CD163+ Foamy Macrophages Are Associated with the Morphogenesis of Oral Verruciform Xanthoma through Angiogenesis by VEGF Expression: An Immunohistochemical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Information
2.2. Morphological Evaluation
2.3. Immunohistochemical Evaluation
2.4. Assessment of Microvessel Density
2.5. Statistical Analysis
3. Results
3.1. Clinical Findings
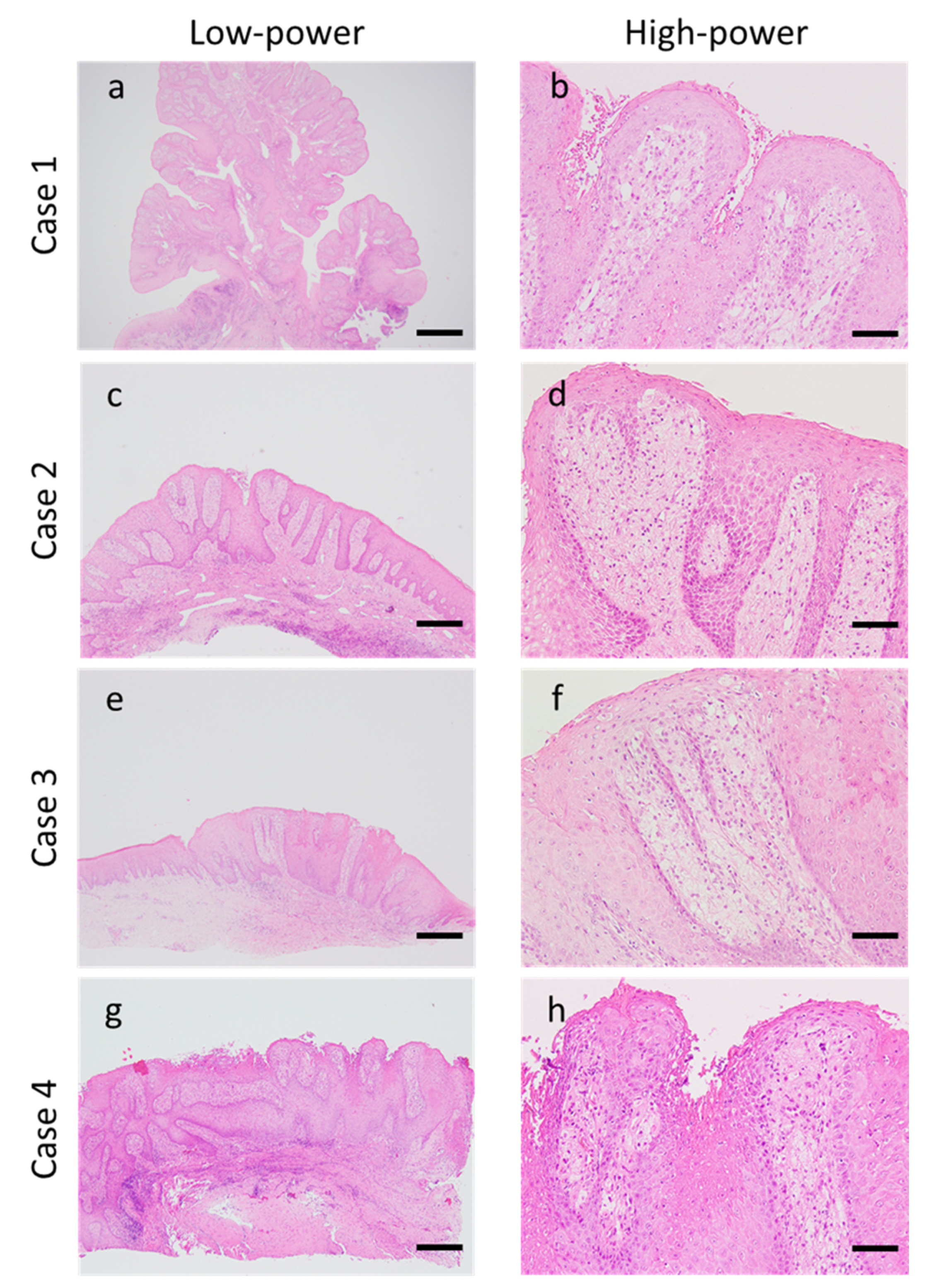
3.2. Morphological Findings
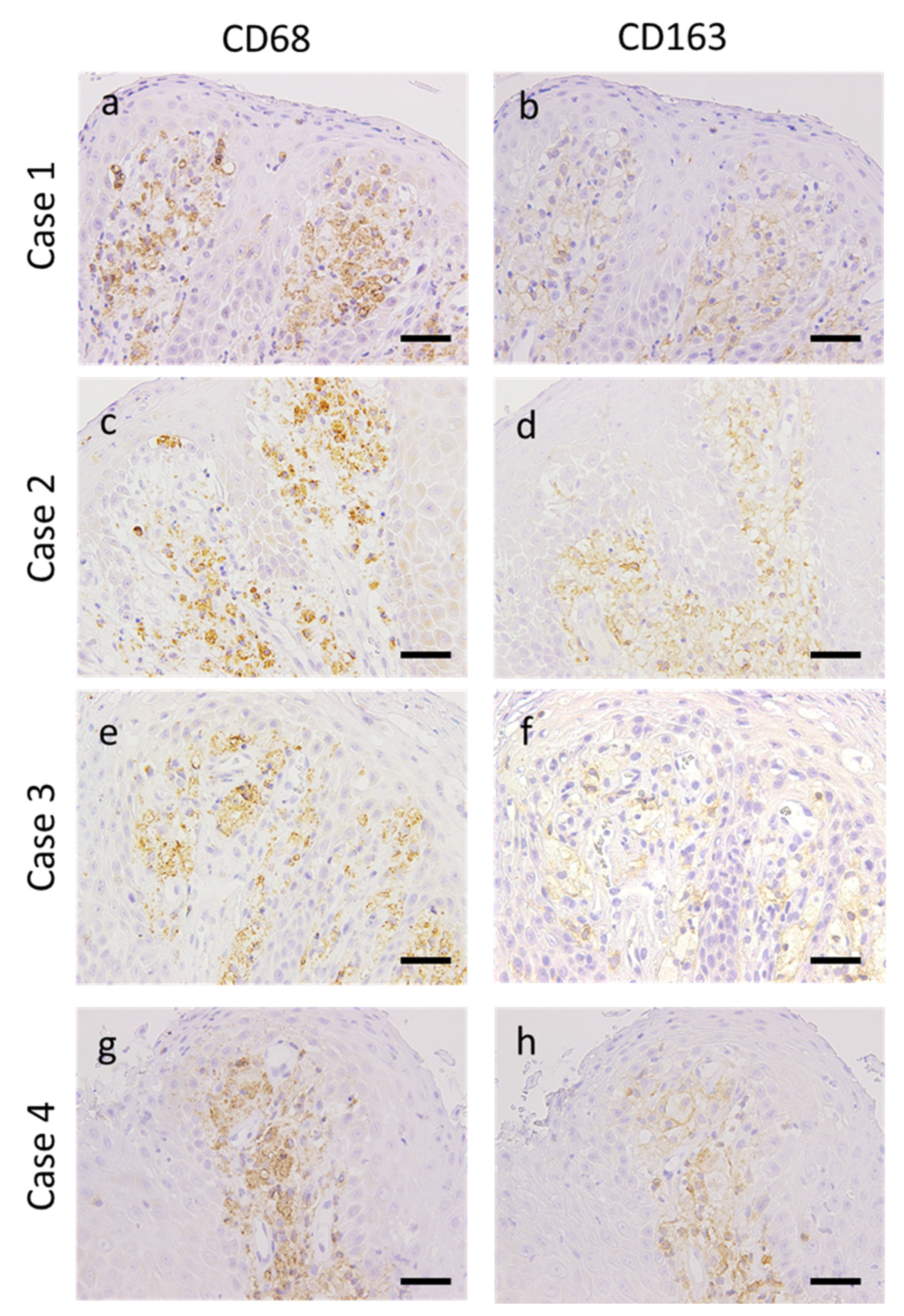
3.3. Expression of Macrophage Markers in OVX
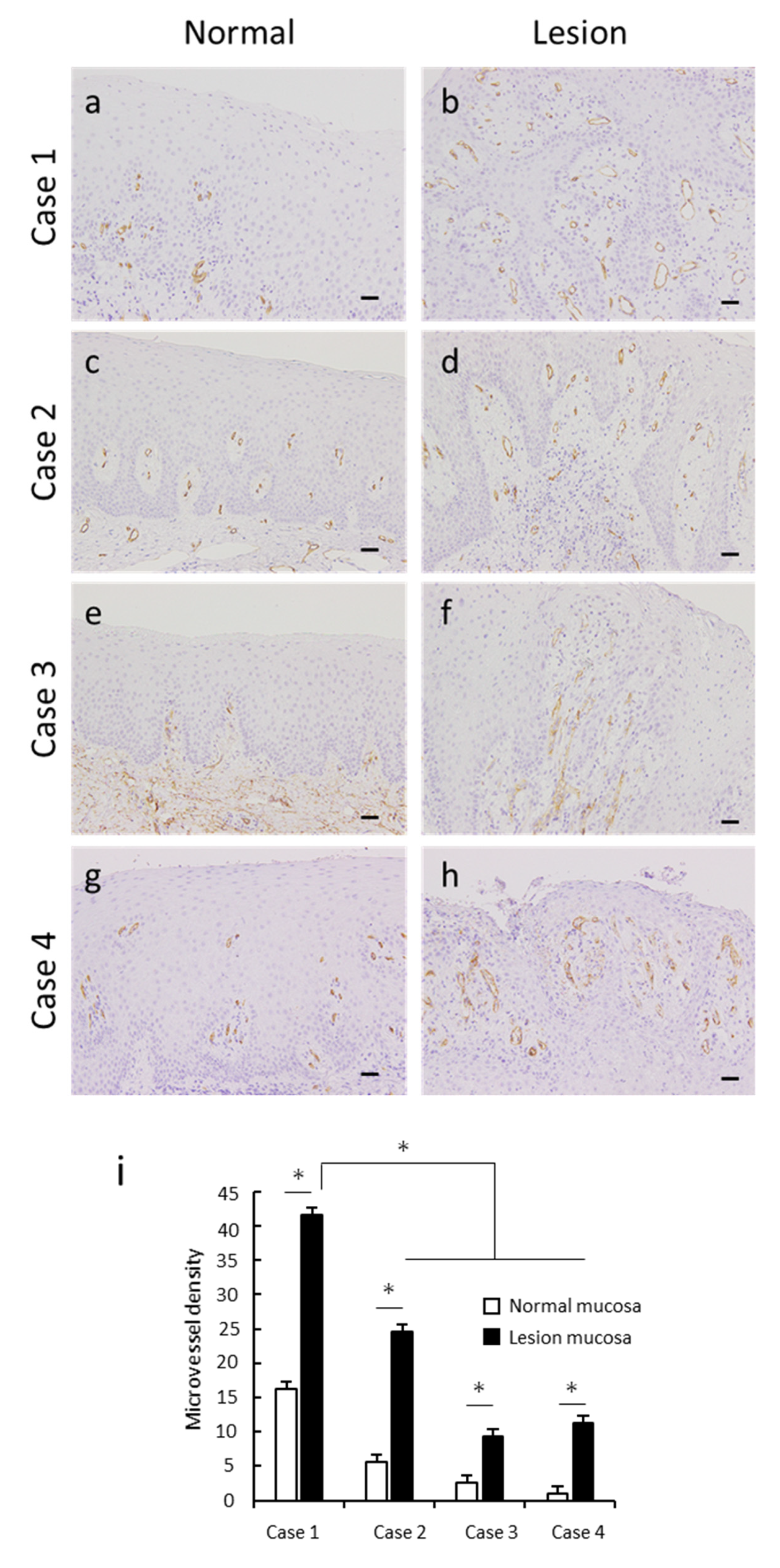
3.4. Angiogenesis in OVX
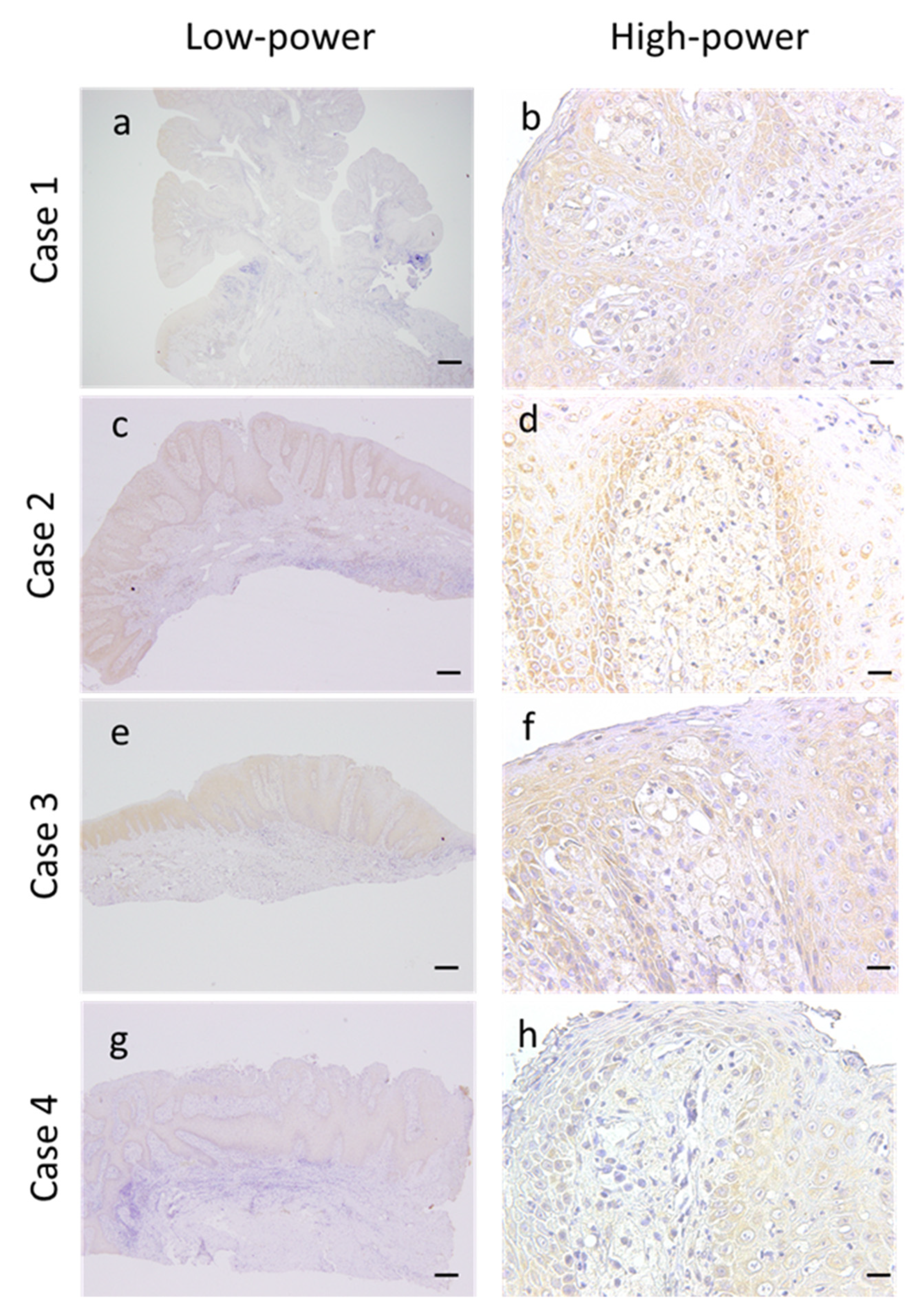
3.5. Expression of VEGF in the OVX Microenvironment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shafer, W.G. Verruciform xanthoma. Oral Surg. Oral Med. Oral Pathol. 1971, 31, 784–789. [Google Scholar] [CrossRef]
- Zegarelli, D.J.; Zegarelli-Schmidt, E.C.; Zegarelli, E.V. Verruciform xanthoma. Further light and electron microscopic studies, with the addition of a third case. Oral Surg. Oral Med. Oral Pathol. 1975, 40, 246–256. [Google Scholar] [CrossRef]
- Mostafa, K.A.; Takata, T.; Ogawa, I.; Ijuhin, N.; Nikai, H. Verruciform xanthoma of the oral mucosa: A clinicopathological study with immunohistochemical findings relating to pathogenesis. Virchows Arch. A Pathol. Anat. Histopathol. 1993, 423, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.T.; Jaeger, R.G.; Cabral, L.A.; Carvalho, Y.R.; Costa, A.L.; Jaeger, M.M. Verruciform xanthoma of the oral mucosa. Report of four cases and a review of the literature. Oral Oncol. 2001, 37, 326–331. [Google Scholar] [CrossRef]
- Yu, C.H.; Tsai, T.C.; Wang, J.T.; Liu, B.Y.; Wang, Y.P.; Sun, A.; Chiang, C.P. Oral verruciform xanthoma: A clinicopathologic study of 15 cases. J. Formos. Med. Assoc. 2007, 106, 141–147. [Google Scholar] [CrossRef][Green Version]
- Nowparast, B.; Howell, F.V.; Rick, G.M. Verruciform xanthoma. A clinicopathologic review and report of fifty-four cases. Oral Surg. Oral Med. Oral Pathol. 1981, 51, 619–625. [Google Scholar] [CrossRef]
- Stein, M.; Keshav, S.; Harris, N.; Gordon, S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: A marker of alternative immunologic macrophage activation. J. Exp. Med. 1992, 176, 287–292. [Google Scholar] [CrossRef]
- Goerdt, S.; Orfanos, C.E. Other functions, other genes: Alternative activation of antigen-presenting cells. Immunity 1999, 10, 137–142. [Google Scholar] [CrossRef]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef]
- Yokozaki, H.; Koma, Y.I.; Shigeoka, M.; Nishio, M. Cancer as a tissue: The significance of cancer-stromal interactions in the development, morphogenesis and progression of human upper digestive tract cancer. Pathol. Int. 2018, 68, 334–352. [Google Scholar] [CrossRef]
- Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunological Rev. 2008, 222, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Shigeoka, M.; Koma, Y.; Nishio, M.; Komori, T.; Yokozaki, H. CD163+ macrophages infiltration correlates with the immunosuppressive cytokine interleukin 10 expression in tongue leukoplakia. Clin. Exp. Dent. Res. 2019, 5, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Shigeoka, M.; Koma, Y.I.; Kodama, T.; Nishio, M.; Akashi, M.; Yokozaki, H. Intraepithelial CD163+ macrophages in tongue leukoplakia biopsy: A promising tool for cancer screening. Oral Dis. 2019, in press. [Google Scholar] [CrossRef]
- De Andrade, B.A.; Agostini, M.; Pires, F.R.; Rumayor, A.; Carlos, R.; de Almeida, O.P.; Romanach, M.J. Oral verruciform xanthoma: A clinicopathologic and immunohistochemical study of 20 cases. J. Cutan. Pathol. 2015, 42, 489–495. [Google Scholar] [CrossRef]
- Koizumi, Y.; Kauzman, A.; Okada, H.; Kuyama, K.; McComb, R.J.; Ymamoto, H. Assesment of Proloferative Activity and Angiogenesis in Ameloblastoma: A Comparison Based on Patient Age. Int. J. Oral-Med. Sci. 2004, 3, 25–33. [Google Scholar] [CrossRef][Green Version]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Shigeoka, M.; Urakawa, N.; Nakamura, T.; Nishio, M.; Watajima, T.; Kuroda, D.; Komori, T.; Kakeji, Y.; Semba, S.; Yokozaki, H. Tumor associated macrophage expressing CD204 is associated with tumor aggressiveness of esophageal squamous cell carcinoma. Cancer Sci. 2013, 104, 1112–1119. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Wright, J.; Lucente, J.; McQuade, M.J. Oral and maxillofacial pathology case of the month. Verruciform xanthoma. Tex. Dent. J. 2010, 127, 126. [Google Scholar]
- Buchner, A.; Hansen, L.S.; Merrell, P.W. Verruciform xanthoma of the oral mucosa. Report of five cases and review of the literature. Arch. Dermatol. 1981, 117, 563–565. [Google Scholar] [CrossRef]
- Tamiolakis, P.; Theofilou, V.I.; Tosios, K.I.; Sklavounou-Andrikopoulou, A. Oral verruciform xanthoma: Report of 13 new cases and review of the literature. Med. Oral Patol. Oral Cir. Bucal. 2018, 23, e429–e435. [Google Scholar] [CrossRef] [PubMed]
- Iamaroon, A.; Vickers, R.A. Characterization of verruciform xanthoma by in situ hybridization and immunohistochemistry. J. Oral Pathol. Med. 1996, 25, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Ide, F.; Obara, K.; Yamada, H.; Mishima, K.; Saito, I.; Kusama, K. Cellular basis of verruciform xanthoma: Immunohistochemical and ultrastructural characterization. Oral Dis. 2008, 14, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Belknap, A.N.; Islam, M.N.; Bhattacharyya, I.; Cohen, D.M.; Fitzpatrick, S.G. Oral Verruciform Xanthoma: A Series of 212 Cases and Review of the Literature. Head Neck Pathol. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Toida, M.; Koizumi, H. Verruciform xanthoma involving the lip: A case report. J. Oral Maxillofac. Surg. 1993, 51, 432–434. [Google Scholar] [CrossRef]
- Shahrabi Farahani, S.; Treister, N.S.; Khan, Z.; Woo, S.B. Oral verruciform xanthoma associated with chronic graft-versus-host disease: A report of five cases and a review of the literature. Head Neck Pathol. 2011, 5, 193–198. [Google Scholar] [CrossRef]
- Getz, G.I.; Parag-Sharma, K.; Reside, J.; Padilla, R.J.; Amelio, A.L. Identification of NSDHL mutations associated with CHILD syndrome in oral verruciform xanthoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 128, 60–69. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Schwartz, E.J.; West, R.B.; Warnke, R.A.; Arber, D.A.; Natkunam, Y. Expression of CD163 (hemoglobin scavenger receptor) in normal tissues, lymphomas, carcinomas, and sarcomas is largely restricted to the monocyte/macrophage lineage. Am. J. Surg. Pathol. 2005, 29, 617–624. [Google Scholar] [CrossRef]
- Sica, A.; Larghi, P.; Mancino, A.; Rubino, L.; Porta, C.; Totaro, M.G.; Rimoldi, M.; Biswas, S.K.; Allavena, P.; Mantovani, A. Macrophage polarization in tumour progression. Semin. Cancer Biol. 2008, 18, 349–355. [Google Scholar] [CrossRef]
- Takeya, M.; Komohara, Y. Role of tumor-associated macrophages in human malignancies: Friend or foe? Pathol. Int. 2016, 66, 491–505. [Google Scholar] [CrossRef]
- Valverde Lde, F.; Pereira Tde, A.; Dias, R.B.; Guimaraes, V.S.; Ramos, E.A.; Santos, J.N.; Gurgel Rocha, C.A. Macrophages and endothelial cells orchestrate tumor-associated angiogenesis in oral cancer via hedgehog pathway activation. Tumour Biol. 2016, 37, 9233–9241. [Google Scholar] [CrossRef] [PubMed]
- Stasikowska-Kanicka, O.; Wagrowska-Danilewicz, M.; Danilewicz, M. Immunohistochemical study on neuropilin 1 (NRP1) immunoexpression in oral squamous cell carcinoma. Folia Histochem. Cytobiol. 2018, 1, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Mao, X.H.; Tian, T.; Wang, W.M.; Su, T.; Jiang, C.H.; Hu, C.Y. Role of PFKFB3 and CD163 in oral squamous cell carcinoma angiogenesis. Curr. Med. Sci. 2019, 39, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Enzan, H.; Yasui, W. Vasculo-smooth muscle hamartomatous structure is linked to morphogenesis of colorectal polypoid adenoma. Med. Mol. Morphol. 2015, 48, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.H.; Hauck, F.; Dreyer, J.H.; Kempkes, B.; Niedobitek, G. Macrophage polarisation: An immunohistochemical approach for identifying M1 and M2 macrophages. PLoS ONE 2013, 8, e80908. [Google Scholar] [CrossRef]
- Drummond, J.F.; White, D.K.; Damm, D.D.; Cramer, J.R. Verruciform xanthoma within carcinoma in situ. J. Oral Maxillofac. Surg. 1989, 47, 398–400. [Google Scholar] [CrossRef]
- Neville, B.W.; Weathers, D.R. Verruciform xanthoma. Oral Surg. Oral Med. Oral Pathol. 1980, 49, 429–434. [Google Scholar] [CrossRef]
- Mannes, K.D.; Dekle, C.L.; Requena, L.; Sangueza, O.P. Verruciform xanthoma associated with squamous cell carcinoma. Am. J. Derm. Pathol. 1999, 21, 66–69. [Google Scholar] [CrossRef]
- Mori, K.; Haraguchi, S.; Hiori, M.; Shimada, J.; Ohmori, Y. Tumor-associated macrophages in oral premalignant lesions coexpress CD163 and STAT1 in a Th1-dominated microenvironment. BMC Cancer 2015, 15, 573. [Google Scholar] [CrossRef]
- Stasikowska-Kanicka, O.; Wagrowska-Danilewicz, M.; Danilewicz, M. T cells are involved in the induction of macrophage phenotypes in oral leukoplakia and squamous cell carcinoma-a preliminary report. J. Oral Pathol. Med. 2018, 47, 136–143. [Google Scholar] [CrossRef]
| Case | Sex | Age (Years) | Region | Size (mm) | Clinical Diagnosis | Histological Classification | Previous Medical History |
|---|---|---|---|---|---|---|---|
| 1 | M | 15 | Gingiva | 5.0 | Epulis | Verrucous | Pneumonia Macroscopic hematuria |
| 2 | M | 54 | Gingiva | 5.0 | Gingival tumor | Flat | Dyslipidemia Hyperuricemia |
| 3 | M | 43 | Palate | 3.0 | Papilloma | Flat | None |
| 4 | F | 48 | Gingiva | 4.0 | Erosion | Flat | Appendicitis Basedow’s disease |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shigeoka, M.; Koma, Y.-i.; Kodama, T.; Nishio, M.; Akashi, M.; Yokozaki, H. CD163+ Foamy Macrophages Are Associated with the Morphogenesis of Oral Verruciform Xanthoma through Angiogenesis by VEGF Expression: An Immunohistochemical Study. Dent. J. 2020, 8, 18. https://doi.org/10.3390/dj8010018
Shigeoka M, Koma Y-i, Kodama T, Nishio M, Akashi M, Yokozaki H. CD163+ Foamy Macrophages Are Associated with the Morphogenesis of Oral Verruciform Xanthoma through Angiogenesis by VEGF Expression: An Immunohistochemical Study. Dentistry Journal. 2020; 8(1):18. https://doi.org/10.3390/dj8010018
Chicago/Turabian StyleShigeoka, Manabu, Yu-ichiro Koma, Takayuki Kodama, Mari Nishio, Masaya Akashi, and Hiroshi Yokozaki. 2020. "CD163+ Foamy Macrophages Are Associated with the Morphogenesis of Oral Verruciform Xanthoma through Angiogenesis by VEGF Expression: An Immunohistochemical Study" Dentistry Journal 8, no. 1: 18. https://doi.org/10.3390/dj8010018
APA StyleShigeoka, M., Koma, Y.-i., Kodama, T., Nishio, M., Akashi, M., & Yokozaki, H. (2020). CD163+ Foamy Macrophages Are Associated with the Morphogenesis of Oral Verruciform Xanthoma through Angiogenesis by VEGF Expression: An Immunohistochemical Study. Dentistry Journal, 8(1), 18. https://doi.org/10.3390/dj8010018





