; Yu, P.C.; Sigmund, W. Reactive oxygen species scavenging properties of ZrO2-CeO2 solid solution nanoparticles. Nanomedicine 2008, 3, 637–645. [Google Scholar] [CrossRef]
- Imran, M.; Riaz, S.; Shah, S.M.H.; Batool, T.; Khan, H.N.; Sabri, A.N.; Naseem, S. In-vitro hemolytic activity and free radical scavenging by sol-gel synthesized Fe3O4 stabilized ZrO2 nanoparticles. Arab. J. Chem. 2020, 13, 7598–7608. [Google Scholar] [CrossRef]
- Atalay, H.; Çelík, A.; Ayaz, F. Investigation of genotoxic and apoptotic effects of zirconium oxide nanoparticles (20 nm) on L929 mouse fibroblast cell line. Chem. Biol. Interact. 2018, 296, 98–104. [Google Scholar] [CrossRef]
- Gunawan, C.; Lord, M.S.; Lovell, E.; Wong, R.J.; Jung, M.S.; Oscar, D.; Mann, R.; Amal, R. Oxygen-Vacancy Engineering of Cerium-Oxide Nanoparticles for Antioxidant Activity. ACS Omega 2019, 4, 9473–9479. [Google Scholar] [CrossRef]
- Alzahrani, F.M.; Katubi, K.M.S.; Ali, D.; Alarifi, S. Apoptotic and DNA-damaging effects of yttriastabilized zirconia nanoparticles on human skin epithelial cells. Int. J. Nanomed. 2019, 14, 7003–7016. [Google Scholar] [CrossRef]
- Münchow, E.A.; Bottino, M.C. Recent Advances in Adhesive Bonding: The Role of Biomolecules, Nanocompounds, and Bonding Strategies in Enhancing Resin Bonding to Dental Substrates. Curr. Oral Heal. Rep. 2017, 4, 215–227. [Google Scholar] [CrossRef]
- Gjorgievska, E.; Nicholson, J.W.; Gabrić, D.; Guclu, Z.A.; Miletić, I.; Coleman, N.J. Assessment of the impact of the addition of nanoparticles on the properties of glass-ionomer cements. Materials 2020, 13, 276. [Google Scholar] [CrossRef]
- Sajjad, A.; Bakar, W.Z.W.; Mohamad, D.; Kannan, T.P. Characterization and enhancement of physico-mechanical properties of glass ionomer cement by incorporating a novel nano zirconia silica hydroxyapatite composite synthesized via sol-gel. AIMS Mater. Sci. 2019, 6, 730–747. [Google Scholar] [CrossRef]
- Alobiedy, A.N.; Alhille, A.H.; Al-Hamaoy, A.R. Mechanical Properties Enhancement of Conventional Glass Ionomer Cement by Adding Zirconium Oxide Micro and Nanoparticles. J. Eng. 2019, 25, 72–81. [Google Scholar] [CrossRef]
- Rahman, I.A.; Ghazali, N.A.M.; Bakar, W.Z.W.; Masudi, S.M. Modification of glass ionomer cement by incorporating nanozirconia-hydroxyapatite-silica nano-powder composite by the one-pot technique for hardness and aesthetics improvement. Ceram. Int. 2017, 43, 13247–13253. [Google Scholar] [CrossRef]
- Gjorgievska, E.; Van Tendeloo, G.; Nicholson, J.W.; Coleman, N.J.; Slipper, I.J.; Booth, S. The Incorporation of Nanoparticles into Conventional Glass-Ionomer Dental Restorative Cements. Microsc. Microanal. 2015, 21, 392–406. [Google Scholar] [CrossRef]
- Li, X.; Yoshihara, K.; De Munck, J.; Cokic, S.; Pongprueksa, P.; Putzeys, E.; Pedano, M.; Chen, Z.; Van Landuyt, K.; Van Meerbeek, B. Modified tricalcium silicate cement formulations with added zirconium oxide. Clin. Oral Investig. 2017, 21, 895–905. [Google Scholar] [CrossRef]
- Rahimi, S.; Salarinasab, S.; Ghasemi, N.; Rahbarghazi, R.; Shahi, S.; Salem Milani, A.; Divband, B.; Davoudi, P. In vitro induction of odontogenic activity of human dental pulp stem cells by white Portland cement enriched with zirconium oxide and zinc oxide components. J. Dent. Res. Dent. Clin. Dent. Prospect. 2019, 13, 3–10. [Google Scholar] [CrossRef]
- Viapiana, R.; Flumignan, D.L.; Guerreiro-Tanomaru, J.M.; Camilleri, J.; Tanomaru-Filho, M. Physicochemical and mechanical properties of zirconium oxide and niobium oxide modified Portland cement-based experimental endodontic sealers. Int. Endod. J. 2014, 47, 437–448. [Google Scholar] [CrossRef]
- Li, Q.; Deacon, A.D.; Coleman, N.J. The impact of zirconium oxide nanoparticles on the hydration chemistry and biocompatibility of white Portland cement. Dent. Mater. J. 2013, 32, 808–815. [Google Scholar] [CrossRef]
- Ilie, N.; Sarosi, C.; Rosu, M.C.; Moldovan, M. Synthesis and characterization of graphene oxide-zirconia (GO-ZrO2) and hydroxyapatite-zirconia (HA-ZrO2) nano-fillers for resin-based composites for load-bearing applications. J. Dent. 2021, 105, 103557. [Google Scholar] [CrossRef]
- Hesaraki, S.; Karimi, M.; Nezafati, N. The synergistic effects of SrF2 nanoparticles, YSZ nanoparticles, and poly-ε-L-lysin on physicomechanical, ion release, and antibacterial-cellular behavior of the flowable dental composites. Mater. Sci. Eng. C 2020, 109, 110592. [Google Scholar] [CrossRef]
- Wu, X.; Dai, S.; Chen, Y.; He, F.; Xie, H.; Chen, C. Reinforcement of dental resin composite via zirconium hydroxide coating and phosphate ester monomer conditioning of nano-zirconia fillers. J. Mech. Behav. Biomed. Mater. 2019, 94, 32–41. [Google Scholar] [CrossRef]
- Furman, B.; Ralph Rawls, H.; Wellinghoff, S.; Dixon, H.; Lankford, J.; Nicolella, D. Metal-oxide nanoparticles for the reinforcement of dental restorative resins. Crit. Rev. Biomed. Eng. 2000, 28, 439–443. [Google Scholar] [CrossRef]
- Provenzi, C.; Collares, F.M.; Cuppini, M.; Samuel, S.M.W.; Alves, A.K.; Bergmann, C.P.; Leitune, V.C.B. Effect of nanostructured zirconium dioxide incorporation in an experimental adhesive resin. Clin. Oral Investig. 2018, 22, 2209–2218. [Google Scholar] [CrossRef]
- Martins, G.C.; Meier, M.M.; Loguercio, A.D.; Cecchin, F.; Gomes, O.M.M.; Reis, A. Effects of zirconia nanoparticles addition to experimental adhesives on radiopacity and microhardness. Braz. J. Oral Sci. 2013, 12, 319–322. [Google Scholar] [CrossRef][Green Version]
- Kaizer, M.R.; Almeida, J.R.; Gonçalves, A.P.R.; Zhang, Y.; Cava, S.S.; Moraes, R.R. Silica coating of nonsilicate nanoparticles for resin-based composite materials. J. Dent. Res. 2016, 95, 1394–1400. [Google Scholar] [CrossRef]
- Nakanishi, L.; Kaizer, M.R.; Brandeburski, S.; Cava, S.S.; Della Bona, A.; Zhang, Y.; Moraes, R.R. Non-silicate nanoparticles for improved nanohybrid resin composites. Dent. Mater. 2020, 36, 1314–1321. [Google Scholar] [CrossRef]
- Hong, G.; Yang, J.; Jin, X.; Wu, T.; Dai, S.; Xie, H.; Chen, C. Mechanical Properties of Nanohybrid Resin Composites Containing Various Mass Fractions of Modified Zirconia Particles. Int. J. Nanomed. 2020, 15, 9891. [Google Scholar] [CrossRef]
- Laiteerapong, A.; Reichl, F.X.; Hickel, R.; Högg, C. Effect of eluates from zirconia-modified glass ionomer cements on DNA double-stranded breaks in human gingival fibroblast cells. Dent. Mater. 2019, 35, 444–449. [Google Scholar] [CrossRef]
- Silva, G.F.; Guerreiro-Tanomaru, J.M.; da Fonseca, T.S.; Bernardi, M.I.B.; Sasso-Cerri, E.; Tanomaru-Filho, M.; Cerri, P.S. Zirconium oxide and niobium oxide used as radiopacifiers in a calcium silicate-based material stimulate fibroblast proliferation and collagen formation. Int. Endod. J. 2017, 50, e95–e108. [Google Scholar] [CrossRef]
- Bosso-Martelo, R.; Guerreiro-Tanomaru, J.M.; Viapiana, R.; Berbert, F.L.C.V.; Basso Bernardi, M.I.; Tanomaru-Filho, M. Calcium Silicate-Based Cements Associated with Micro- and Nanoparticle Radiopacifiers: Physicochemical Properties and Bioactivity. Int. Sch. Res. Not. 2015, 2015, 1–7. [Google Scholar] [CrossRef]


| Specimen | t | c |
|---|---|---|
| nY-ZrO800 | 79.65% | 20.35% |
| nY-ZrO1000 | 95.13% | 4.87% |
| nY-ZrO1200 | 100.00% | 0% |
| Ring | Diam. cm | R cm | dspac A° | ICDD #82-1241 | St.Dev. | St.Dev % | hkl |
|---|---|---|---|---|---|---|---|
| Ring 1 | 6.5 | 3.250 | 2.892 | 2.963 | 0.0239 | 2.3924 | 101 |
| Ring 2 | 7.6 | 3.800 | 2.474 | 2.559 | 0.0332 | 3.3151 | 110 |
| Ring 3 | 10.3 | 5.150 | 1.825 | 1.818 | 0.0043 | 0.4260 | 112 |
| Ring 4 | 12.45 | 6.225 | 1.510 | 1.544 | 0.0221 | 2.2058 | 103 |
| Ring 5 | 13 | 6.500 | 1.446 | 1.482 | 0.0239 | 2.3924 | 202 |
| Ring 6 | 15.65 | 7.825 | 1.201 | 1.212 | 0.0085 | 0.8503 | 104 |
| Ring 7 | 17.35 | 8.675 | 1.084 | 1.144 | 0.0530 | 5.2986 | 310 |
| Specimen | Size (d.nm) | Standard Deviation | %Std Deviation | Pdl |
|---|---|---|---|---|
| nY-ZrO800 | 243.890 | 2.345 | 0.961 | 0.265 |
| nY-ZrO1000 | 188.956 | 5.831 | 3.086 | 0.277 |
| nY-ZrO1200 | 364.003 | 8.216 | 2.257 | 0.376 |
| Authors | Zirconia Nanoparticles Type/Size (nm) | Amount of Filler (%) w.t. | Evaluated Property | Main Results |
|---|---|---|---|---|
| Dental Cements | ||||
| Gjorgievska et al. [81] | ZrO2 (80 nm) * Glass Ionomer Cement | 2, 5, 10 | -compressive strength element release profile | Increased compressive strength, no release of Al, Zr or Ti |
| Sajjad et al. [82] | ZrO2 (114 nm) * Glass Ionomer Cement | 3, 5, 7, 9 | -compressive strength -flexural strength -surface roughness | Increase in compressive and flexural strength |
| Alobiedy et al. [83] | ZrO2 (20 nm) * Glass Ionomer Cement | 3, 5, 7 | -compressive strength -micro-hardness -biaxial flexural strength wear rate loss | Favorable effect on biaxial flexural strength, micro-hardness, wear rate loss |
| Ab Rahman et al. [84] | ZrO2 (40 nm) * Glass Ionomer Cement | 1, 3, 5, 7, 9, 15, 20 | -hardness color | Increased hardness and aesthetics |
| Gjorgievska et al. [85] | ZrO2 (80 nm) * Glass Ionomer Cement | 10 | -compressive strength | Increased compressive strength |
| Li et al. [86] | ZrO2 (200 nm) * Tricalcium Cement | 5, 10, 20, 30, 50 | -mini-fracture toughness -bioactivity -cytotoxicity | Increase in biocompatibility |
| Rahimi et al. [87] | ZrO2 (<100 nm) * Portland cement | 30 | -viability of human dental pulp cells | Increased alkaline phosphatase activity in human dental pulp cells |
| Viapiana et al. [88] | ZrO2 (nanosize is not reported) * Portland cement | 30 | -Setting time -compressive strength -flow -film thickness -radiopacity -solubility -dimensional stability -formaldehyde release | Film thickness requires further reduction |
| Li et al. [89] | ZrO2 (50–75 nm) * Portland cement | 20 | -hydration chemistry | Biocompatibility not compromised. Accelerated hydration |
| Dental Composites | ||||
| Ilie et al. [90] | GO-ZrO2 HA-ZrO2 (10–40 nm) | 0.3 GO-ZrO2 15 HA-ZrO2 | -light transmittance -flexural strength, modulus, Weibull parameters -plastic and elastic deformation parameters | -Improved mechanical properties -Optical properties require adjustments |
| Hesaraki et al. [91] | 3-YSZ (≤100 nm) | 5, 10 | -flexural strength compressive strength | -Increase in mechanical strength |
| Wu et al. [92] | ZrO2 coated with Zr (OH)4 (50 nm) | 10 | -flexural strength -elastic modulus -Weibull analysis | -Improvement of mechanical properties |
| Dai et al. [30] | ZrO2 coated with Zr (OH)4 (50 nm) | 2.5, 5, 7.5 | -flexural strength -translucency | -5% wt presented the highest strength |
| Furman et al. [93] | Zirconium propoxide (<100 nm) | 10, 20, 30 | -flexural strength -fracture toughness | -Reduced flexural strength |
| Dental Adhesives | ||||
| Provenzi et al. [94] | ZrO2 (<25 nm) | 0.5, 1, 4.8, 9.1 | -degree of conversion -radiopacity -tensile bond strength softening in solvent | 1 wt% led to a significantly higher degree of conversion |
| Martins et al. [95] | ZrO2 (20–30 nm) | 15, 25, 30, 50 | -micro-hardness radiopacity | Increased micro-hardness and radiopacity |
| Lohbauer et al. [32] | YSZ (20–50 nm) | 5, 10, 15, 20 | -micro-tensile -bond strength | Increasing concentration led to higher bond strength values |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beketova, A.; Theocharidou, A.; Tsamesidis, I.; Rigos, A.E.; Pouroutzidou, G.K.; Tzanakakis, E.-G.C.; Kourtidou, D.; Liverani, L.; Ospina, M.A.; Anastasiou, A.; et al. Sol–Gel Synthesis and Characterization of YSZ Nanofillers for Dental Cements at Different Temperatures. Dent. J. 2021, 9, 128. https://doi.org/10.3390/dj9110128
Beketova A, Theocharidou A, Tsamesidis I, Rigos AE, Pouroutzidou GK, Tzanakakis E-GC, Kourtidou D, Liverani L, Ospina MA, Anastasiou A, et al. Sol–Gel Synthesis and Characterization of YSZ Nanofillers for Dental Cements at Different Temperatures. Dentistry Journal. 2021; 9(11):128. https://doi.org/10.3390/dj9110128
Chicago/Turabian StyleBeketova, Anastasia, Anna Theocharidou, Ioannis Tsamesidis, Athanasios E. Rigos, Georgia K. Pouroutzidou, Emmanouil-George C. Tzanakakis, Dimitra Kourtidou, Liliana Liverani, Marcela Arango Ospina, Antonios Anastasiou, and et al. 2021. "Sol–Gel Synthesis and Characterization of YSZ Nanofillers for Dental Cements at Different Temperatures" Dentistry Journal 9, no. 11: 128. https://doi.org/10.3390/dj9110128
APA StyleBeketova, A., Theocharidou, A., Tsamesidis, I., Rigos, A. E., Pouroutzidou, G. K., Tzanakakis, E.-G. C., Kourtidou, D., Liverani, L., Ospina, M. A., Anastasiou, A., Tzoutzas, I. G., & Kontonasaki, E. (2021). Sol–Gel Synthesis and Characterization of YSZ Nanofillers for Dental Cements at Different Temperatures. Dentistry Journal, 9(11), 128. https://doi.org/10.3390/dj9110128









