Impact of Bone Augmentation of Facial Bone Defect around Osseointegrated Implant: A Three Dimensional Finite Element Analysis
Abstract
:1. Introduction
2. Materials and Methods
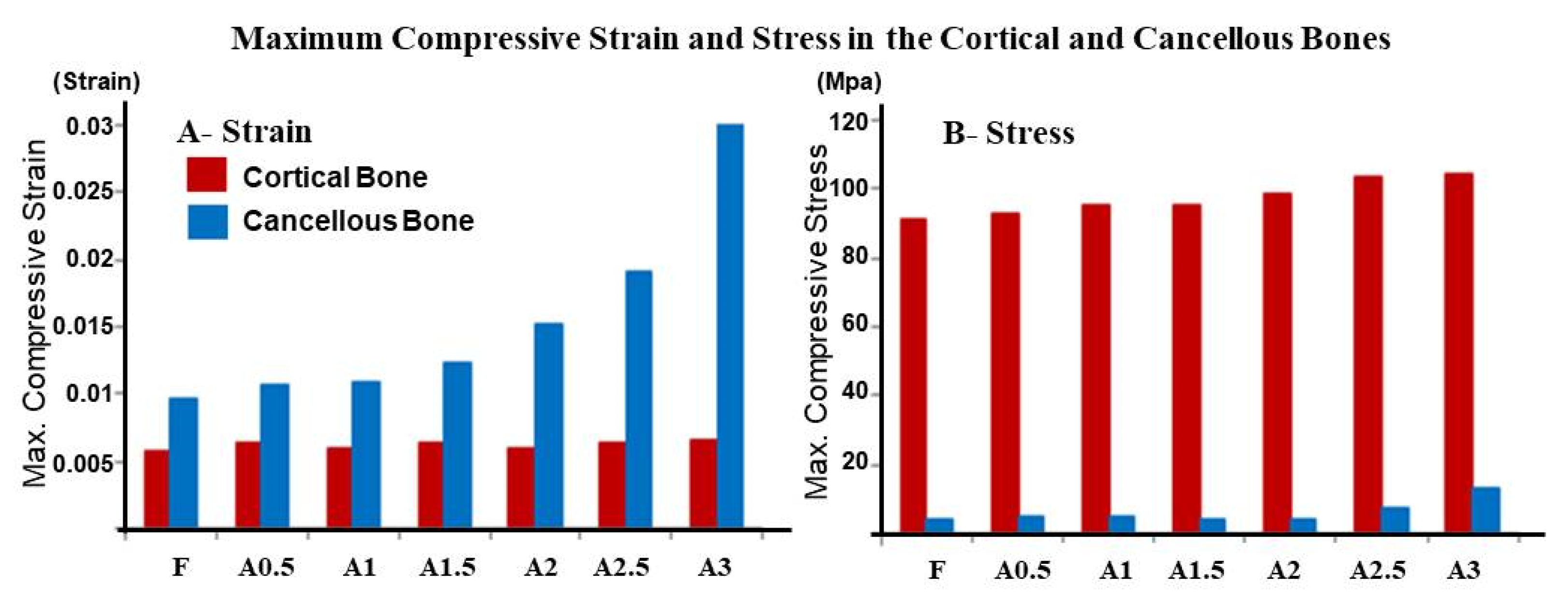
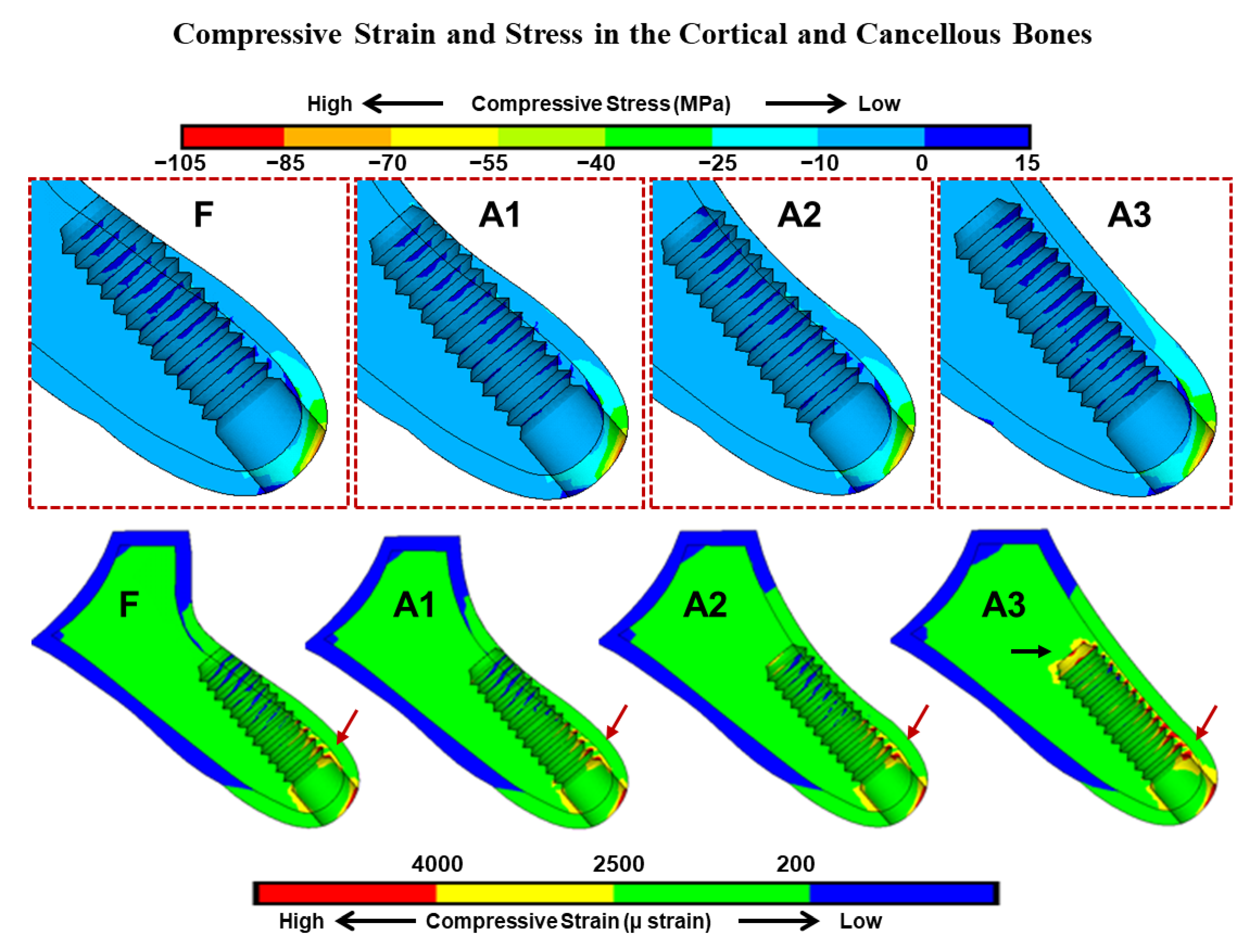
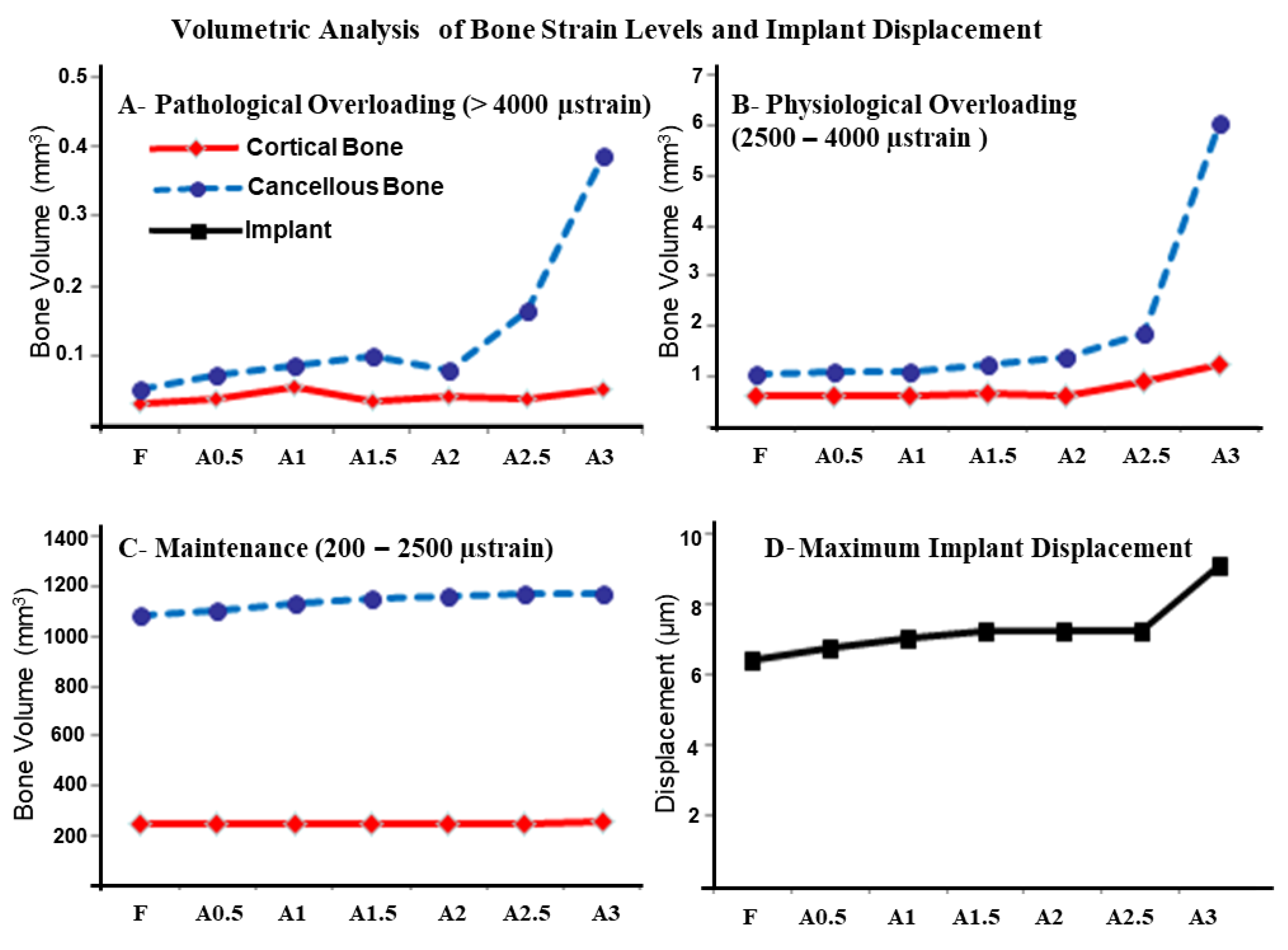
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gallucci, G.O.; Hamilton, A.; Zhou, W.; Buser, D.; Chen, S. Implant placement and loading protocols in partially edentulous patients: A systematic review. Clin. Oral Implant. Res. 2018, 29, 106–134. [Google Scholar] [CrossRef] [Green Version]
- Blanco, J.; Carral, C.; Argibay, O.; Liñares, A. Implant placement in fresh extraction sockets. Periodontol. 2000 2019, 79, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Polyzos, I.P.; Felice, P.; Worthington, H.V. Interventions for replacing missing teeth: Dental implants in fresh extraction sockets (immediate, immediate-delayed and delayed implants). Cochrane Database Syst. Rev. 2010, CD005968. [Google Scholar] [CrossRef] [PubMed]
- Weigl, P.; Strangio, A. The impact of immediately placed and restored single-tooth implants on hard and soft tissues in the anterior maxilla. Eur. J. Oral Implantol. 2016, 9 (Suppl. 1), S89–S106. [Google Scholar] [PubMed]
- Chen, S.T.; Buser, D. Esthetic Outcomes Following Immediate and Early Implant Placement in the Anterior Maxilla—A Systematic Review. Int. J. Oral Maxillofac. Implant. 2014, 29, 186–215. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-Y.; Pan, W.-L.; Wang, H.-L. Facial Fenestration and Dehiscence Defects Associated With Immediate Implant Placement Without Flap Elevation in Anterior Maxillary Ridge: A Preliminary Cone Beam Computed Tomography Study. Int. J. Oral Maxillofac. Implant. 2018, 33, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Skrypczak, A.; Weltman, R. Anterior maxilla alveolar ridge dimension and morphology measurement by cone beam computerized tomography (CBCT) for immediate implant treatment planning. BMC Oral Health 2015, 15, 65. [Google Scholar] [CrossRef] [Green Version]
- Chan, H.-L.; Garaicoa-Pazmino, C.; Suarez, F.; Monje, A.; Benavides, E.; Oh, T.-J.; Wang, H.-L. Incidence of implant buccal plate fenestration in the esthetic zone: A cone beam computed tomography study. Int. J. Oral Maxillofac. Implant. 2014, 29, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Aloy-Prósper, A.; Peñarrocha-Oltra, D.; Peñarrocha-Diago, M.; Peñarrocha-Diago, M. Dental implants with versus without peri-implant bone defects treated with guided bone regeneration. J. Clin. Exp. Dent. 2015, 7, e361–e368. [Google Scholar] [CrossRef]
- Holmes, D.C.; Loftus, J.T. Influence of bone quality on stress distribution for endosseous implants. J. Oral Implantol. 1997, 23, 104–111. [Google Scholar]
- Hudieb, M.; Wakabayashi, N.; Suzuki, T.; Kasugai, S. Morphologic classification and stress analysis of the mandibular bone in the premolar region for implant placement. Int. J. Oral Maxillofac. Implant. 2010, 25, 482–490. [Google Scholar]
- Duyck, J.; Naert, I.; Ronold, H.J.; Ellingsen, J.E.; Van Oosterwyck, H.; Vander Sloten, J. The influence of static and dynamic loading on marginal bone reactions around osseointegrated implants: An animal experimental study. Clin. Oral. Implant. Res. 2001, 12, 207–218. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, T.-J.; Misch, C.E.; Wang, H.-L. Occlusal considerations in implant therapy: Clinical guidelines with biomechanical rationale. Clin. Oral Implant. Res. 2004, 16, 26–35. [Google Scholar] [CrossRef]
- Geng, J.-P.; Tan, K.B.C.; Liu, G.-R. Application of finite element analysis in implant dentistry: A review of the literature. J. Prosthet. Dent. 2001, 85, 585–598. [Google Scholar] [CrossRef] [Green Version]
- Al-Sukhun, J.; Kelleway, J. Biomechanics of the mandible: Part II. Development of a 3-dimensional finite element model to study mandibular functional deformation in subjects treated with dental implants. Int. J. Oral Maxillofac. Implant. 2007, 22, 455–466. [Google Scholar]
- O’Mahony, A.M.; Williams, J.; Spencer, P. Anisotropic elasticity of cortical and cancellous bone in the posterior mandible increases peri-implant stress and strain under oblique loading. Clin. Oral Implant. Res. 2001, 12, 648–657. [Google Scholar] [CrossRef]
- Saab, X.E.; Griggs, J.A.; Powers, J.M.; Engelmeier, R.L. Effect of abutment angulation on the strain on the bone around an implant in the anterior maxilla: A finite element study. J. Prosthet. Dent. 2007, 97, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, E.; Stegaroiu, R.; Nomura, S.; Miyakawa, O. Influence of marginal bone resorption on stress around an implant—A three-dimensional finite element analysis. J. Oral Rehabil. 2005, 32, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-L.; Fuh, L.-J.; Ko, C.-C.; Hsu, J.-T.; Chen, C.-C. Biomechanical effects of a maxillary implant in the augmented sinus: A three-dimensional finite element analysis. Int. J. Oral Maxillofac. Implant. 2009, 24, 455–462. [Google Scholar]
- Frost, H.M. A 2003 update of bone physiology and Wolff’s Law for clinicians. Angle Orthod. 2004, 74, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Mosley, J.; Lanyon, L. Strain rate as a controlling influence on adaptive modeling in response to dynamic loading of the ulna in growing male rats. Bone 1998, 23, 313–318. [Google Scholar] [CrossRef]
- Hirata, Y.; Inaba, Y.; Kobayashi, N.; Ike, H.; Fujimaki, H.; Saito, T. Comparison of Mechanical Stress and Change in Bone Mineral Density Between Two Types of Femoral Implant Using Finite Element Analysis. J. Arthroplast. 2013, 28, 1731–1735. [Google Scholar] [CrossRef] [PubMed]
- Meijer, H.J.; Starmans, F.J.; Steen, W.H.; Bosman, F. A three-dimensional, finite-element analysis of bone around dental implants in an edentulous human mandible. Arch. Oral Biol. 1993, 38, 491–496. [Google Scholar] [CrossRef]
- Cawood, J.I.; Howell, R.A. A classification of the edentulous jaws. Int. J. Oral Maxillofac. Surg. 1988, 17, 232–236. [Google Scholar] [CrossRef]
- Flemmig, T.; Beikler, T. Decision making in implant dentistry: An evidence-based and decision-analysis approach. Periodontol. 2000 2009, 50, 154–172. [Google Scholar] [CrossRef]
- Schwarz, M.S. Mechanical complications of dental implants. Clin. Oral Implant. Res. 2000, 11, 156–158. [Google Scholar] [CrossRef]
- Tallarico, M.; Martinolli, M.; Kim, Y.-J.; Cocchi, F.; Meloni, S.M.; Alushi, A.; Xhanari, E. Accuracy of Computer-Assisted Template-Based Implant Placement Using Two Different Surgical Templates Designed with or without Metallic Sleeves: A Randomized Controlled Trial. Dent. J. 2019, 7, 41. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hudieb, M.; AlKhader, M.; Mortaja, S.; Abusamak, M.; Wakabayashi, N.; Kasugai, S. Impact of Bone Augmentation of Facial Bone Defect around Osseointegrated Implant: A Three Dimensional Finite Element Analysis. Dent. J. 2021, 9, 114. https://doi.org/10.3390/dj9100114
Hudieb M, AlKhader M, Mortaja S, Abusamak M, Wakabayashi N, Kasugai S. Impact of Bone Augmentation of Facial Bone Defect around Osseointegrated Implant: A Three Dimensional Finite Element Analysis. Dentistry Journal. 2021; 9(10):114. https://doi.org/10.3390/dj9100114
Chicago/Turabian StyleHudieb, Malik, Mustafa AlKhader, Salah Mortaja, Mohammad Abusamak, Noriyuki Wakabayashi, and Shohei Kasugai. 2021. "Impact of Bone Augmentation of Facial Bone Defect around Osseointegrated Implant: A Three Dimensional Finite Element Analysis" Dentistry Journal 9, no. 10: 114. https://doi.org/10.3390/dj9100114
APA StyleHudieb, M., AlKhader, M., Mortaja, S., Abusamak, M., Wakabayashi, N., & Kasugai, S. (2021). Impact of Bone Augmentation of Facial Bone Defect around Osseointegrated Implant: A Three Dimensional Finite Element Analysis. Dentistry Journal, 9(10), 114. https://doi.org/10.3390/dj9100114





