Anti-Bacterial and Anti-Inflammatory Effects of Toothpaste with Swiss Medicinal Herbs towards Patients Suffering from Gingivitis and Initial Stage of Periodontitis: From Clinical Efficacy to Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Products for Examination
2.2. Patients and Study Design
2.3. Clinical Assessment
2.4. Biological Material Collection and Processing
2.5. Reagents and Assay Kits
2.6. Bacterial Strains and Growth Conditions
2.7. Phagocytosis and Post-Phagocytosis Bacterial Survival Assays
2.8. Bacterial Catalase Assay
2.9. Differential Bacterial Concentrations in GCF and Plaque Determined by a Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (qrPCR) Method
2.10. Reduction-Oxidation (Redox) Assays
2.11. Cytokine Assays
2.12. Statistical Analysis
3. Results
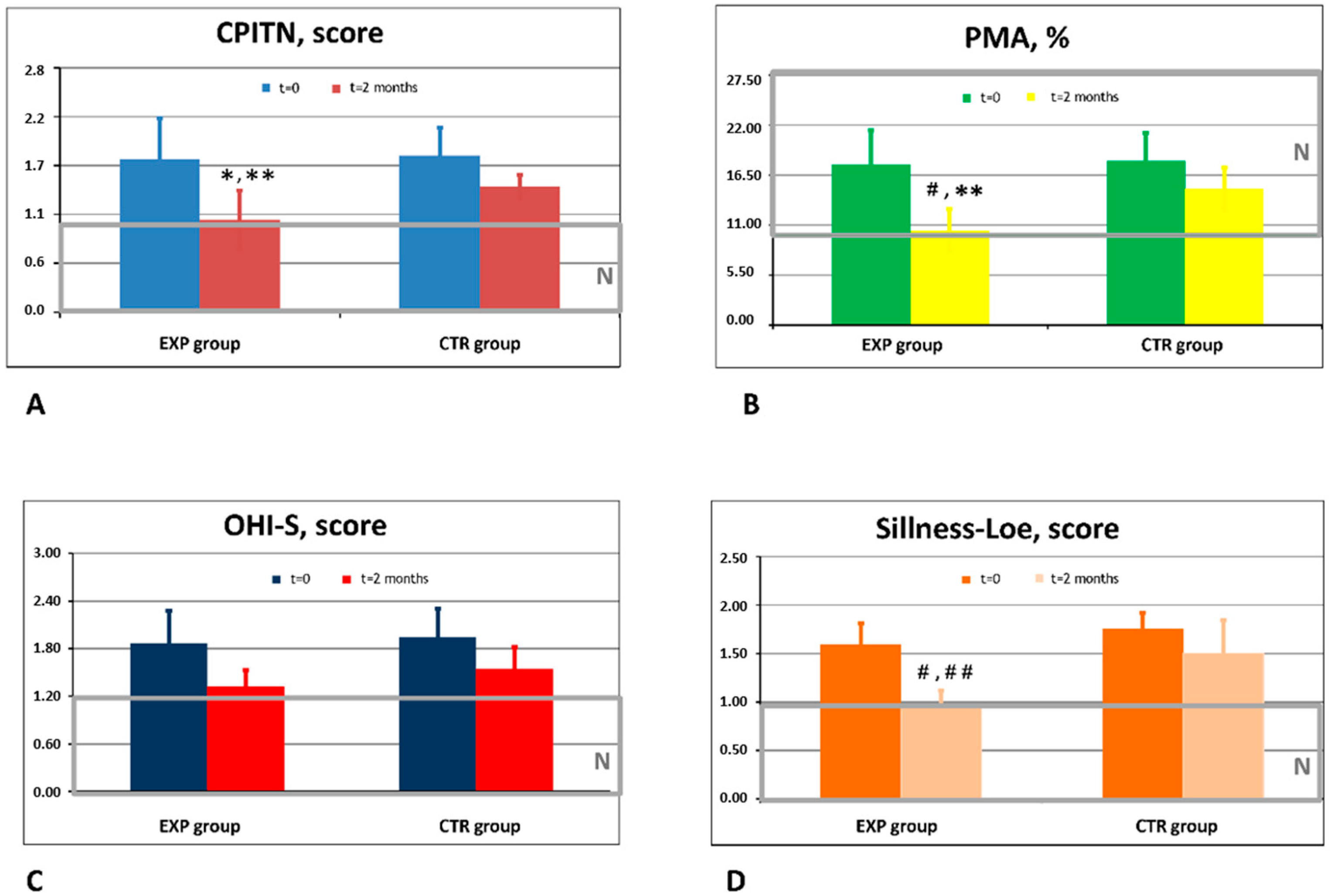
3.1. Clinical Efficacy of Experimental and Placebo Toothpastes
3.2. Effects of ETP and CTP on Pro- and Anti-Inflammatory Cytokines in GCF
3.3. Comparison of In Vivo Effects of ETP and CTP on Nitrite/Nitrate Content and Total Antioxidant Activity in GCF
3.4. Differential Count of Periodontal Pathogens in GCF: In Vivo Effects of ETP and CTP
3.5. The In Vitro Effects of CTP, ETP, and Its Active Herbal Ingredients on Bacterial Count and Bacterial Survival within Phagocytes (Intracellular Bacterial Killing)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marsh, P.D. The significance of maintaining the stability of the natural microflora of the mouth. Br. Dent. J. 1991, 171, 174–177. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T.; Hajishengallis, E.; Lambris, J.D. Neutrophil homeostasis and inflammation: Novel paradigms from studying periodontitis. J. Leukoc. Biol. 2015, 98, 539–548. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2014, 15, 30–44. [Google Scholar] [CrossRef]
- Sommer, M.E.; Dalia, R.A.; Nogueira, A.V.; Cirelli, J.A.; Vinolo, M.A.; Fachi, J.L.; Oliveira, C.A.; Andrade, T.A.; Mendonça, F.A.; Santamaria, M., Jr.; et al. Immune response mediated by Th1/IL-17/caspase-9 promotes evolution of periodontal disease. Arch. Oral Biol. 2019, 97, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kistler, J.O.; Booth, V.; Bradshaw, D.J.; Wade, W.G. Bacterial community development in experimental gingivitis. PLoS ONE 2013, 8, e71227. [Google Scholar] [CrossRef] [PubMed]
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Dosseva-Panova, V.T.; Popova, C.L.; Panov, V.E. Subgingival microbial profile and production of pro inflammatory cytokines in chronic periodontitis. Folia Med. 2014, 56, 152–160. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, W.; Hao, L.; Zhu, G.; Lu, Y.; Li, S.; Wang, L.; Li, Y.P. Ac45 silencing mediated by AAV-sh-Ac45-RNAi prevents both bone loss and inflammation caused by periodontitis. J. Clin. Periodontol. 2015, 42, 599–608. [Google Scholar] [CrossRef]
- Haffajee, A.D.; Socransky, S.S.; Patel, M.R.; Song, X. Microbial complexes in supragingival plaque. Oral Microbiol. Immunol. 2008, 23, 196–205. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Giuffrè, A.; Borisov, V.B.; Arese, M.; Sarti, P.; Forti, E. Cytochrome bd oxidase and bacterial tolerance to oxidative and nitrosative stress. Biochim. Biophys. Acta 2014, 1837, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, E.; Gerdes, K. Molecular mechanisms underlying bacterial persisters. Cell 2014, 157, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Douglas, C.W.; Naylor, K.; Phansopa, C.; Frey, A.M.; Farmilo, T.; Stafford, G.P. Physiological adaptations of key oral bacteria. Adv. Microb. Physiol. 2014, 65, 257–335. [Google Scholar] [PubMed]
- Heindorf, M.; Kadari, M.; Heider, C.; Skiebe, E.; Wilharm, G. Impact of Acinetobacter baumannii superoxide dismutase on motility, virulence, oxidative stress resistance and susceptibility to antibiotics. PLoS ONE 2014, 9, e101033. [Google Scholar] [CrossRef]
- Ezzo, P.J.; Culter, C.W. Microorganisms as risk indicators for periodontal disease. Periodontology 2003, 32, 24–35. [Google Scholar] [CrossRef]
- Nonnenmacher, C.; Dalpke, A.; Mutters, R.; Heeg, K. Quantitative detection of periodontopathogens by real-time PCR. J. Microbiol. Methods 2004, 59, 117–125. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, J.K.; Cho, J.Y.; Lee, J.M.; Hong, S.H. Quantification of subgingival bacterial pathogens at different stages of periodontal diseases. Curr. Microbiol. 2012, 65, 22–27. [Google Scholar] [CrossRef]
- Tuter, G.; Kurtis, B.; Serdar, M. Interleukin-1beta and thiobarbituric acid reactive substance (TBARS) levels after phase I periodontal therapy in patients with chronic periodontitis. J. Periodontol. 2001, 72, 883–888. [Google Scholar] [CrossRef]
- De Luca, C.; Kharaeva, Z.; Korkina, L. Is there a role for antioxidants in the prevention of infection-associated carcinogenesis and in the treatment of infection-driven tumours? Curr. Top. Med. Chem. 2015, 15, 120–135. [Google Scholar] [CrossRef]
- Painter, K.L.; Strange, E.; Parkhill, J.; Bamford, K.B.; Armstrong-James, D.; Edwards, A.M. Staphylococcus aureus adapts to oxidative stress by producing H2O2-resistant small-colony variants via the SOS response. Infect. Immun. 2015, 83, 1830–1844. [Google Scholar] [CrossRef]
- Prabhu, A.; Michalowicz, B.S.; Mathur, A. Detection of local and systemic cytokines in adult periodontitis. J. Periodontol. 1996, 67, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Slotwinska, S.M. Cytokines and periodontitis. Part I: Interleukin-1 and interleukin-1 receptor antagonist. Cent. Eur. J. Immunol. 2012, 37, 173–177. [Google Scholar]
- Liu, Z.; Liu, Y.; Song, Y.; Zhang, X.; Wang, S.; Wang, Z. Systemic oxidative stress biomarkers in chronic periodontitis: A meta-analysis. Dis. Markers 2014, 2014, 931083. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Damrongrungruang, T.; Hori, S.; Nouno, K.; Minagawa, K.; Sato, M.; Miyazaki, H. Effect of periodontal treatment on adipokines in type 2 diabetes. World J. Diabetes 2014, 5, 924–931. [Google Scholar] [CrossRef]
- Baltacioglu, E.; Kehribar, M.A.; Yuva, P.; Alver, A.; Atagün, O.S.; Karabulut, E.; Akalın, F.A. Total oxidant status and bone resorption biomarkers in serum and gingival crevicular fluid of patients with periodontitis. J. Periodontol. 2014, 85, 317–326. [Google Scholar] [CrossRef]
- Scott, D.A.; Krauss, J.L. Neutrophils in periodontal inflammation. Front. Oral Biol. 2012, 15, 56–83. [Google Scholar]
- Tenuta, L.M.; Cury, J.A. Fluoride: Its role in dentistry. Braz. Oral Res. 2010, 24, 9–17. [Google Scholar] [CrossRef]
- Randall, J.P.; Seow, W.K.; Walsh, L.J. Antibacterial activity of fluoride compounds and herbal toothpastes on Streptococcus mutants: An in vitro study. Aust. Dent. J. 2015, 60, 368–374. [Google Scholar] [CrossRef]
- American Academy of Pediatric Dentistry. Guideline on xylitol use in caries prevention. Pediatr. Dent. 2011, 33, 157–160. [Google Scholar]
- Chi, D.L.; Tut, O.; Milgrom, P. Cluster-randomized xylitol toothpaste trial for early childhood caries prevention. J. Dent. Child. 2014, 81, 27–32. [Google Scholar]
- Maden, E.A.; Allun, C.; Ozmen, B.; Bazak, P. Antimicrobial effect of toothpaste containing fluoride, xylitol, or xylitol-probiotic on salivary Streptococcus metans and Lactobacillus in children. Niger. J. Clin. Pract. 2018, 21, 134–138. [Google Scholar] [PubMed]
- Ullah, R.; Zafar, M.S.; Shahani, N. Potential fluoride toxicity from oral medicaments: A review. Iran. J. Basic Med. Sci. 2017, 20, 841–848. [Google Scholar] [PubMed]
- Zuo, H.; Chen, L.; Kong, M.; Qui, L.; Lu, P.; Wu, P.; Yang, Y.; Chen, K. Toxic effects of fluoride on organisms. Life Sci. 2018, 198, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.; Lamont, T. Triclosan/copolymer containing toothpastes for oral health. Cochrane Database Syst. Rev. 2013, 12, CD010514. [Google Scholar] [CrossRef]
- Al Habashneh, R.; Farasin, R.; Khader, Y. The effect of a triclosan/copolymer/fluoride toothpaste on plaque formation, gingivitis, and dentin hypersensitivity: A single-blinded randomized clinical study. Quintessence Int. 2017, 48, 123–130. [Google Scholar] [PubMed]
- Muller, H.P.; Barrieshi-Nusair, K.M.; Kononen, E.; Yang, M. Effect of triclosan/copolymer-containing toothpaste on the association between plaque and gingival bleeding: A randomized controlled clinical trial. J. Clin. Periodontol. 2006, 33, 811–818. [Google Scholar] [CrossRef]
- Sandborgh-Englund, G.; Adolfsson-Erici, M.; Odham, G.; Ekstrand, J. Pharmacokinetics of triclosan following oral ingestion in humans. J. Toxicol. Environ. Health A 2006, 69, 1861–1873. [Google Scholar] [CrossRef]
- Weatherly, L.M.; Gosse, J.A. Triclosan exposure, transformation, and human health effects. J. Toxicol. Environ. Health B Crit. Rev. 2017, 20, 447–469. [Google Scholar] [CrossRef]
- Weatherly, L.M.; Shim, J.; Hashmi, H.N.; Kennedy, R.H.; Hess, S.T.; Gosse, J.A. Antimicrobial agent triclosan is a proton ionophore uncoupler of mitochondria in living rat and human mast cells and in primary human keratinocytes. J. Appl. Toxicol. 2016, 36, 777–789. [Google Scholar] [CrossRef]
- Ruszkiewicz, J.A.; Li, S.; Rodriguez, M.B.; Aschner, M. Is triclosan a neurotoxic agent? J. Toxicol. Environ. Health B Crit. Rev. 2017, 20, 104–117. [Google Scholar] [CrossRef]
- Kharaeva, Z.F.; Zhanimova, L.R.; Mustafaev, M.S.; De Luca, C.; Mayer, W.; Thai, J.C.S.; Tuan, R.T.S.; Korkina, L.G. Effects of standardised fermented papaya gel on clinical symptoms, inflammatory cytokines, and nitric oxide metabolites in patients with chronic periodontitis: An open randomised clinical study. Mediat. Inflamm. 2016, 2016, 9379840. [Google Scholar] [CrossRef] [PubMed]
- Geidel, A.; Kruger, M.; Schrodl, W.; Jentsch, H. Control of plaque and gingivitis by an herbal toothpaste: A randomised controlled study. Oral Health Prev. Dent. 2017, 15, 407–413. [Google Scholar] [PubMed]
- Tekko, I.A.; Bohner, M.C.; Bowen, R.D.; Williams, A.C. Permeation of bioactive constituents from Arnica montana preparations through human skin in-vitro. J. Pharm. Pharmacol. 2006, 58, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.E. Martindale: The Extra Pharmacopoeia, 34th ed.; Sweetman, S.C., Ed.; The Pharmaceutical Press: London, UK, 2005; pp. 1656–1657. [Google Scholar]
- Korkina, L.G. Phenylpropanoids as naturally occurring antioxidants: From plant defence to human health. Cell. Mol. Biol. (Noisy-le-grand) 2007, 53, 15–25. [Google Scholar]
- Willuhn, G. Arnica flowers, pharmacology, toxicology and analysis of sesquiterpene lactones, their main active substances. In Phytomedicine in Europe, Chemistry and Biological Activity; Lawson, L.D., Bauer, R., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1998; Volume 691, pp. 118–132. [Google Scholar]
- Baumann, L.S. Less-known botanical cosmeceuticals. Dermatol. Ther. 2007, 20, 330–342. [Google Scholar] [CrossRef]
- Lee, K.G.; Shibamoto, T. Determination of antioxidant potential of volatile extracts isolated from various herbs and spices. J. Agric. Food Chem. 2002, 50, 4947–4952. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilzadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- European Medicines Agency. Community Herbal Monograph on Salvia officinalis L., Folium; European Medicines Agency: London, UK, 2009. [Google Scholar]
- Lima, C.F.; Valentao, P.C.R.; Andrade, P.B.; Seabra, R.M.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Water and methanolic extracts of Salvia officinalis protect HepG2 cells from t-BHP induced oxidative damage. Chem. Biol. Interact. 2007, 167, 107–115. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Rangarajan, M.; Shao, Y.; LaVoie, J.; Huang, T.C.; Ho, C.T. Antioxidative phenolic compounds from Sage (Salvia officinalis). J. Agric. Food Chem. 1998, 46, 4869–4873. [Google Scholar] [CrossRef]
- Mitic-Culafic, D.; Vukovic-Gacic, B.; Knezevic-Vukcevic, J.; Stankovic, S.; Simic, D. Comparative study on the antibacterial activity of volatiles from sage (Salvia officinalis L.). Arch. Biol. Sci. 2005, 57, 173–178. [Google Scholar] [CrossRef]
- Safarabadi, M.; Ghaznavi-Rad, E.; Pakniyat, A.; Rezaie, K.; Jadidi, A. Comparing the effects of Echinacea and chlorhexidine mouthwash on the microbial flora of intubated patients admitted to the intensive care unit. Iran. J. Nurs. Midwifery Res. 2017, 22, 481–485. [Google Scholar] [PubMed]
- Fu, A.; Wang, Y.; Wu, Y.; Chen, H.; Zheng, S.; Li, Y.; Xu, X.; Li, W. Echinacea purpurea extract polarizes M1 macrophages in murine bone marrow-derived macrophages through the activation of JNK. Cell Biochem. 2017, 118, 2664–2671. [Google Scholar] [CrossRef] [PubMed]
- Pires, C.; Martins, N.; Carvalho, A.M.; Barros, L.; Ferreira, I.C. Phytopharmacological preparations as predictors of plant bioactivity: A particular approach to Echinacea purpurea (L.) Moench antioxidant properties. Nutrition 2016, 32, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.Y.; Chung, K.S.; Jin, J.S.; Bang, K.S.; Eom, Y.J.; Hong, C.H.; Nugroho, A.; Park, H.J.; An, H.J. Effect of chicoric acid on mast cell-mediated allergic inflammation in vitro and in vivo. J. Nat. Prod. 2015, 78, 2956–2962. [Google Scholar] [CrossRef]
- Barnett, M.L. Suitability of gingival indices for use in therapeutic trials. Is bleeding a sine qua non? J. Clin. Periodontol. 1996, 23, 582–586. [Google Scholar] [CrossRef]
- Marks, R.G.; Magnusson, I.; Taylor, M.; Clouser, B.; Maruniak, J.; Clark, W.B. Evaluation of reliability and reproducibility of dental indices. J. Clin. Periodontol. 1993, 20, 54–58. [Google Scholar] [CrossRef]
- Missiakas, D.M.; Schneewind, O. Growth and Laboratory Maintenance of Staphylococcus aureus. Curr. Protoc. Microbiol. 2013, 28, 9C.1.1–9C.1.9. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Meth. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Buommino, E.; Scognamiglio, M.; Donnarumma, G.; Fiorentino, A.; D’Abrosca, B. Recent advances in natural product-based anti-biofilm approaches to control infections. Mini Rev. Med. Chem. 2014, 14, 1169–1182. [Google Scholar] [CrossRef]
- Haught, J.C.; Xie, S.; Circello, B.; Tansky, C.S.; Khambe, D.; Sun, Y.; Lin, Y.; Sreekrishna, K.; Klukowska, M.; Higgins, T.; et al. Lipopolysaccharide and lipoteichoic acid binding by antimicrobials used in oral care formulations. Am. J. Dent. 2016, 29, 328–332. [Google Scholar]
- Dickinson, S.E.; Wondrak, G.T. TLR4-directed molecular strategies targeting skin photodamage and carcinogenesis. Curr. Med. Chem. 2018, 25, 5487–5502. [Google Scholar] [CrossRef]
- Imlay, J.A. Diagnosing oxidative stress in bacteria: Not as easy as you might think. Curr. Opin. Microbiol. 2015, 24, 124–131. [Google Scholar] [CrossRef]
- Imlay, J.A. Where in the world do bacteria experience oxidative stress? Environ. Microbiol. 2019, 21, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, K.; Coutts, G.; Jonsson, I.M. Catalase (KatA) and alkyl hydroperoxide reductase (AhpC) have compensatory roles in peroxide stress resistance and are required for survival, persistence, and nasal colonization in Staphylococcus aureus. J. Bacteriol. 2007, 189, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Huseby, D.L.; Brandis, G.; Hughes, D. Alternative evolutionary pathways for drug-resistant small colony variant mutants in staphylococcus aureus. MBio 2017, 8, e00358-17. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Zheng, C. OxyR activation in Porphyromonas gingivalis in response to a hemin-limited environment. Infect. Immun. 2012, 80, 3471–3480. [Google Scholar] [CrossRef]
- Gaupp, R.; Ledala, N.; Somerville, G.A. Staphylococcal response to oxidative stress. Front. Cell. Infect. Microbiol. 2012, 2. [Google Scholar] [CrossRef]
- Giuffrè, A.; Borisov, V.B.; Mastronicola, D.; Sarti, P.; Forte, E. Cytochrome bd oxidase and nitric oxide: From reaction mechanisms to bacterial physiology. FEBS Lett. 2012, 586, 622–629. [Google Scholar] [CrossRef]
- Harms, A.; Maisonneuve, E.; Gerdes, K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 2016, 354, aaf4268. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Dawson, D., 3rd; Emecen-Huja, P.; Nagarajan, R.; Howard, K.; Grady, M.E.; Thompson, K.; Peyyala, R.; Al-Attar, A.; Lethbridge, K.; et al. The periodontal war: Microbes and immunity. Periodontology 2000 2017, 75, 52–115. [Google Scholar] [CrossRef]
- Proctor, R.A.; Kriegeskorte, A.; Kahl, B.C.; Becker, K.; Löffler, B.; Peters, G. Staphylococcus aureus Small Colony Variants (SCVs): A road map for the metabolic pathways involved in persistent infections. Front. Cell. Infect. Microbiol. 2014, 4, 99. [Google Scholar] [CrossRef] [PubMed]
- Henry, L.G.; McKenzie, R.M.; Robles, A.; Fletcher, H.M. Oxidative stress resistance in Porphyromonas gingivalis. Future Microbiol. 2012, 7, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Alfano, J.R.; Becker, D.F. Proline metabolism increases katG expression and oxidative stress resistance in Escherichia coli. J. Bacteriol. 2014, 197, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. Transcription factors that defend bacteria against reactive oxygen species. Ann. Rev. Microbiol. 2015, 69, 93–108. [Google Scholar] [CrossRef]
- Fernandez, E.; Sanchez, M.D.C.; Llama-Palacios, A.; Sanz, M.; Herrera, D. Antibacterial effects of toothpastes evaluated in an in vitro biofilm model. Oral Health Prev. Dent. 2017, 15, 251–257. [Google Scholar]
- Garcia-Godoy, C.; Rothrock, J.; Gurich, N.; Anastasia, M.K.; Gerlach, R.W. Post-prophylaxis gingivitis prevention with two-step stannous fluoride dentifrice plus whitening gel sequence or chlorhexidine gluconate mouth rinse. Am. J. Dent. 2018, 31, 18A–23A. [Google Scholar]
- de Oliveira, J.R.; de Jesus, D.; Figueira, L.W.; de Oliveira, F.E.; Pacheco Soares, C.; Camargo, S.E.; Jorge, A.O.; de Oliveira, L.D. Biological activities of Rosmarinus officinalis L. (rosemary) extract as analyzed in microorganisms and cells. Exp. Biol. Med. 2017, 242, 625–634. [Google Scholar] [CrossRef]
- Elgamily, H.; Moussa, A.; Elboraey, A.; El-Sayed, H.; Al-Moghazy, M.; Abdalla, A. Microbiological assessment of Moringa oleifera extracts and its incorporation in novel dental remedies against some oral pathogens. Open Access Maced. J. Med. Sci. 2016, 4, 585–590. [Google Scholar] [CrossRef]
- Stoeken, J.E.; Paraskevas, R.; van der Weijen, G.A. The long-term effect of a mouth rinse containing essential oils on dental plaque and gingivitis: A systematic review. J. Periodontol. 2007, 78, 1218–1228. [Google Scholar] [CrossRef]




| Group | Patients | Age, Years | Sex | Smokers | Diagnosis | ||
|---|---|---|---|---|---|---|---|
| M | F | Gingivitis | Initial PD | ||||
| Experimental (conventional treatment + ETP twice a day for 60 days) | 35 | 35–55 | 12 | 23 | 5 | 8 | 27 |
| Control (conventional treatment + CTP twice a day for 60 days) | 15 | 36–55 | 7 | 8 | 3 | 3 | 12 |
| Bacteria, Risk Grade | Experimental Group (n = 15) | Control Group (n = 5) | ||
|---|---|---|---|---|
| Before (Cells/mL) | After 2 Months (Cells/mL) | Before (Cells/mL) | After 2 Months (Cells/mL) | |
| Aggregatibacter actinomycetem comitans, high risk | 7.9 × 105 2.2 × 106 1.3 × 107 1.9 × 105 1.3 × 105 1.2 × 105 | 2.8 × 104 2.5 × 104 1.7 × 106 0 1.1 × 105 0 | 1.6 × 103 2.4 × 104 | 0 6.5 × 104 |
| Porphyromonas gingivalis, high risk | 2.8 × 106 1.2 × 106 1.9 × 105 1.2 × 106 2.7 × 107 | 0 1.4 × 103 0 0 3.3 × 106 | 2.9 × 106 2.0 × 104 1.6 × 105 8.5 × 103 | 2.1 × 104 1.0 × 103 4.8 × 104 0 |
| Porphyromonas endodontalis, moderate risk | 7.8 × 103 1.2 × 104 1.2 × 104 8.4 × 105 6.9 × 105 1.2 × 104 1.2 × 104 1.4 × 104 1.8 × 104 3.7 × 106 | 0 0 0 1.4 × 105 0 0 0 0 0 9.8 × 103 | 1.2 × 104 1.2 × 104 | 0 0 |
| Treponema denticola, high risk | 3.3 × 105 1.8 × 106 3.3 × 105 7.9 × 105 3.8 × 105 3.3 × 105 1.8 × 105 1.3 × 105 2.3 × 107 | 0 3.7 × 103 0 0 1.1 × 103 0 0 1.1 × 102 2.7 × 106 | 5.9 × 105 2.0 × 103 3.3 × 105 3.3 × 105 | 1.9 × 105 0 0 0 |
| Tanerella forsythia, high risk | 1.2 × 106 2.6 × 105 3.2 × 105 3.7 × 104 5.8 × 105 4.8 × 106 5.4 × 103 6.0 × 105 7.5 × 104 7.5 × 104 3.2 × 107 | 0 1.4 × 103 0 0 3.5 × 105 2.2 × 105 0 3.4 × 104 0 0 6.6 × 104 | 1.9 × 106 1.3 × 106 4.6 × 104 | 0 8.7 × 105 5.9 × 103 |
| Prevotella intermedia, moderate risk | 5.8 × 105 4.5 × 105 5.8 × 105 4.0 × 103 1.2 × 105 1.9 × 107 | 0 0 0 0 6.4 × 104 0 | 1.4 × 105 | 4.5 × 106 |
| Fusobacterium nucleatum, moderate risk | 6.6 × 105 1.0 × 103 1.4 × 105 1.2 × 105 1.4 × 105 1.6 × 105 4.2 × 104 6.4 × 105 8.3 × 106 6.1 × 105 2.1 × 105 3.6 × 105 6.6 × 106 4.6 × 106 6.2 × 105 | 1.6 × 102 0 0 1.5 × 102 2.4 × 102 0 0 3.6 × 104 1.6 × 105 1.4 × 104 1.4 × 105 0 2.6 × 102 2.1 × 102 0 | 4.4 × 105 1.7 × 105 7.9 × 104 8.0 × 104 5.3 × 103 | 0 3.5 × 104 5.9 × 103 3.2 × 105 5.0 × 103 |
 | |||
| Sample | Direct Anti-Bacterial Effect | Bacterial Catalase Activity after Pre-Treatment with Toothpaste/Herbal Extract (% of Inhibition) | Number of Bacteria Survived in Phagocytes after Pre-Treatment, Cells/mL |
|---|---|---|---|
| Negative control (no pre-treatment) | 5 × 106 | 5.1 (0%) | 5 × 106 |
| Control toothpaste (CTP) | 105 | 4.5 (11.8%) | 5 × 105 |
| Experimental toothpaste (ETP) | 5 × 103 | 3.1 (39.2%) | 5 × 102 |
| Chamomile leaves extract | 106 | 2.7 (47.0%) | 104 |
| Salvia leaves extract | 106 | 1.9 (63.7%) | 102 |
| Arnica flower extract | 106 | 2.5 (50.9%) | 103 |
| Echinacea flower extract | 106 | 2.8 (54.9%) | 103 |
| Strain Number | Source of Isolation | Resistance to 5–10 Antibiotics | Catalase Activity (Units/2 × 107 Bacteria) |
|---|---|---|---|
| 1523 | Throat, tonsils (chronic tonsillitis) | +++ | 5.1 |
| 1546 | Oral epithelia (stomatitis) | − | 2.1 |
| 1549 | Throat, tonsils (chronic tonsillitis) | ++ | 3.7 |
| 1555 | Oral epithelia (stomatitis) | + | 2.3 |
| 1561 | Nasal sinuses (sinusitis) | − | 2.2 |
| 1612 | Oral epithelia (stomatitis) | − | 2.2 |
| 1620 | Throat, tonsils (chronic tonsillitis) | + | 2.6 |
| 1643 | Throat, tonsils (chronic tonsillitis) | − | 2.2 |
| 1670 | Oral epithelia (stomatitis) | − | 2.1 |
| 1780 | Throat, tonsils (chronic tonsillitis) | − | 2.0 |
| Sample | Initial Catalase Activity (U/mL) in S. aureus Strains (Mean ± S.D.) | Catalase Activity (U/mL) in S. aureus Strains after Pre-Treatment with Toothpastes or Active Herbal Ingredients |
|---|---|---|
| Control toothpaste (CTP) | 2.38 ± 0.90 | 2.27 ± 0.93 |
| Experimental toothpaste (ETP) | 2.38 ± 0.90 | 1.52 ± 0.13 * |
| Chamomile leaves extract | 2.38 ± 0.90 | 1.81 ± 0.21 |
| Salvia leaves extract | 2.38 ± 0.90 | 1.28 ± 0.71 * |
| Arnica flower extract | 2.38 ± 0.90 | 1.68 ± 0.70 |
| Echinacea flower extract | 2.38 ± 0.90 | 1.27 ± 0.71 * |
| Mixture of extracts | 2.38 ± 0.90 | 1.51 ± 0.42 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharaeva, Z.F.; Mustafaev, M.S.; Khazhmetov, A.V.; Gazaev, I.H.; Blieva, L.Z.; Steiner, L.; Mayer, W.; De Luca, C.; Korkina, L.G. Anti-Bacterial and Anti-Inflammatory Effects of Toothpaste with Swiss Medicinal Herbs towards Patients Suffering from Gingivitis and Initial Stage of Periodontitis: From Clinical Efficacy to Mechanisms. Dent. J. 2020, 8, 10. https://doi.org/10.3390/dj8010010
Kharaeva ZF, Mustafaev MS, Khazhmetov AV, Gazaev IH, Blieva LZ, Steiner L, Mayer W, De Luca C, Korkina LG. Anti-Bacterial and Anti-Inflammatory Effects of Toothpaste with Swiss Medicinal Herbs towards Patients Suffering from Gingivitis and Initial Stage of Periodontitis: From Clinical Efficacy to Mechanisms. Dentistry Journal. 2020; 8(1):10. https://doi.org/10.3390/dj8010010
Chicago/Turabian StyleKharaeva, Zaira F., Magomet Sh. Mustafaev, Anzor V. Khazhmetov, Ismail H. Gazaev, Larisa Z. Blieva, Lukas Steiner, Wolfgang Mayer, Chiara De Luca, and Liudmila G. Korkina. 2020. "Anti-Bacterial and Anti-Inflammatory Effects of Toothpaste with Swiss Medicinal Herbs towards Patients Suffering from Gingivitis and Initial Stage of Periodontitis: From Clinical Efficacy to Mechanisms" Dentistry Journal 8, no. 1: 10. https://doi.org/10.3390/dj8010010
APA StyleKharaeva, Z. F., Mustafaev, M. S., Khazhmetov, A. V., Gazaev, I. H., Blieva, L. Z., Steiner, L., Mayer, W., De Luca, C., & Korkina, L. G. (2020). Anti-Bacterial and Anti-Inflammatory Effects of Toothpaste with Swiss Medicinal Herbs towards Patients Suffering from Gingivitis and Initial Stage of Periodontitis: From Clinical Efficacy to Mechanisms. Dentistry Journal, 8(1), 10. https://doi.org/10.3390/dj8010010





