3. Results
3.1. Sample Description
The frequency of participants was statistically equivalent between both patient samples by the binomial test (
p = 0.306, exact two-sided
p-value). At the same time, the frequencies of both sexes were statistically equivalent between both samples by the chi-square test with Yates correction (χ
2[1,
N = 61] = 0.44,
p = 0.505, asymptotic two-sided
p-value). The proportion of both sexes it was statistically equivalent in the pooled sample by the binomial test (
p = 1, exact two-sided
p-value), therefore half of the participants are women and the other half men. The frequencies of the five categories of civil status were statistically equivalent between both samples by the chi-square test (χ
2[4,
N = 61] = 6.44,
p = 0.177, exact two-sided
p-value). Levels of schooling and subjective socioeconomic status were statistically equivalent using the Mann–Whitney U-test (Z
U = −0.05,
p = 0.958, and Z
U = −1.31,
p = 0.191, respectively) and the average age using Student’s
t-test (t [59] = 1.43,
p = 0.159). The average age in the pooled sample was 46.36 years (SD = 7.57) with a minimum of 34 and a maximum of 63. However, there were differences in occupation (χ
2[2,
N = 61] = 6.98,
p = 0.030 exact bilateral). When performing the pairwise comparisons applying the Bonferroni correction, there was only a significant difference in the category of blue-collar employees. The frequency of unskilled workers/technical workers was higher in the sample of dental patients than in that of mental health patients (
Table 1).
In the sample of dental patients, 51.4% (18 out of 35) of participants had severe periodontitis (5 mm or more of clinical attachment loss) and 48.6% (17 out of 35) moderate (3–4 mm). Regarding extension of periodontitis, 97.1% had generalized periodontitis (≥30% of the sites are involved with clinical attachment loss ≥ 1 mm) and 2.9% localized (<30%). In the sample of mental health patients, 7.7% (2 out of 26) of participants had severe periodontitis and 92.3% (24 out of 26) had moderate periodontitis. There was not absence or mild cases of periodontitis in either of the two samples. Regarding extension of periodontitis in mental health patients, 50% had generalized periodontitis and 50% localized. The severity (χ2[1, N = 58] = 9.28 with Yates’s correction for continuity, p = 0.002 two-tailed asymptotic probability) and extension of periodontitis (χ2[1, N = 58] = 17.46 with Yates’s correction for continuity, p < 0.001) were significantly higher in dental patients than in mental health patients.
The average gingival bleeding index was significantly higher (Welch’s t [46.37] = 3.78, p < 0.000) in dental patients (M = 15.41%, 95% CI: 10.13, 20.69) than in mental health patients (M = 4.67%, 95% CI: 2.29, 7.06). Moreover, the average probing depth was significantly greater (Welch’s t [46.37] = 3.78, p < 0.001) in dental patients (M = 4.57, 95% CI: 4.47, 4.66) than mental health patients (M = 4.27, 95% CI: 4.19, 4.35).
3.2. Randomness of the Analyzed Variables
When using the mean as a cut-off point, the assumption of randomness was maintained in the two patient samples with a significance level of 0.05 for all variables. In the pooled sample, the assumption was also maintained with this level of significance, except for the variables IL-1β and clinical attachment loss that should be reduced to 0.01. Although if the median with clinical attachment loss (
Z = 1.42,
p = 0.156) and the mode with IL-1β (
Z = 0.19,
p = 0.850) are used as the cut-off point, it would remain with a significance level of 0.05 (
Table 2).
3.3. Reliability of Estimates of IL-1β, IL-6, MMP-8, and Scores in Psychometric Scales
The correlations between the average absorbancy and the antibody concentrations for IL-1β, IL-6 and MMP-8 in the pooled sample varied from 0.97 to 1. In their 95% confidence interval estimation, the three correlations included 1, that is, they were unitary. For these measurements, both samples were joined (58 patients for IL-1β, and 59 for IL-6 and MMP-8), since each kit had a 96-well microplate. Three cases for IL-1β and two for MMP-8 were eliminated by invalid estimates.
In the pooled sample of 61 participants and in each sample of patients, the levels of reliability of the 21 items composing of the BDI-II, the eight items composing of the OHHS and the 14 items composing of the OHIP-14-PD varied from good to excellent when they were estimated using the ordinal coefficient alpha (
Table 3).
3.4. Test of the Normal Distribution Assumption
The transformation of the Napierian logarithm was applied to the values in the 3 biomarkers to correct positive skewness and achieve normality (ln_IL-1β, ln_IL-6, and ln_MMP-8). Except for BDI-II and ln_MMP-8, the distributions were adjusted to normality in the pooled sample of 58 patients (three cases were lost due to invalid biomarker data) by the Kolmogorov–Smirnov test with the Lilliefors correction. The BDI-II presented a slight positive skewness (Z
SK = Sk/SE = 3.24), but its distribution was mesokurtic (Z
K3 = K3/SE = 0.95), which has a minor impact on the estimate of correlations when it is not accomplished the assumption of normal distribution. The distribution of ln_MMP-8 showed symmetry (Z
SK = −1.80) and mesokurtosis (Z
K3 = 0.26), adjusting to a normal distribution by the D’Agostino–Pearson test (
K2 = 3.31,
p = 0.191) (
Table 4).
In the sample of 34 dental patients, 6 of the 7 variables were adjusted to normal by the Shapiro–Wilk test. In the case of BDI-II, the null hypothesis of normality was sustained with a level of significance of 0.01 by this same test. The distribution of scores in BDI-II showed symmetry (ZSK = 0.48), mesokurtosis (ZK3 = −1.62), and the null hypothesis of normal distribution was accepted with a significance level of 0.05 by the D’Agostino–Pearson test (K2 = 2.84, p = 0.241).
In the sample of 24 mental health patients, the normality assumption was maintained by the Shapiro–Wilk test with a level of significance at the rate 0.01 in all variables, and at the rate 0.05 in four of them (
Table 4).
Regarding multivariate normality, the Mardia’s standardized coefficient of multivariate kurtosis with the seven variables was 2.61 in the pooled sample of 58 patients. Its value was 0.78 in the sample of 34 dental patients and 1.65 in the sample of 24 mental health patients. Therefore, these data reflect an acceptable approximation to multivariate normality in the pooled sample and good in the two patient samples.
3.5. Test of the Hypothetical Model in the Pooled Sample of Patients
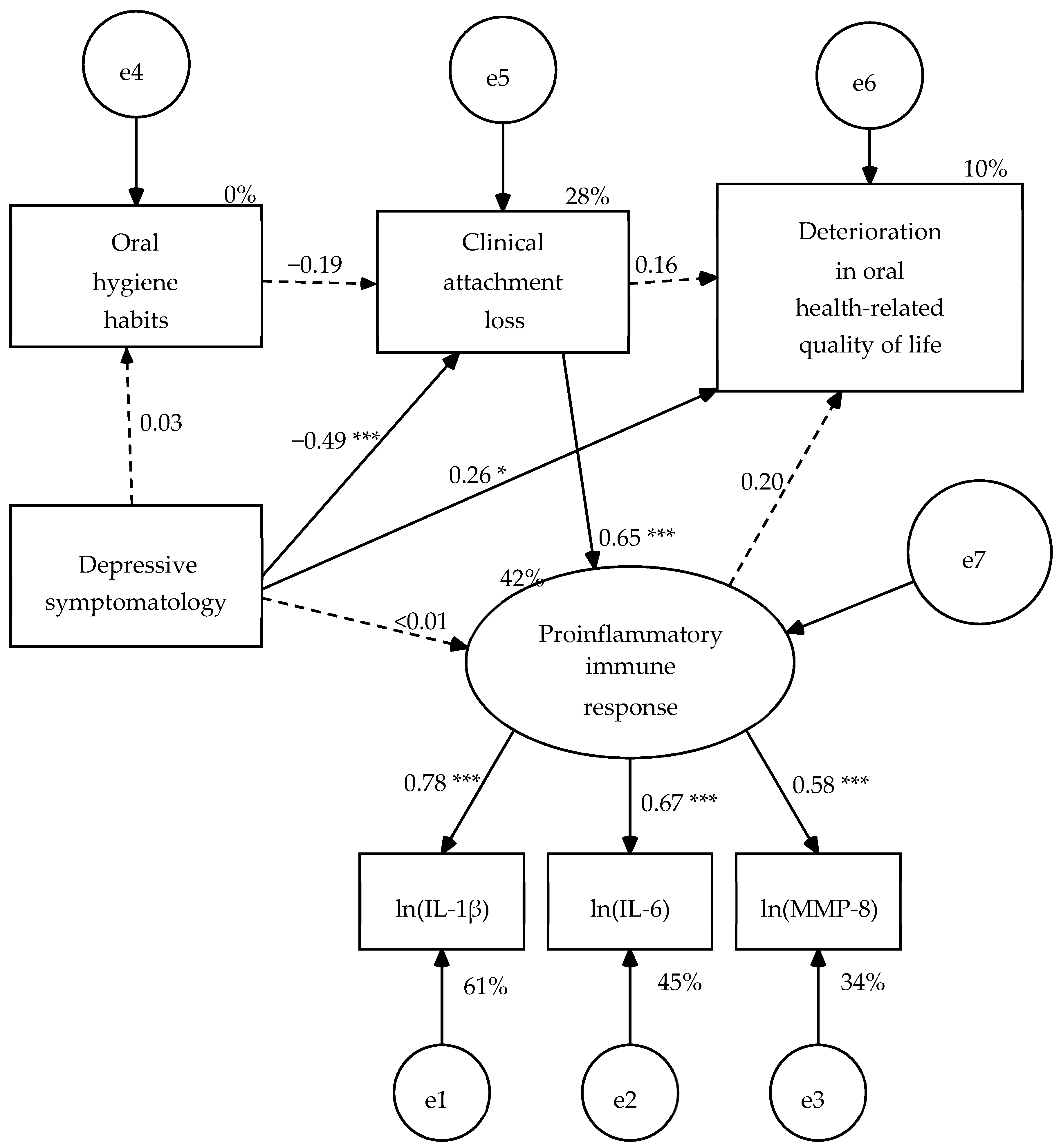
A structural model consisting of five variables was specified: one manifest exogenous variable (depressive symptomatology = BDI-II total score), three manifest endogenous variables (oral hygiene habits = OHHS total score, clinical attachment loss, and oral health-related quality of life = OHIP-14-PD total score), and one latent endogenous variable (proinflammatory immune response). The measurement model of this latent variable was made up of three indicators (IL-1β, IL-6, and MMP-8 with their values transformed using Napierian logarithm). In the structural model, depressive symptomatology predicts the 4 endogenous variables, the variable of oral hygiene habits predicts clinical attachment loss, clinical attachment loss predicts proinflammatory immune response and OHRQoL, and finally the proinflammatory immune response predicts OHRQoL (
Figure 1). When the parameters were estimated in the pooled sample of 58 patients (three cases were lost due to invalid estimates in the concentration of IL-1β), five structural weights were not significant (
Figure 1). Consequently, these paths were eliminated, except for the weight of the clinical attachment loss on the OHRQoL that became significant once the other four structural weights were eliminated (
Figure 2).
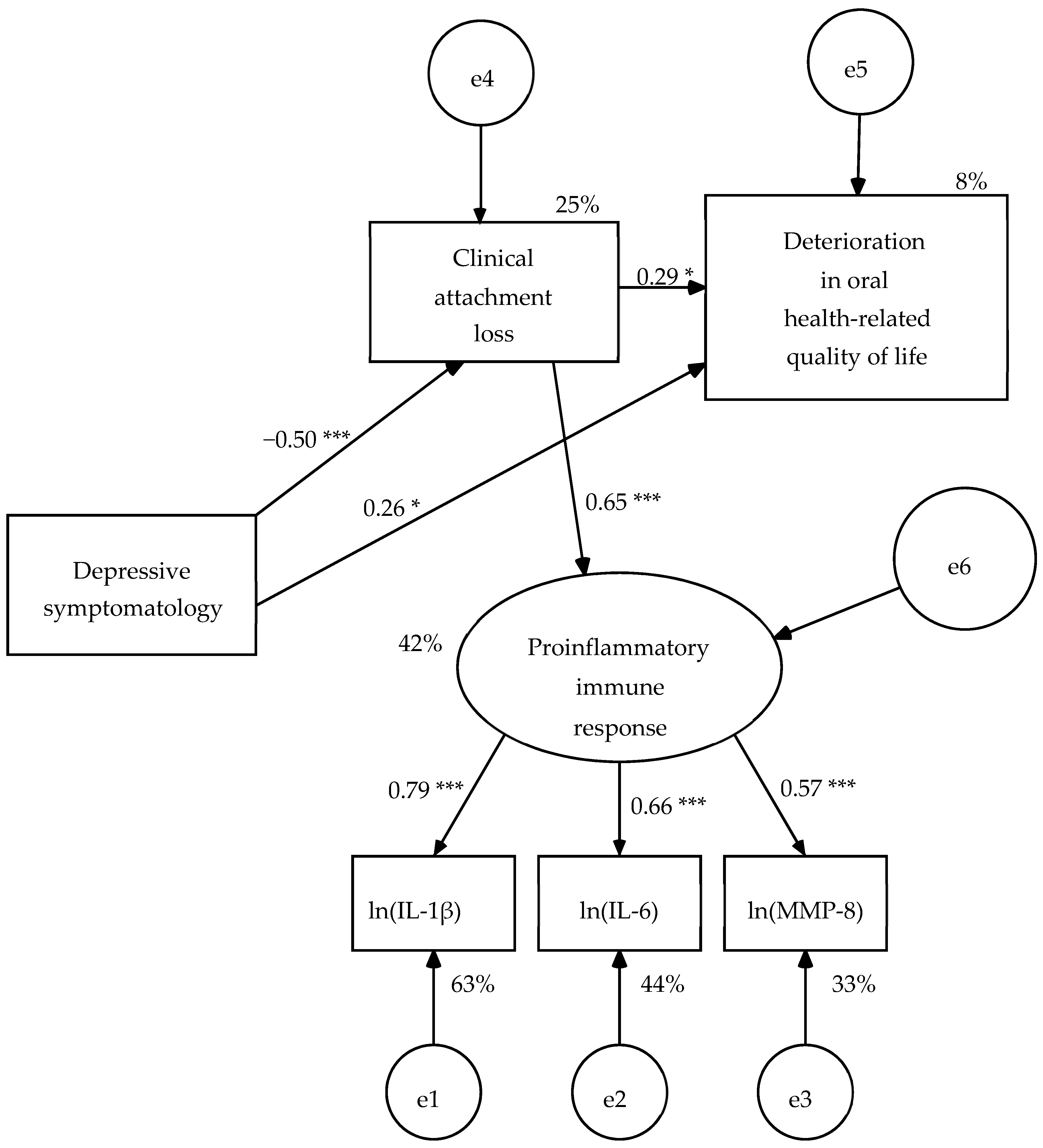
In this revised model (R1), all structural weights were significant (
Figure 2). The AVE of the measurement model was 0.46, its composite reliability (ω) of 0.72 and its measurement weights varied from 0.57 to 0.79, therefore it presented convergent validity. The effect size of the model was large on proinflammatory immunological response (42% of explained variance) and depressive symptomatology (25% of explained variance), and small on OHRQoL (8% of explained variance) (
Figure 2). The null hypothesis of goodness of fit was maintained by the likelihood ratio chi-square test (χ
2[8,
N = 58] = 6.45,
p = 0.597) and the Bollen–Stine bootstrap probability value (BS
p = 0.614). In addition, GFI, IFI, CFI, and RMSEA showed good fit values, and SRMR, AGFI, and NFI reached acceptable fit values (
Table 5).
3.6. Test of the Invariance of the Model Across the Two Types of Patients Using Multi-Group Analysis
The invariance of the revised model (R1) was tested across dental patients with periodontitis and mental health patients with depressive symptomatology, specifying six nested models in constraints. The solution was admissible in the six nested models in the two samples (34 dental patients and 24 mental health patients). There was a significant difference in two parameters (
Table 6). On the one hand, the structural weight of depressive symptomatology on the clinical attachment loss. In the unconstrained model (UC), the weight was negative and significant in dental patients (β = −0.35,
Z = −2.16,
p = 0.031), but it was positive and not significant in mental health patients (β = 0.14,
Z = 0.68,
p = 0.500). On the other hand, the structural variance of depressive symptomatology was greater in mental health patients (S
2 = 87.58, 95% CI: 37.15, 138.01) than in dental patients (S
2 = 17.62, 95% CI: 9.09, 26.14) in the UC model. The model with constraints in all parameters (MR) had two non-significant structural weights: the effect of depressive symptomatology on the clinical attachment loss (β = −0.03,
Z = −0.24,
p = 0.812) and on the OHRQoL (
β = 0.11,
Z = 0.84,
p = 0.402).
The null hypothesis of goodness of fit was accepted by the likelihood ratio chi-square test and the Bollen–Stine bootstrap probability value, and χ
2/df, IFI, CFI, and RMSEA had good fit values in the unconstrained model (UC), as well as in the models with constraints on the measurement weights (MW) and on structural weights (SW) (
Table 5). In addition, the goodness of fit was statistically equivalent between these three models nested by the chi-square difference test: χ
2[2] = 0.15,
p = 0.929 between UC and MW; χ
2[6] = 6.97,
p = 0.324 between UC and SW; χ
2[4] = 6.82,
p = 0.146 between MW and SW. However, the fit indices were poor in the models with constraints on structural covariances (SC), structural residuals (SR) and measurement residuals (MR), and the goodness of fit of these three models was significantly lower than that of the previous three models (
Table 7).
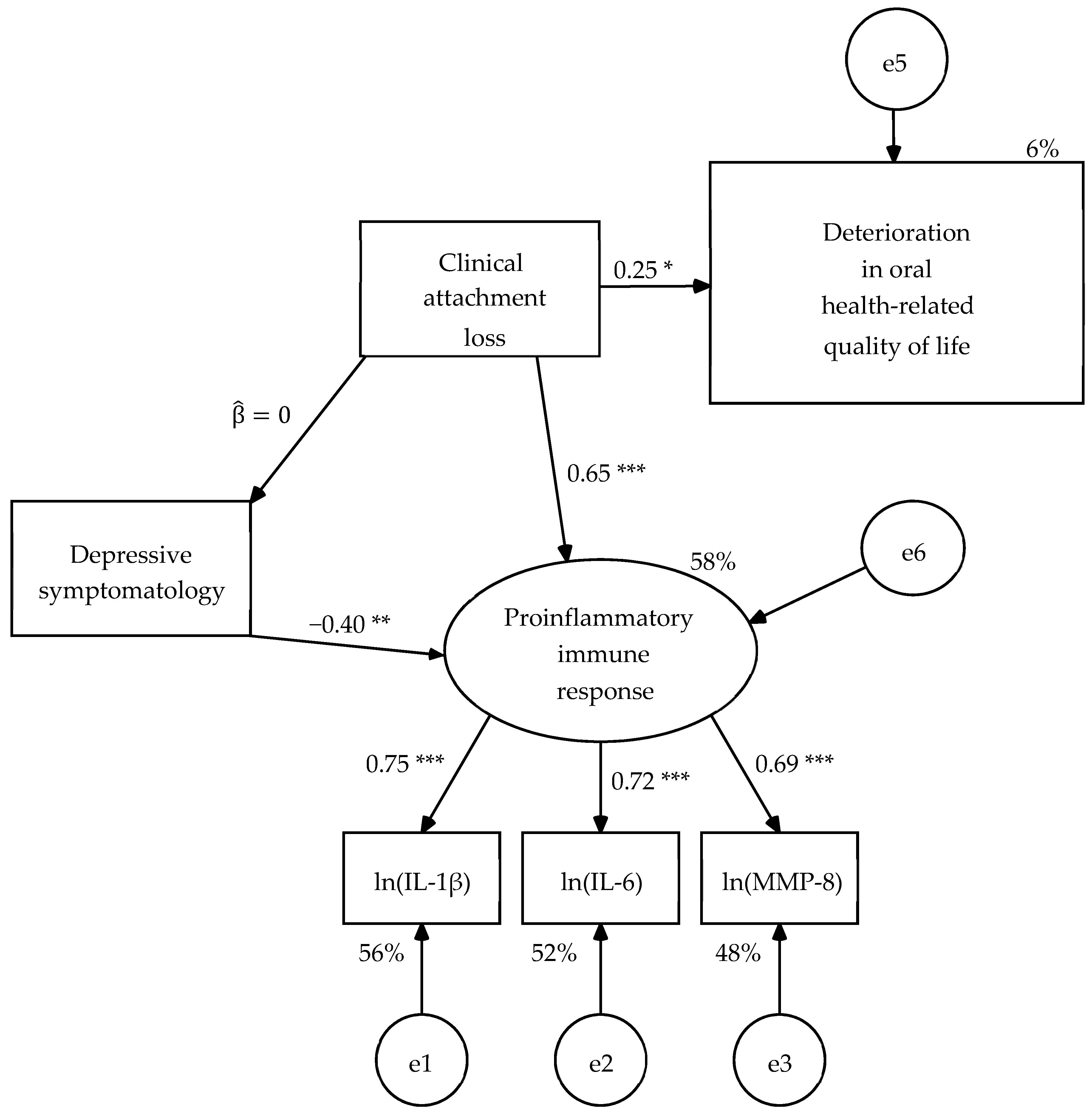
The R1 model was modified to achieve its partial or incomplete invariance between both populations. First, the non-significant path of the effect of depressive symptomatology on OHRQoL was eliminated. Next, two population singularities were introduced in relation to depressive symptomatology. When reviewing the modification indices, the suggestion of introducing the effect of depressive symptomatology on proinflammatory immunological response in mental health patients was found, which is consistent with the hypotheses. This path was uniquely specified in the sample of mental health patients. The second singularity arose from the fact that the weight of depressive symptomatology on the clinical attachment loss was not significant in mental health patients, but it was significant and with an opposite sign to the expected one in dental patients. Therefore, it was decided to contemplate it in a singular way as a consequence in dental patients and omit it in mental health patients. This proposal as a consequence implies a hypothesis. Dental consultation and diagnosis, probably in the expectation of successful treatment, seem to generate a feeling of euphoria or antidepressant as the patient presents more periodontal injury.
When estimating the parameters of R2 model, the structural weight of the clinical attachment loss on the OHRQoL was significant with significant level of 0.01 in the unconstrained model (UC) and in the model with constraints on the measurement weights (SW) of in both samples, and in the other four nested models with significant level of 0.05 (
Figure 3 and
Figure 4). The parameters subjected to restriction were statistically equivalent between both types of patients in the nested models (
Table 8). The six nested models had good fit to the data (
Table 9) and the goodness of fit was equivalent between them by the chi-square difference test (
Table 6). Therefore, the R2 model was partially invariant, that is, it was invariant once that a singularity for each population in relation to the depressive symptomatology was specified.
4. Discussion
The first objective was to test a model to predict clinical attachment loss and OHRQoL. The model was formulated based on five hypotheses. The first hypothesis was about the convergent validity of proinflammatory immune response as a latent variable, when measured by three biomarkers (IL-1β, IL-6, and MMP-8), that is, it was intended to find out if the data provide evidence that the three indicators assess a same construct. Although Fornell and Larcker in the 1980s proposed that an AVE greater than 0.50 allows us to state that a factor or measurement model has convergent validity, this criterion has been revised; and recently it has been suggested that three criteria must be accomplished to state the convergent validity is acceptably reached: measuring weights greater than or equal to 0.50, coefficient ω or H greater than or equal to 0.70, and AVE not less than 0.25 [
18]. In this methodological study, it is shown that when the factor has three indicators, these three conditions are met with an AVE greater than or equal to 0.44 [
18]. Measuring weights > 0.50, AVE > 0.44 and ω > 0.70 were conditions satisfied by the latent variable called proinflammatory immune response, and thus the hypothesis was fulfilled. Moreover, we can be affirmed that this measurement model is valid when considering the fact that the levels of IL-1β, IL-6, and MMP-8 were chosen as indicators of this latent variable, since they mediate inflammatory processes and activation of the immune system against periodontal disease [
12] and depression [
1,
3,
6].
The second hypothesis to specify the model stated that a higher level of depressive symptomatology would be a predictor of loss of OHRQoL [
16], clinical attachment loss [
8,
9,
11], poor oral hygiene habits [
13], and increased proinflammatory immune response [
1,
2]. The hypothesis was fulfilled very partially. It was fulfilled that a higher level of depressive symptomatology predicts worse OHRQoL. Depressive symptomatology also predicted the clinical attachment level, but against the expectation a higher level of depressive symptomatology predicted less clinical attachment loss. On the other hand, depressive symptomatology was independent of oral hygiene habits and proinflammatory immune response. It should be noted that the estimates of these last two structural weights were virtually nil and, thus, it is a clear result and not attributable to a small sample size (n = 58). When combining the first datum consistent with its hypothesis (significant positive effect on OHRQoL), the second discordant with its hypothesis (significant negative effect on clinical attachment loss) and the last two that did not confirm their hypotheses (non-significant weights on oral hygiene habits and proinflammatory immune response), the pooled sample seems to support a psychosomatic model in which there is no correspondence between the patient’s complaint and the evidence that is provided by clinical exams and laboratory tests [
40,
41]. This same expressed with other words. When both patient samples are analyzed together, the model reflects that people with more depressive symptoms complain of a lower level of OHRQoL as the periodontal damage is less; in addition, their depressive symptoms are independent of the activation of proinflammatory processes and oral hygiene habits.
The third hypothesis to specify the model stated that poor oral hygiene habits are predictors of clinical attachment loss [
19]. In the present sample, oral hygiene habits were not a significant predictor with a significance level of 0.05. A very pointed distribution around the maximum value could explain this lack of significance [
39]. This would mean that all participants reported very good oral hygiene habits. Nevertheless, this was not the case, since 38% of the participants showed bad habits (OHHS total scores between 0 and 0.999), 57% regular (between 1 and 2.124) and 5% good (between 2.125 and 4). Nor can it be attributed to the lack of reliability of the OHHS, since this was good in the sample of 58 participants (ordinal α = 0.86 and standardized α = 0.80), as in other studies [
36]. However, the limited sample size of 58 participants could affect. Altman and Krzywinski [
42] point out that when the size of the population effect is small, it may not be significant in small samples [
42]. In this regard, in a meta-analysis study, it was found that the size of the effect of oral hygiene habits on clinical attachment loss is small when regular habits dominate: OR = 2.04 (95% CI: 1.65–2.53) [
43]. Precisely, if the determination of the statistical significance of the structural weight is performed using a one-tailed test, then the null hypothesis that the parameter is equal to zero would be rejected with a significance level of 0.05 (
Z = −1.69,
p = 0.046). Therefore, these data are consistent with the small population effect.
The fourth hypothesis stated that clinical attachment loss predicts an increase in proinflammatory immune response [
12] and deterioration of OHRQoL [
14]. This hypothesis did fulfill with a large effect size on proinflammatory immunological response and small on OHRQoL. The deterioration of the periodontium due to infection activates the inflammatory and immune response of the organism, and also deteriorates the quality of life of the person [
14].
The fifth hypothesis that proposed that the increase in proinflammatory immune response predicts impairment of OHRQoL was not accomplish. As previously noted, the sample size could affect, since the direct effect of the increase in proinflammatory immunological response on OHRQoL is small at the population level and mediated by a complexity of factors [
20].
The second objective of the study was to contrast the invariance of the model across the two types of patients: dental patients with periodontitis and mental health patients with depressive symptoms. The model reduced to its significant paths was not strictly invariant and it was decided to define a model that included a singularity in relation to the depressive symptomatology for each population, that is, a partially invariant model. The first singularity introduced in the revision of the model for multi-group analysis was in relation to the effect of depressive symptomatology on clinical attachment level in dental patients. Since depressive symptomatology did not have significant effect in mental health patients, but did have a significant and opposite effect to that expected in dental patients, it was decided to keep this path only in the latter patients, but by reversing its sense. Now a lower depressive symptomatology, which has a minimum level in the sample of dental patients, is a euphoric consequence of the diagnosis of periodontal pathology and the expectation of recovery of oral health [
44]. The second singularity introduced in the model was to recover the effect of depressive symptomatology on proinflammatory immune response only in mental health patients, where this path makes more sense [
1,
3,
6].
With the inclusion of the singularity in relation to the depressive symptomatology for each of two patient populations, the six nested models had good fit to the data, the goodness of fit was equivalent between them, and the free estimates of the parameters subject to restriction also they were equivalent between both types of patients (in the nested models in which these parameters were not constrained). This final model indicates that the clinical attachment loss activates proinflammatory immune processes and impairs OHRQoL in both patients, the increase in depressive symptomatology activates proinflammatory immune processes only in mental health patients, and the severity of periodontal tissue damage has a euphoric effect on the situation of periodontal diagnosis and expectation of recovery of oral health only in dental patients. On the other hand, the measurement model for the proinflammatory immune response had convergent validity in all nested models, as in the one-group analysis; moreover the estimates of measurement weights and variance of factor were equivalent between both types of patients.
One limitation of the study is the use of non-probability sampling, hence inferences should be taken with due caution in the populations of dental patients with periodontitis and mental health patients with depressive symptoms, all of them resident in Monterrey and its metropolitan area, middle-aged adults corresponding to a subjective socioeconomic status varying from low to middle-middle. Sample size was limited, which was due to the difficulty of finding mental health patients who met the inclusion and exclusion criteria. This implies that the parameter estimates have to be higher to reach statistical significance; as a consequence, trivial or weak relations at the population level may be non-significant in the sample. However, the end was to find substantial effects with a standardized value of at least 0.20. The design was cross-sectional non-experimental, therefore, no causal inferences can be made and only predictive relationships are discussed. Despite the efforts made in the data analysis to overcome the limitations (verification of assumptions of randomness and multivariate normality, continuous monotonous transformations applied to the data of the three biomarkers to achieve greater proximity to multivariate normality, repetitive sampling procedures with a high number of bootstrap samples to generate errors in the estimates), this research should be considered as a pilot study.
5. Conclusions
In the analysis of the pooled sample of dental patients with periodontitis and mental health patients with depressive symptomatology, a psychosomatic complaint model appears. Depressive symptomatology predicts greater complaints or loss of OHRQoL with a small effect size, but less clinical attachment loss with a large effect size, that is, a discordance between the complaint and the clinically observable. On the other hand, depressive symptomatology has no effect on proinflammatory immune response. However, according to expectations, clinical attachment loss predicts the activation of proinflammatory immune processes with a large effect size, as well as a greater complaint of loss of OHRQoL with a small effect size. In addition, the measurement model of this response based on the levels of three proteins (IL-1β, IL-6, and MMP-8) shows convergent validity. The effect of bad oral hygiene habits on the clinical attachment loss is not significant in a two-tailed test and, thus, this path was eliminated, although this effect of small size would be significant in a one-tailed test. The model reduced to its significant paths shows good fit to the data in the one-group analysis, but is not invariant between both types of patients.
In the multi-group analysis, the effect of depressive symptomatology on OHRQoL is not significant in both a one-tailed test and a two-tailed test. Although the effect of the clinical attachment loss on the OHRQoL is not significant in two nested models, it is significant in the four models with more constraints, hence, the elimination of this path is not justified. When specifying a singularity in relation to depressive symptomatology for each population, a model with a good fit to the data and a goodness of fit equivalent between its six specifications nested in constraints is achieved; moreover, parameters subject to constrain in each nested model were statistically equivalent between both groups of patients. Due to this singularity for each population, it should be named as a strong but partial invariance. A singularity refers to the effect of depressive symptomatology on the clinical attachment loss disappears in patients with depressive symptomatology and its sense is reversed in dental patients, that is, it is contemplated as a euphoric consequence of diagnosis and expectations on treatment in dental patients with periodontitis. The other singularity is with respect to the effect of depressive symptomatology on proinflammatory immune response, eliminated due to lack of significance in the one-group analysis, is recovered for the sample of patients with depressive symptomatology in the multi-group analyses. The common paths between both types of patients in the final model are the large-size effect of clinical attachment loss on the increase in proinflammatory immune response and the small-size effect of clinical attachment loss on OHRQoL. As in the one-group analysis (when pooling both samples), the measurement model of this latent variable has convergent validity and its measurement weights and variance are equivalent between both types of patients.