A Preshaped Titanium Mesh for Guided Bone Regeneration with an Equine-Derived Bone Graft in a Posterior Mandibular Bone Defect: A Case Report
Abstract
:1. Introduction
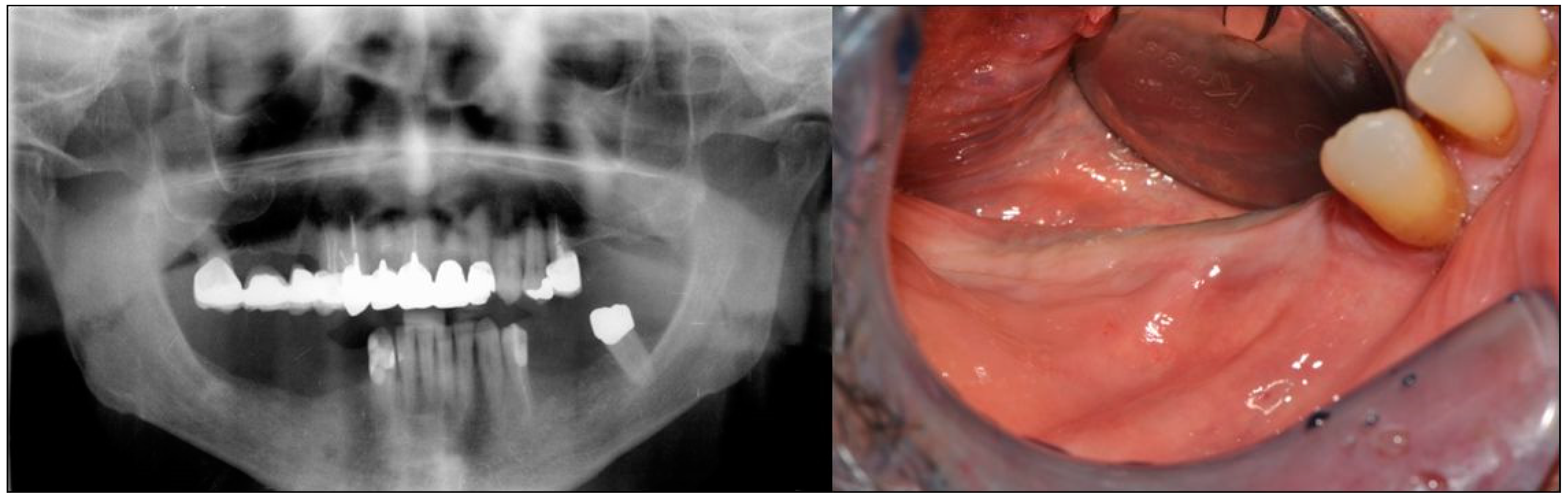
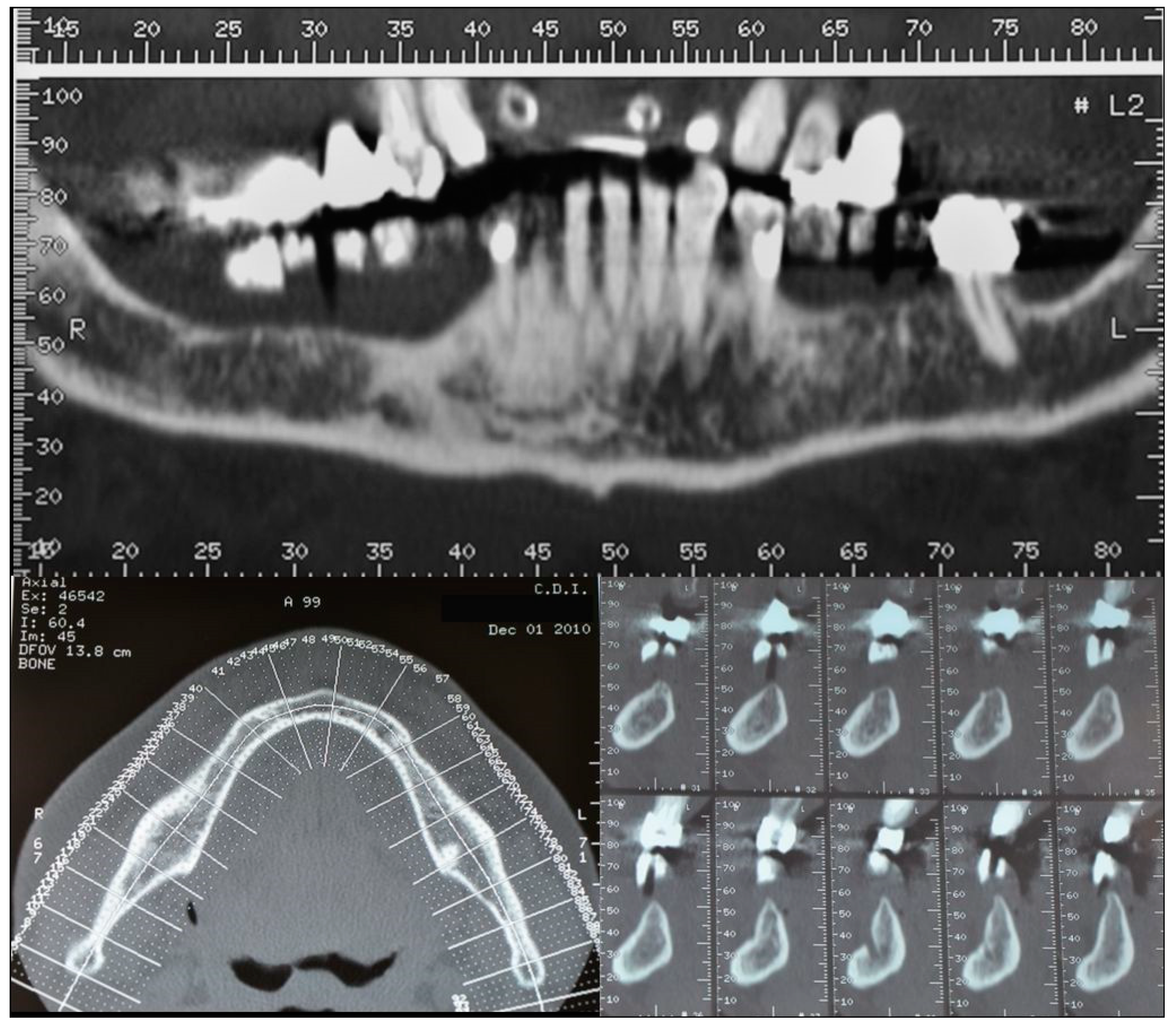
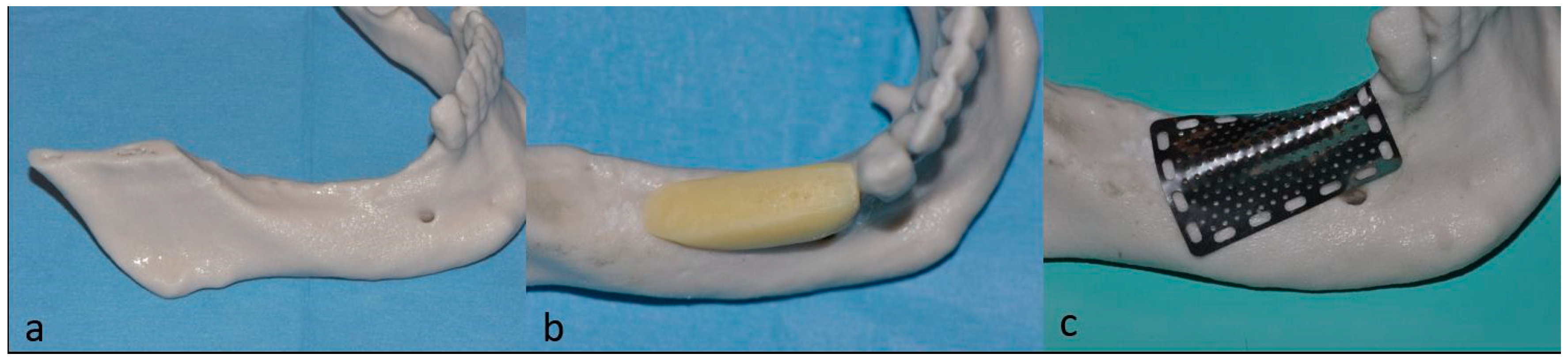
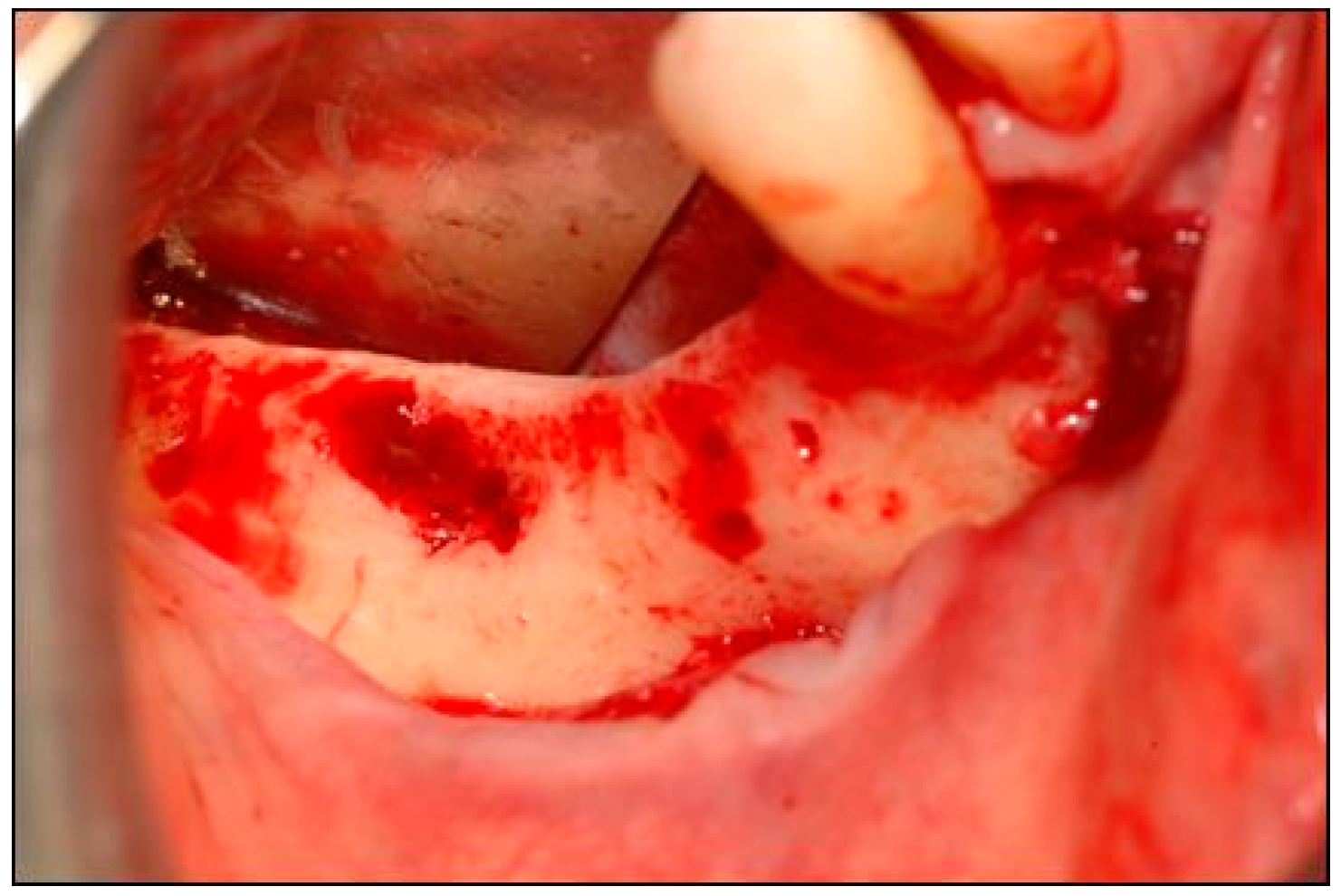
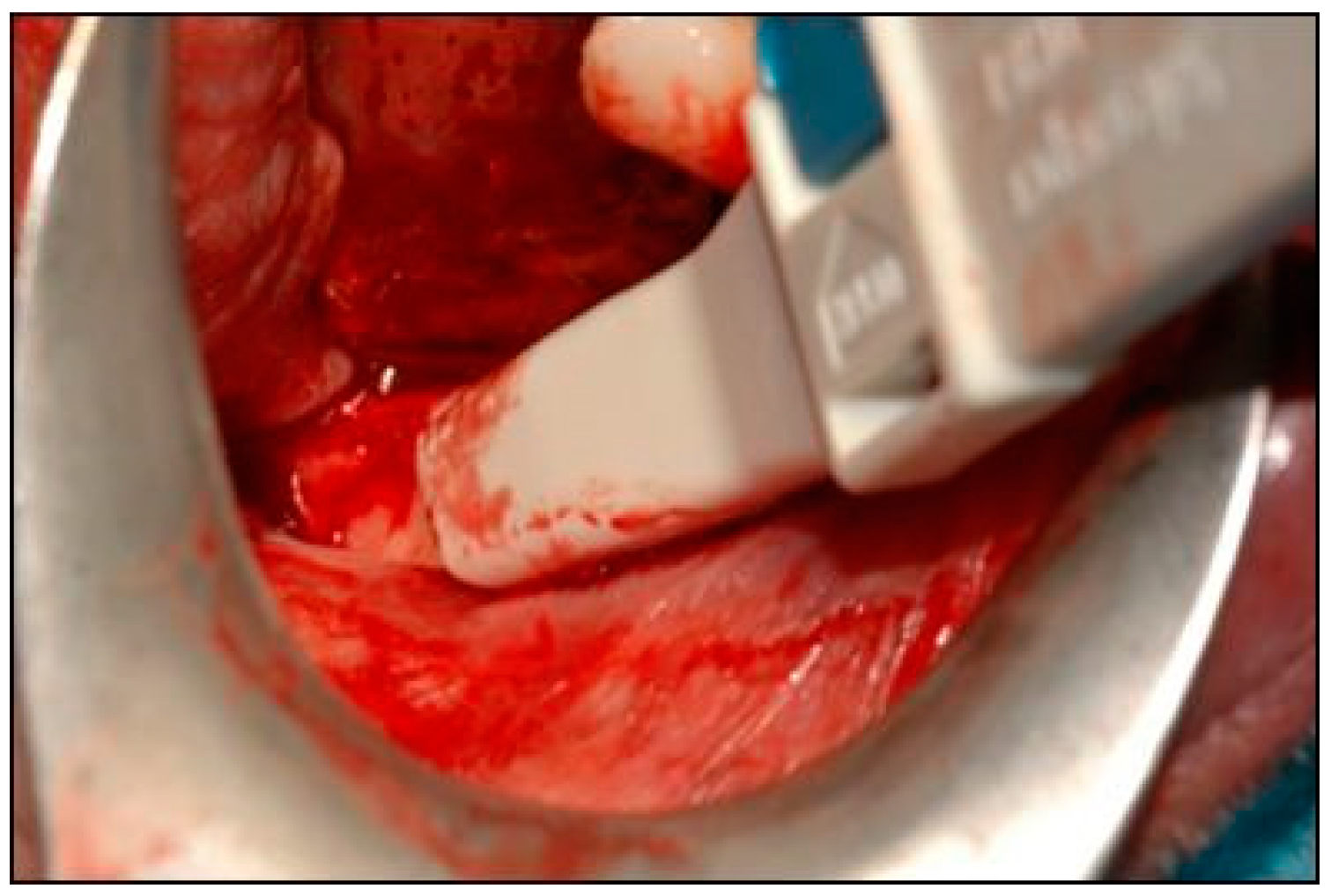
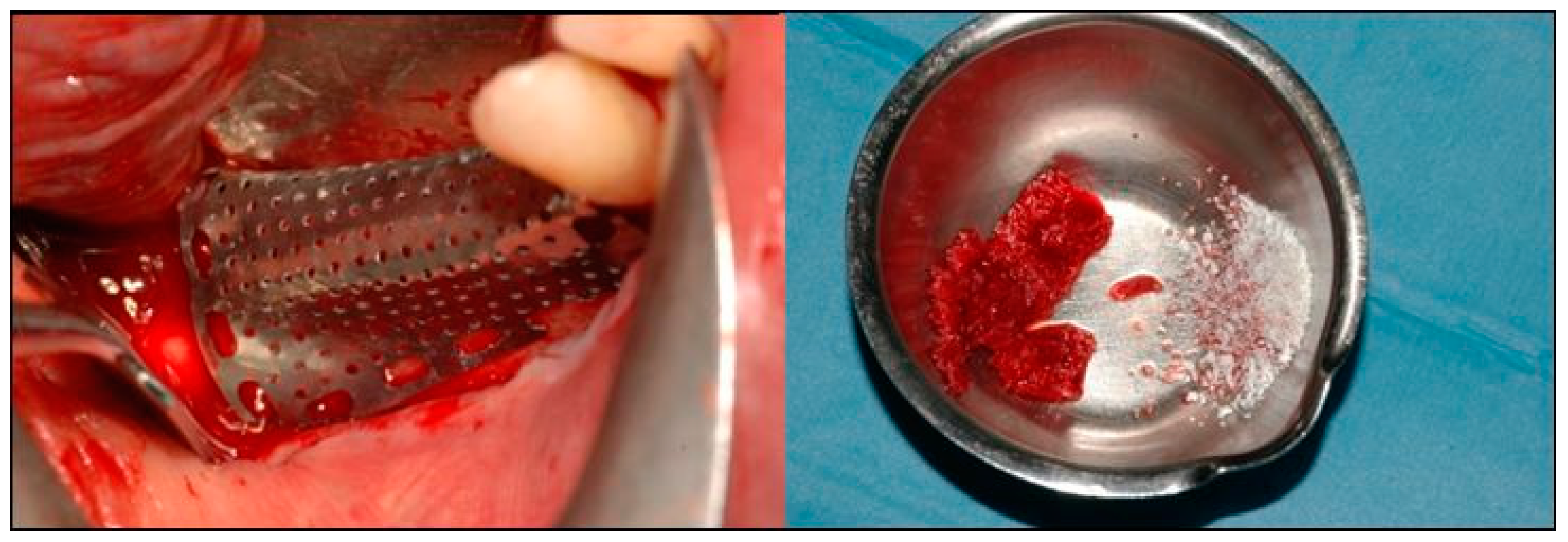
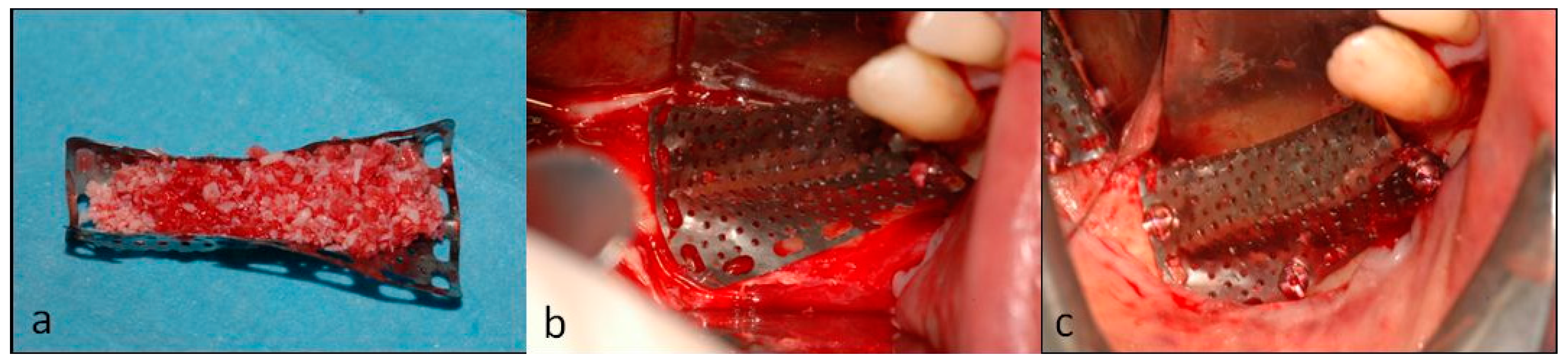
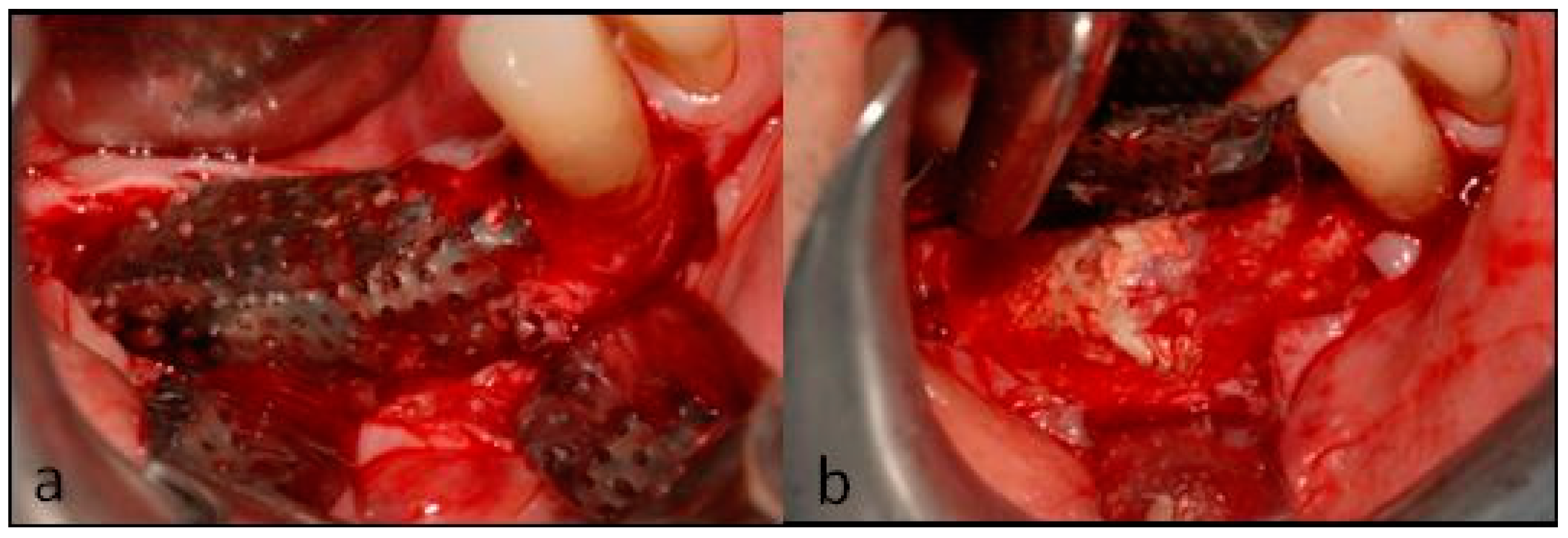
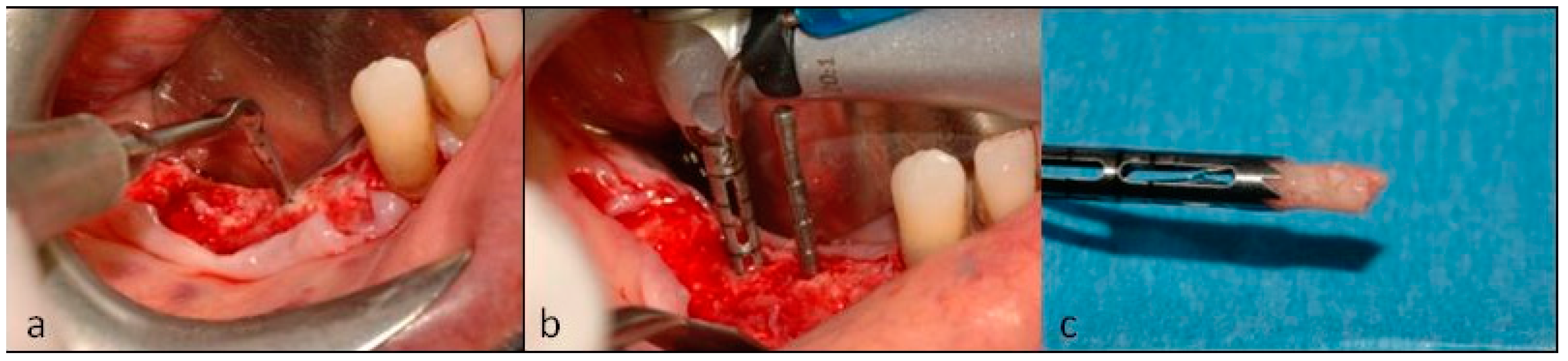
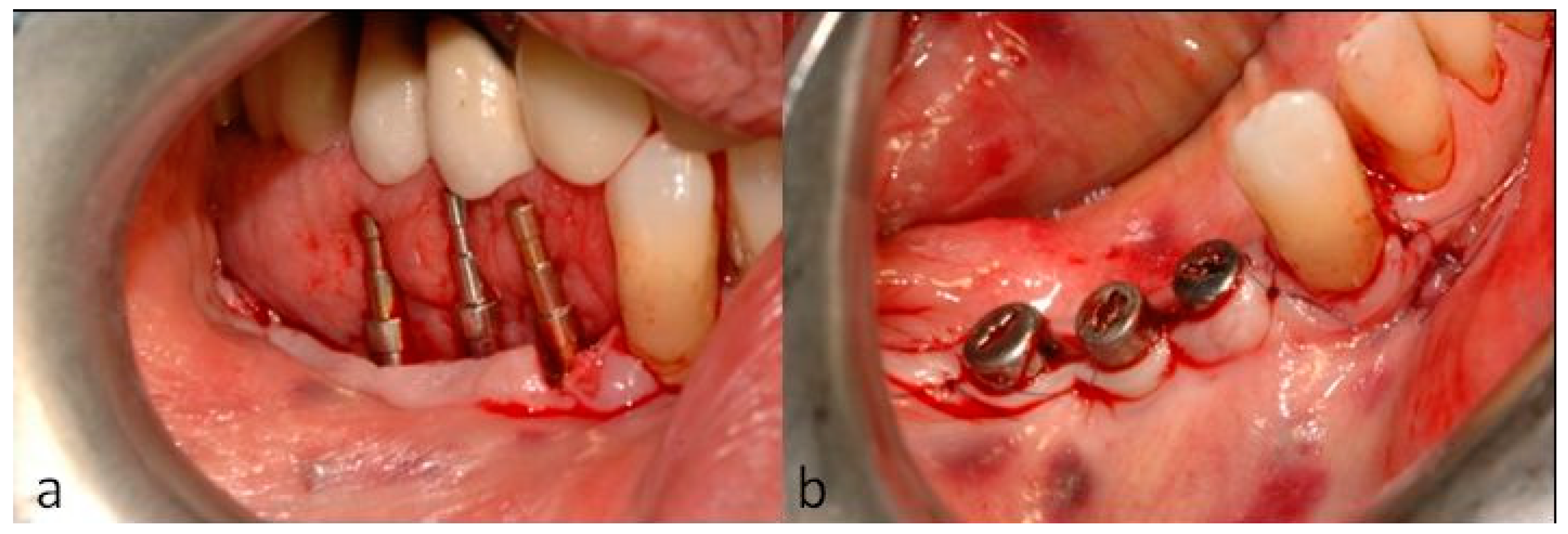
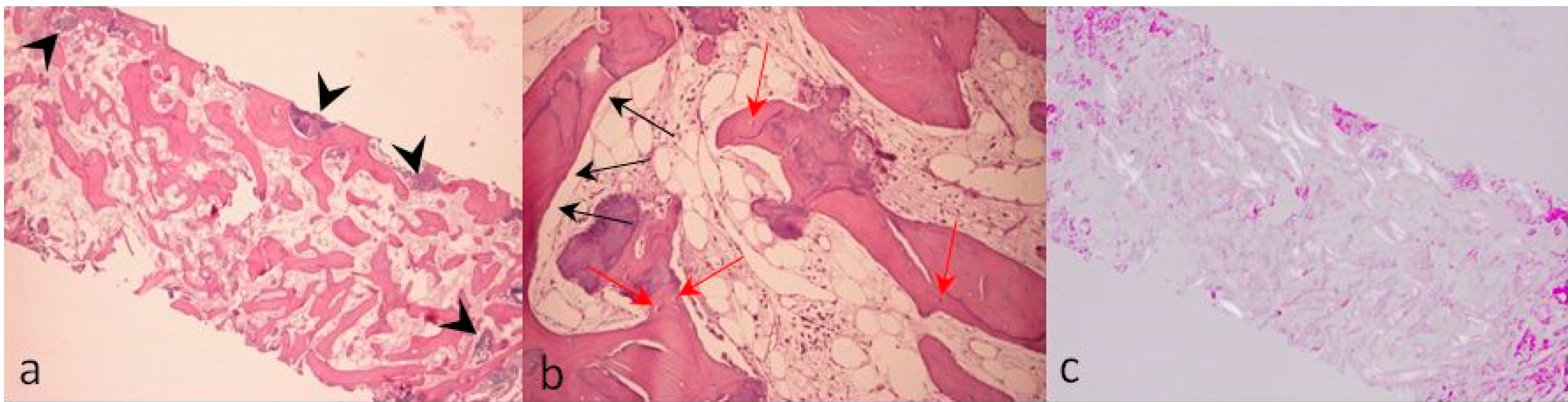
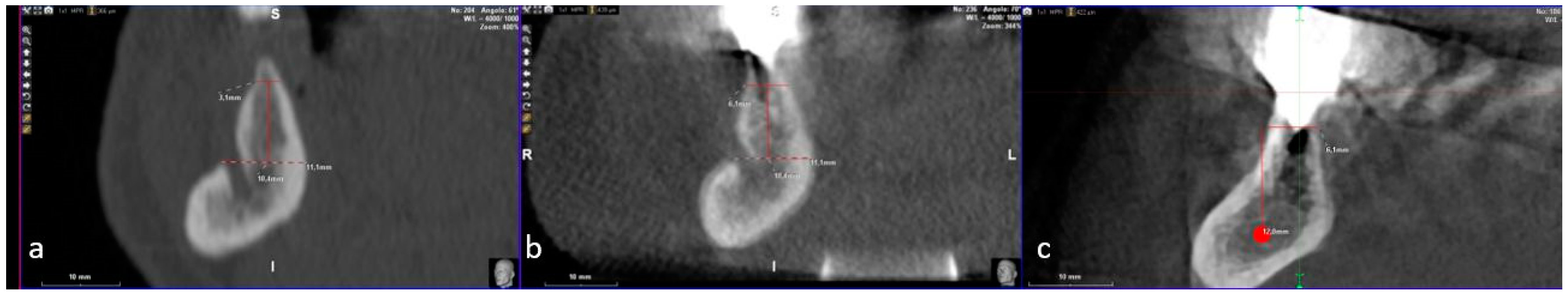
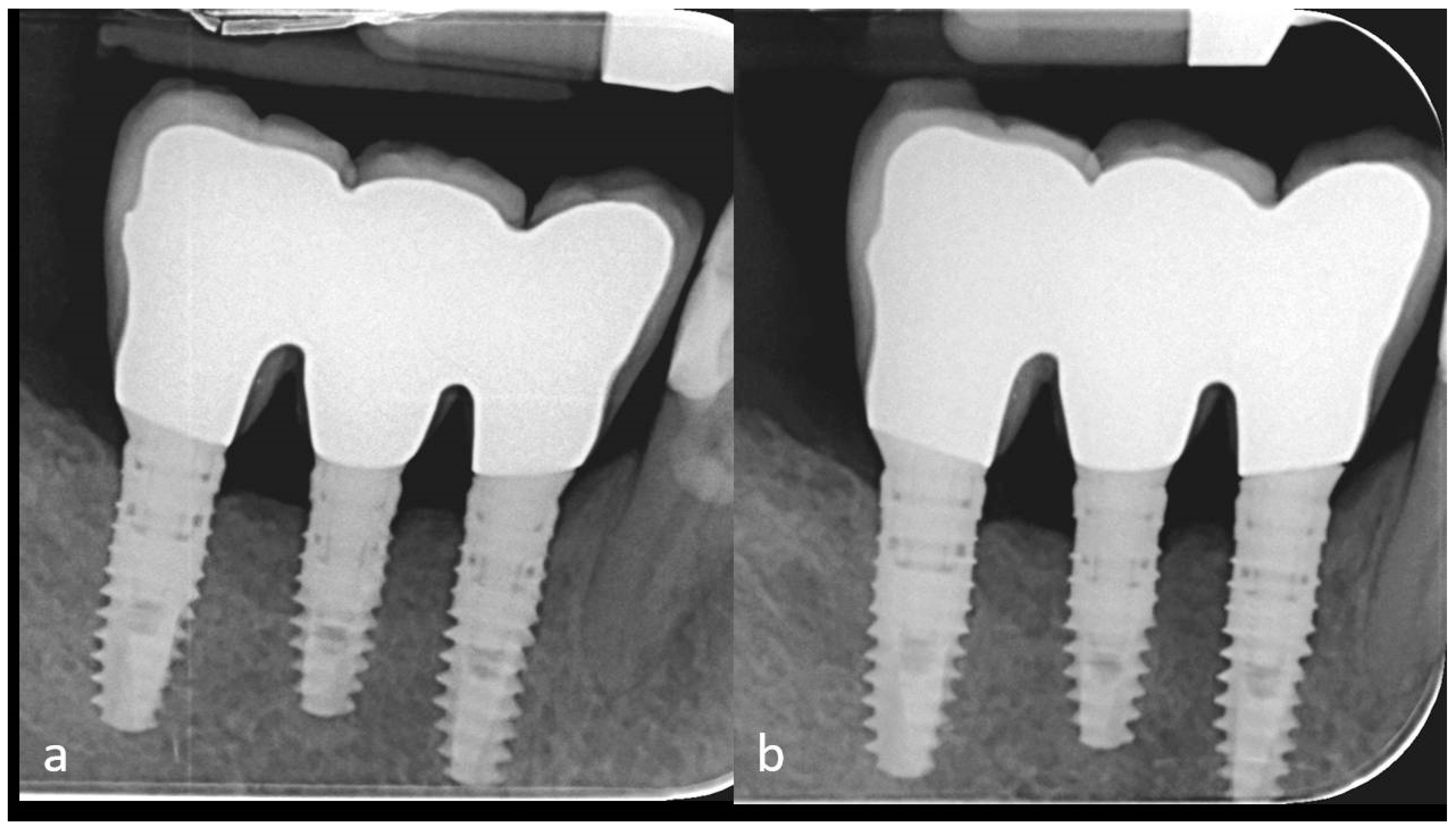
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chappuis, V.; Rahman, L.; Buser, R.; Janner, S.F.M.; Belser, U.C.; Buser, D. Effectiveness of Contour Augmentation with Guided Bone Regeneration: 10-Year Results. J. Dent. Res. 2018, 97, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Benic, G.I.; Hämmerle, C.H. Horizontal bone augmentation by means of guided bone regeneration. Periodontol. 2000 2014, 66, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, N.U.; Schärer, P.; Marinello, C.P. Long-term results of implants treated with guided bone regeneration: A 5-year prospective study. Int. J. Oral Maxillofac. Implant. 2001, 16, 355–366. [Google Scholar]
- Beitlitum, I.; Artzi, Z.; Nemcovsky, C.E. Clinical evaluation of particulate allogeneic with and without autogenous bone grafts and resorbable collagen membranes for bone augmentation of atrophic alveolar ridges. Clin. Oral Implant. Res. 2010, 21, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lee, K.W.; Oh, T.J.; Misch, C.E.; Shotwell, J.; Wang, H.L. Effect of absorbable membranes on sandwich bone augmentation. Clin. Oral Implant. Res. 2008, 19, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Mordenfeld, A.; Johansson, C.B.; Albrektsson, T.; Hallman, M. A randomized and controlled clinical trial of two different compositions of deproteinized bovine bone and autogenous bone used for lateral ridge augmentation. Clin. Oral Implant. Res. 2014, 25, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Van Assche, N.; Michels, S.; Naert, I.; Quirynen, M. Randomized controlled trial to compare two bone substitutes in the treatment of bony dehiscences. Clin. Implant Dent. Relat. Res. 2013, 15, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Kim, S.G.; Lim, S.C.; Lee, H.J.; Yun, P.Y. A clinical study on bone formation using a demineralized bone matrix and resorbable membrane. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2010, 109, e6–e11. [Google Scholar] [CrossRef]
- Sisti, A.; Canullo, L.; Mottola, M.P.; Covani, U.; Barone, A.; Botticelli, D. Clinical evaluation of a ridge augmentation procedure for the severely resorbed alveolar socket: Multicenter randomized controlled trial, preliminary results. Clin. Oral Implant. Res. 2012, 23, 526–535. [Google Scholar] [CrossRef]
- Hammerle, C.H.; Jung, R.E. Bone augmentation by means of barrier membranes. Periodontol. 2000 2003, 33, 36–53. [Google Scholar] [CrossRef] [Green Version]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Soldatos, N.K.; Stylianou, P.; Koidou, V.P.; Angelov, N.; Yukna, R.; Romanos, G.E. Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence Int. 2017, 48, 131–147. [Google Scholar] [PubMed]
- Wessing, B.; Lettner, S.; Zechner, W. Guided Bone Regeneration with Collagen Membranes and Particulate Graft Materials: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Corinaldesi, G.; Pieri, F.; Marchetti, C.; Fini, M.; Aldini, N.N.; Giardino, R. Histologic and histomorphometric evaluation of alveolar ridge augmentation using bone grafts and titanium micromesh in humans. J. Periodontol. 2007, 78, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Pieri, F.; Corinaldesi, G.; Fini, M.; Aldini, N.N.; Giardino, R.; Marchetti, C. Alveolar ridge augmentation with titanium mesh and a combination of autogenous bone and anorganic bovine bone: A 2-year prospective study. J. Periodontol. 2008, 79, 2093–2103. [Google Scholar] [CrossRef]
- Roccuzzo, M.; Ramieri, G.; Bunino, M.; Berrone, S. Autogenous bone graft alone or associated with titanium mesh for vertical alveolar ridge augmentation: A controlled clinical trial. Clin. Oral Implant. Res. 2007, 18, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Ricci, L.; Perrotti, V.; Ravera, L.; Scarano, A.; Piattelli, A.; Iezzi, G. Rehabilitation of deficient alveolar ridges using titanium grids before and simultaneously with implant placement: A systematic review. J. Periodontol. 2013, 84, 1234–1242. [Google Scholar] [CrossRef]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Cawood, J.I.; Howell, R.A. A classification of the edentulous jaws. Int. J. Oral Maxillofac. Surg. 1988, 17, 232–236. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; Moreno-Riestra, I.; Avila, G.; Fernández-Barbero, J.E.; Mesa, F.; Aguilar, M.; Wang, H.L.; O’Valle, F. Histomorphometric comparison of maxillary pristine bone and composite bone graft biopsies obtained after sinus augmentation. Clin. Oral Implant. Res. 2010, 21, 122–128. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; Moreno-Riestra, I.; Avila, G.; Padial-Molina, M.; Paya, J.A.; Wang, H.L.; O’Valle, F. Effect of anorganic bovine bone to autogenous cortical bone ratio upon bone remodeling patterns following maxillary sinus augmentation. Clin. Oral Implant. Res. 2011, 22, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Baljoon, M.; Natto, S.; Bergstrom, J. Occurrence of vertical bone defects in dentally aware individuals. Acta Odontol. Scand. 2003, 61, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Penarrocha-Diago, M.; Gomez-Adrian, M.D.; Garcia-Garcia, A.; Camacho-Alonso, F.; Rambla-Ferrer, J. Vertical mandibular alveolar bone distraction and dental implant placement: A case report. J. Oral Implantol. 2006, 32, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.A.; Jovanovic, S.A.; Lozada, J.L. Vertical ridge augmentation using guided bone regeneration (GBR) in three clinical scenarios prior to implant placement: A retrospective study of 35 patients 12 to 72 months after loading. Int. J. Oral Maxillofac. Implant. 2009, 24, 502–510. [Google Scholar]
- Saadoun, A.P.; LeGall, M.; Touati, B. Selection and ideal tridimensional implant position for soft tissue aesthetics. Pract. Periodontics Aesthet. Dent. 1999, 11, 1063–1072. [Google Scholar] [PubMed]
- Wang, H.L.; Boyapati, L. “PASS” principles for predictable bone regeneration. Implant Dent. 2006, 15, 8–17. [Google Scholar] [CrossRef]
- Urban, I.A.; Monje, A. Guided Bone Regeneration in Alveolar Bone Reconstruction. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 331–338. [Google Scholar] [CrossRef]
- Roccuzzo, M.; Ramieri, G.; Spada, M.C.; Bianchi, S.D.; Berrone, S. Vertical alveolar ridge augmentation by means of a titanium mesh and autogenous bone grafts. Clin. Oral Implant. Res. 2004, 15, 73–81. [Google Scholar] [CrossRef]
- Artzi, Z.; Dayan, D.; Alpern, Y.; Nemcovsky, C.E. Vertical ridge augmentation using xenogenic material supported by a configured titanium mesh: Clinicohistopathologic and histochemical study. Int. J. Oral Maxillofac. Implant. 2003, 18, 440–446. [Google Scholar]
- Proussaefs, P.; Lozada, J.; Kleinman, A.; Rohrer, M.D.; McMillan, P.J. The use of titanium mesh in conjunction with autogenous bone graft and inorganic bovine bone mineral (bio-oss) for localized alveolar ridge augmentation: A human study. Int. J. Periodontics Restor. Dent. 2003, 23, 185–195. [Google Scholar]
- Sagheb, K.; Schiegnitz, E.; Moergel, M.; Walter, C.; Al-Nawas, B.; Wagner, W. Clinical outcome of alveolar ridge augmentation with individualized CAD-CAM-produced titanium mesh. Int. J. Implant Dent. 2017, 3, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poli, P.P.; Beretta, M.; Cicciù, M.; Maiorana, C. Alveolar ridge augmentation with titanium mesh. A retrospective clinical study. Open Dent. J. 2014, 8, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Basle, M.F.; Lesourd, M.; Grizon, F.; Pascaretti, C.; Chappard, D. Type I collagen in xenogenic bone material regulates attachment and spreading of osteoblasts over the beta1 integrin subunit. Orthopade 1998, 27, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Schotland, S.; Stauber, D.J.; Kleeman, C.R.; Clemens, T.L. Cell-matrix interaction in bone: Type I collagen modulates signal transduction in osteoblast-like cells. Am. J. Physiol. 1995, 268, C1090–C1103. [Google Scholar] [CrossRef] [PubMed]
- Gungormus, M. The effect on osteogenesis of type I collagen applied to experimental bone defects. Dent. Traumatol. 2004, 20, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Gungormus, M.; Kaya, O. Evaluation of the effect of heterologous type I collagen on healing of bone defects. J. Oral Maxillofac. Surg. 2002, 60, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Hu, Y.Y.; Zhao, J.N.; Wu, S.J.; Xiong, Z.; Lu, R. Effect of type I collagen on the adhesion, proliferation, and osteoblastic gene expression of bone marrow-derived mesenchymal stem cells. Chin. J. Traumatol. 2004, 7, 358–362. [Google Scholar] [PubMed]
- Mizuno, M.; Fujisawa, R.; Kuboki, Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-alpha2beta1 integrin interaction. J. Cell. Physiol. 2000, 184, 207–213. [Google Scholar] [CrossRef]
- Regazzoni, C.; Winterhalter, K.H.; Rohrer, L. Type I collagen induces expression of bone morphogenetic protein receptor type II. Biochem. Biophys. Res. Commun. 2001, 283, 316–322. [Google Scholar] [CrossRef]
- Toroian, D.; Lim, J.E.; Price, P.A. The size exclusion characteristics of type I collagen: Implications for the role of noncollagenous bone constituents in mineralization. J. Biol. Chem. 2007, 282, 22437–22447. [Google Scholar] [CrossRef]
- Arosio, P.; Greco, G.B.; Zaniol, T.; Iezzi, G.; Perrotti, V.; Di Stefano, D.A. Sinus augmentation and concomitant implant placement in low bone-density sites. A retrospective study on an undersized drilling protocol and primary stability. Clin. Implant Dent. Relat. Res. 2018, 20, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, D.A.; Gastaldi, G.; Vinci, R.; Polizzi, E.M.; Cinci, L.; Pieri, L.; Gherlone, E. Bone Formation Following Sinus Augmentation with an Equine-Derived Bone Graft: A Retrospective Histologic and Histomorphometric Study with 36-Month Follow-up. Int. J. Oral Maxillofac. Implant. 2016, 31, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, D.A.; Greco, G.B.; Riboli, F. Guided Bone Regeneration of an Atrophic Mandible with a Heterologous Bone Block. Craniomaxillofac. Trauma Reconstr. 2016, 9, 88–93. [Google Scholar] [PubMed] [Green Version]
- Di Stefano, D.A.; Greco, G.B.; Cinci, L.; Pieri, L. Horizontal-guided Bone Regeneration using a Titanium Mesh and an Equine Bone Graft. J. Contemp. Dent. Pract. 2015, 16, 154–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pistilli, R.; Signorini, L.; Pisacane, A.; Lizio, G.; Felice, P. Case of severe bone atrophy of the posterior maxilla rehabilitated with blocks of equine origin bone: Histological results. Implant Dent. 2013, 22, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, D.A.; Cazzaniga, A.; Andreasi Bassi, M.; Ludovichetti, M.; Ammirabile, G.; Celletti, R. The use of cortical heterologous sheets for sinus lift bone grafting: A modification of Tulasne’s technique with 7-year follow-up. Int. J. Immunopathol. Pharmacol. 2013, 26, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, D.A.; Garagiola, U.; Bassi, M.A. Preserving the Bone Profile in Anterior Maxilla using an Equine Cortical Bone Membrane and an Equine Enzyme-treated Bone Graft: A Case Report with 5-year Follow-up. J. Contemp. Dent. Pract. 2017, 18, 614–621. [Google Scholar] [CrossRef]
- Artese, L.; Piattelli, A.; Di Stefano, D.A.; Piccirilli, M.; Pagnutti, S.; D’Alimonte, E.; Perrotti, V. Sinus lift with autologous bone alone or in addition to equine bone: An immunohistochemical study in man. Implant Dent. 2011, 20, 383–388. [Google Scholar] [CrossRef]
- Ludovichetti, M.; Di Stefano, D.A.; Pagnutti, S.; Vaccari, E.; Ludovichetti, F.S.; Celletti, R. Vertical ridge augmentation using a flexible heterologous cortical bone sheet: Three-year follow-up. Int. J. Periodontics Restor. Dent. 2011, 31, 401–407. [Google Scholar]
- Di Stefano, D.A.; Artese, L.; Iezzi, G.; Piattelli, A.; Pagnutti, S.; Piccirilli, M.; Perrotti, V. Alveolar ridge regeneration with equine spongy bone: A clinical, histological, and immunohistochemical case series. Clin. Implant Dent. Relat. Res. 2009, 11, 90–100. [Google Scholar] [CrossRef]
- Di Stefano, D.A.; Gastaldi, G.; Vinci, R.; Cinci, L.; Pieri, L.; Gherlone, E. Histomorphometric Comparison of Enzyme-Deantigenic Equine Bone and Anorganic Bovine Bone in Sinus Augmentation: A Randomized Clinical Trial with 3-Year Follow-Up. Int. J. Oral Maxillofac. Implant. 2015, 30, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Benke, D.; Olah, A.; Mohler, H. Protein-chemical analysis of Bio-Oss bone substitute and evidence on its carbonate content. Biomaterials 2001, 22, 1005–1012. [Google Scholar] [CrossRef]
- Mordenfeld, A.; Hallman, M.; Johansson, C.B.; Albrektsson, T. Histological and histomorphometrical analyses of biopsies harvested 11 years after maxillary sinus floor augmentation with deproteinized bovine and autogenous bone. Clin. Oral Implant. Res. 2010, 21, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Sartori, S.; Silvestri, M.; Forni, F.; Icaro Cornaglia, A.; Tesei, P.; Cattaneo, V. Ten-year follow-up in a maxillary sinus augmentation using anorganic bovine bone (Bio-Oss). A case report with histomorphometric evaluation. Clin. Oral Implant. Res. 2003, 14, 369–372. [Google Scholar] [CrossRef] [Green Version]
- Schlegel, A.K.; Donath, K. BIO-OSS—A resorbable bone substitute? J. Long-Term Eff. Med. Implant. 1998, 8, 201–209. [Google Scholar]
- Traini, T.; Valentini, P.; Iezzi, G.; Piattelli, A. A histologic and histomorphometric evaluation of anorganic bovine bone retrieved 9 years after a sinus augmentation procedure. J. Periodontol. 2007, 78, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, N.U.; Scharer, P.; Marinello, C.P.; Schupbach, P.; Berglundh, T. Alveolar ridge augmentation with Bio-Oss: A histologic study in humans. Int. J. Periodontics Restor. Dent. 2001, 21, 288–295. [Google Scholar]
- Perrotti, V.; Nicholls, B.M.; Horton, M.A.; Piattelli, A. Human osteoclast formation and activity on a xenogenous bone mineral. J. Biomed. Mater. Res. A 2009, 90, 238–246. [Google Scholar] [CrossRef]
- Perrotti, V.; Nicholls, B.M.; Piattelli, A. Human osteoclast formation and activity on an equine spongy bone substitute. Clin. Oral Implant. Res. 2009, 20, 17–23. [Google Scholar] [CrossRef]
- Helfrich, M.H.; Nesbitt, S.A.; Lakkakorpi, P.T.; Barnes, M.J.; Bodary, S.C.; Shankar, G.; Mason, W.T.; Mendrick, D.L.; Vaananen, H.K.; Horton, M.A. β1 integrins and osteoclast function: Involvement in collagen recognition and bone resorption. Bone 1996, 19, 317–328. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Stefano, D.A.; Greco, G.; Gherlone, E. A Preshaped Titanium Mesh for Guided Bone Regeneration with an Equine-Derived Bone Graft in a Posterior Mandibular Bone Defect: A Case Report. Dent. J. 2019, 7, 77. https://doi.org/10.3390/dj7030077
Di Stefano DA, Greco G, Gherlone E. A Preshaped Titanium Mesh for Guided Bone Regeneration with an Equine-Derived Bone Graft in a Posterior Mandibular Bone Defect: A Case Report. Dentistry Journal. 2019; 7(3):77. https://doi.org/10.3390/dj7030077
Chicago/Turabian StyleDi Stefano, Danilo Alessio, Gianbattista Greco, and Enrico Gherlone. 2019. "A Preshaped Titanium Mesh for Guided Bone Regeneration with an Equine-Derived Bone Graft in a Posterior Mandibular Bone Defect: A Case Report" Dentistry Journal 7, no. 3: 77. https://doi.org/10.3390/dj7030077



