Chemical, Clinical and Histomorphometric Comparison between Equine Bone Manufactured through Enzymatic Antigen-Elimination and Bovine Bone Made Non-Antigenic Using a High-Temperature Process in Post-Extractive Socket Grafting. A Comparative Retrospective Clinical Study
Abstract
1. Introduction
2. Materials and Methods
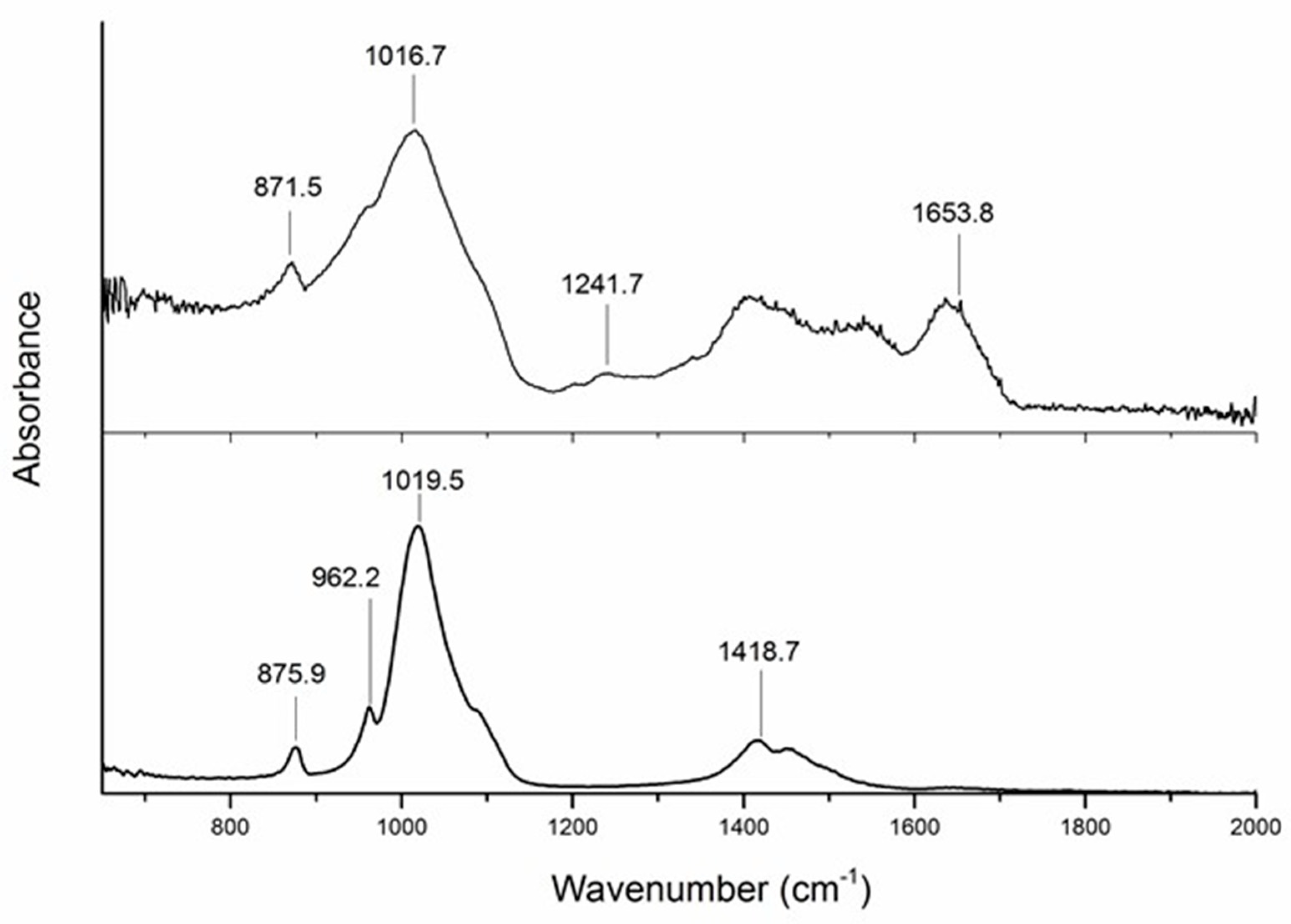
2.1. ATR-FTIR Spectroscopy
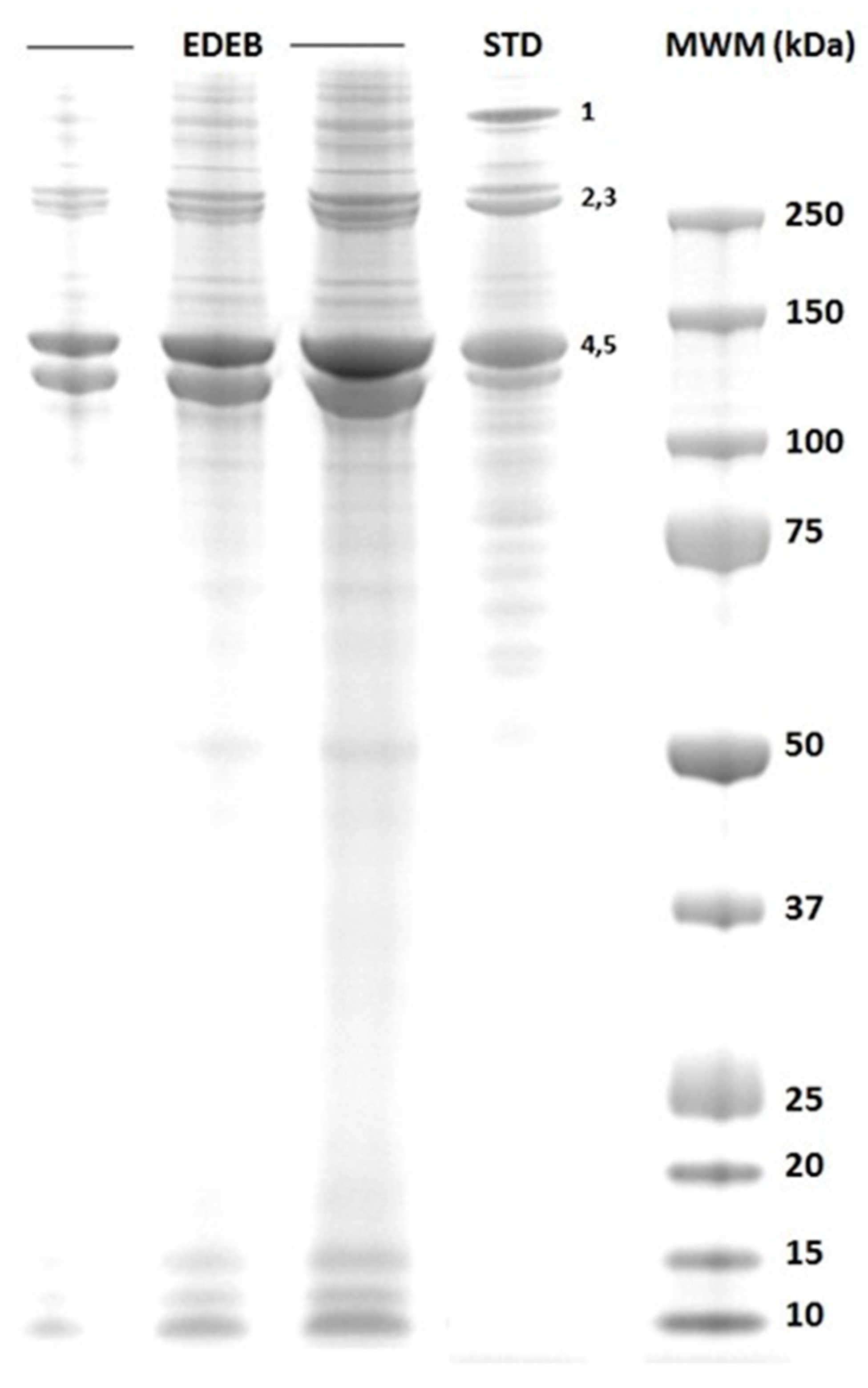
2.2. SDS-PAGE Analysis
2.3. Retrospective Data Assessment
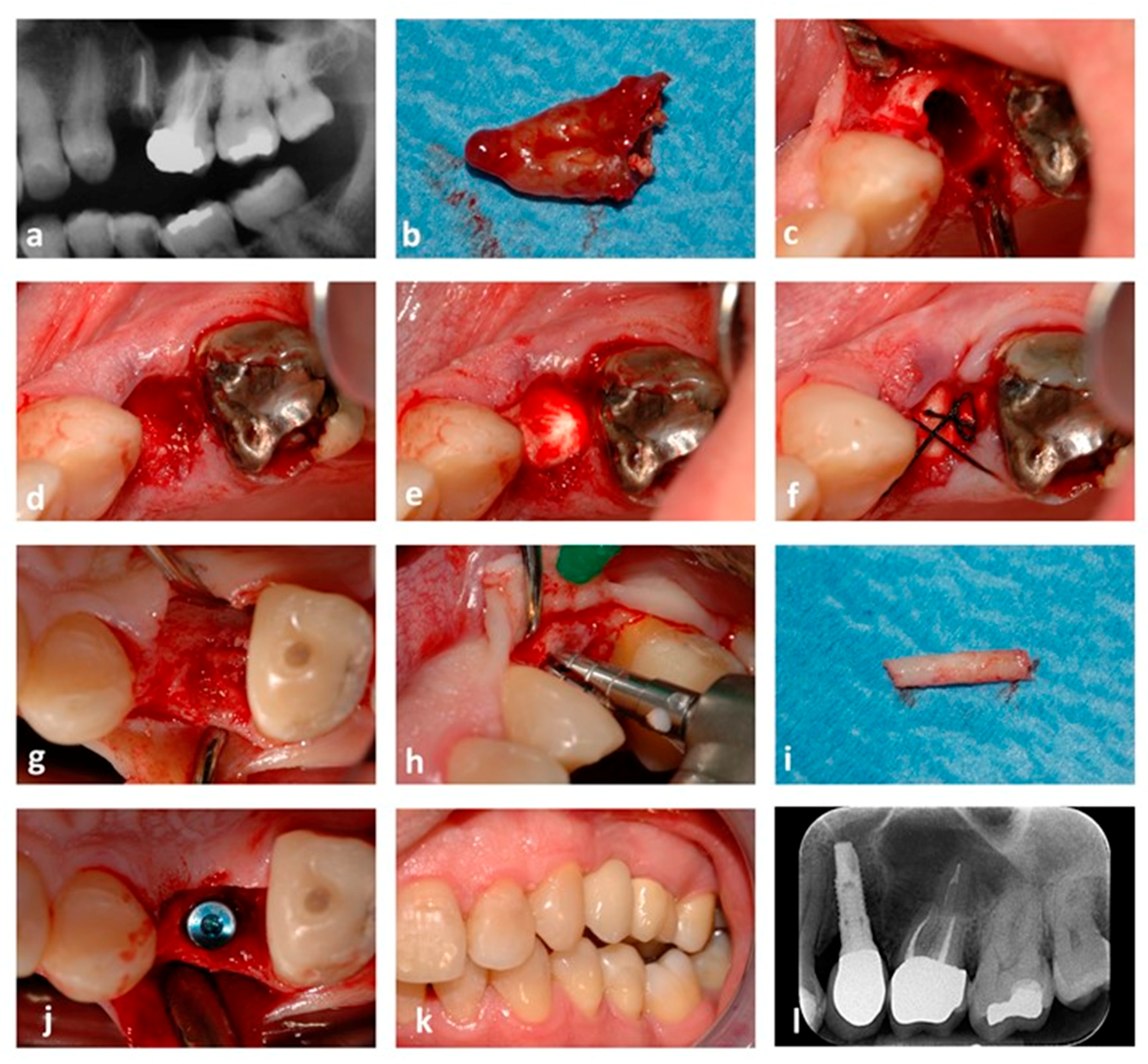
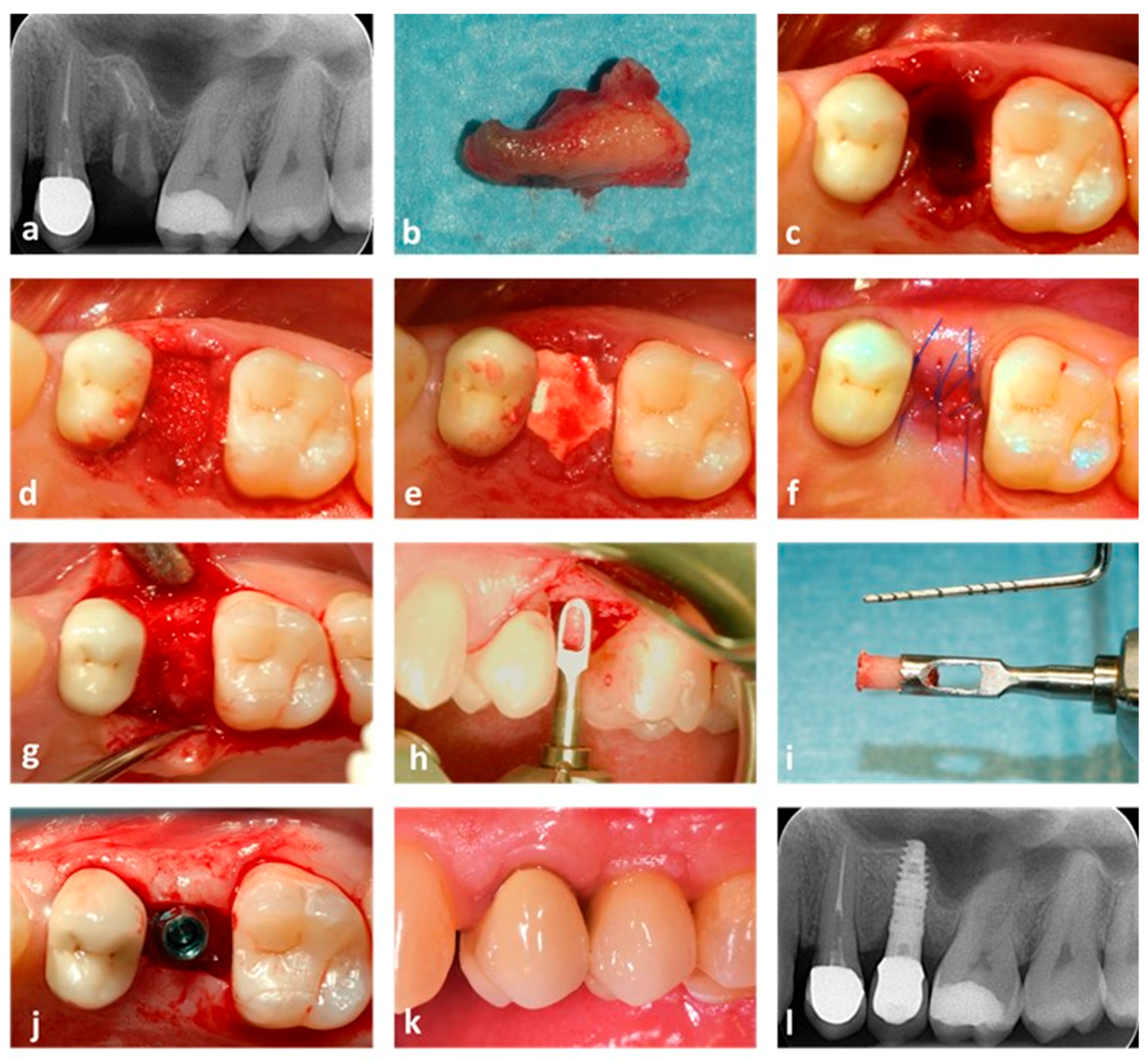
2.4. Surgical Procedure
2.5. Histology and Histomorphometry
2.6. Statistical Analyses
3. Results
3.1. ATR-FTIR Spectroscopy Assessment
3.2. SDS-PAGE Analysis
3.3. Histology and Histomorphometry
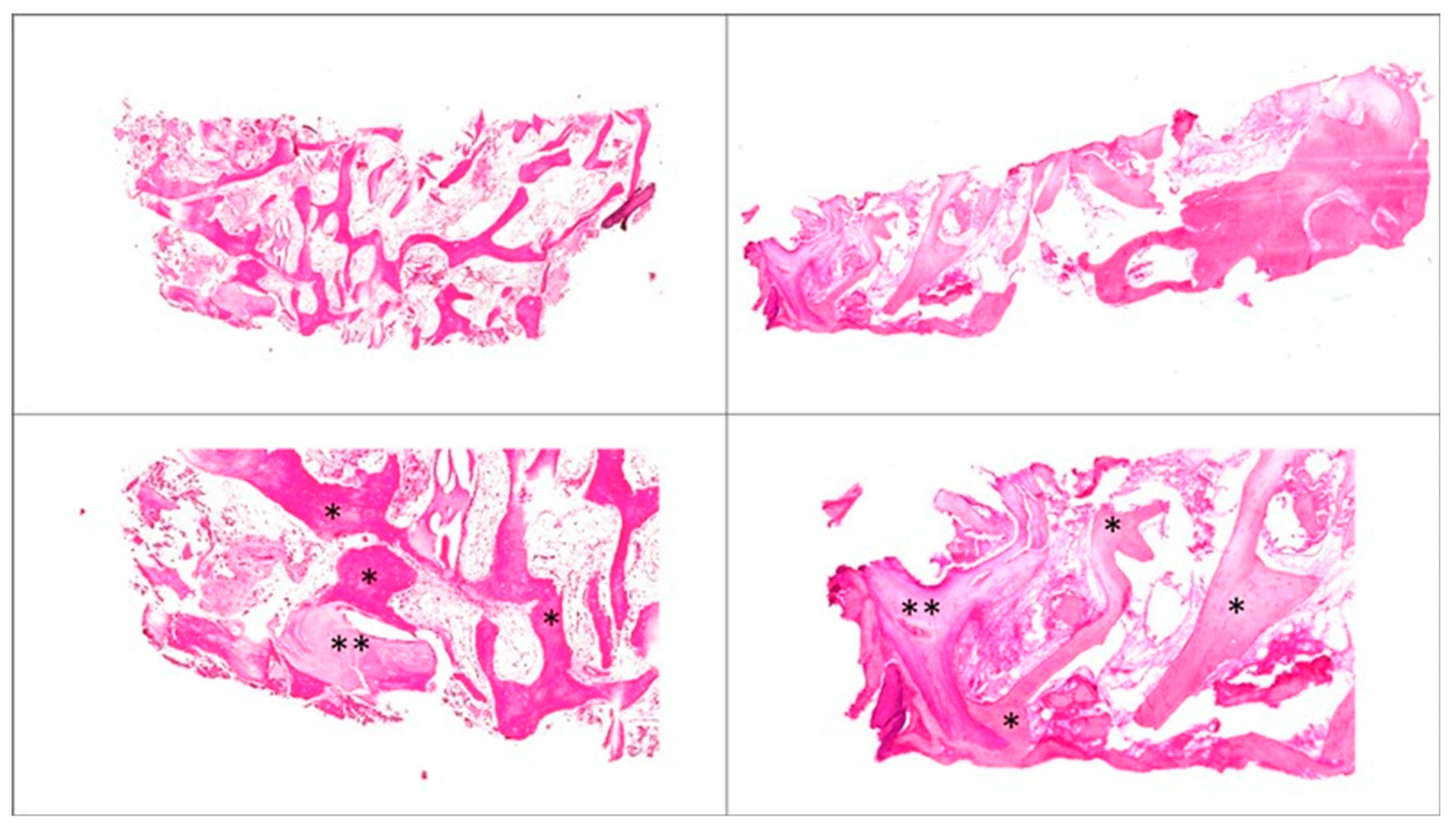
3.4. Qualitative Histologic Analyses
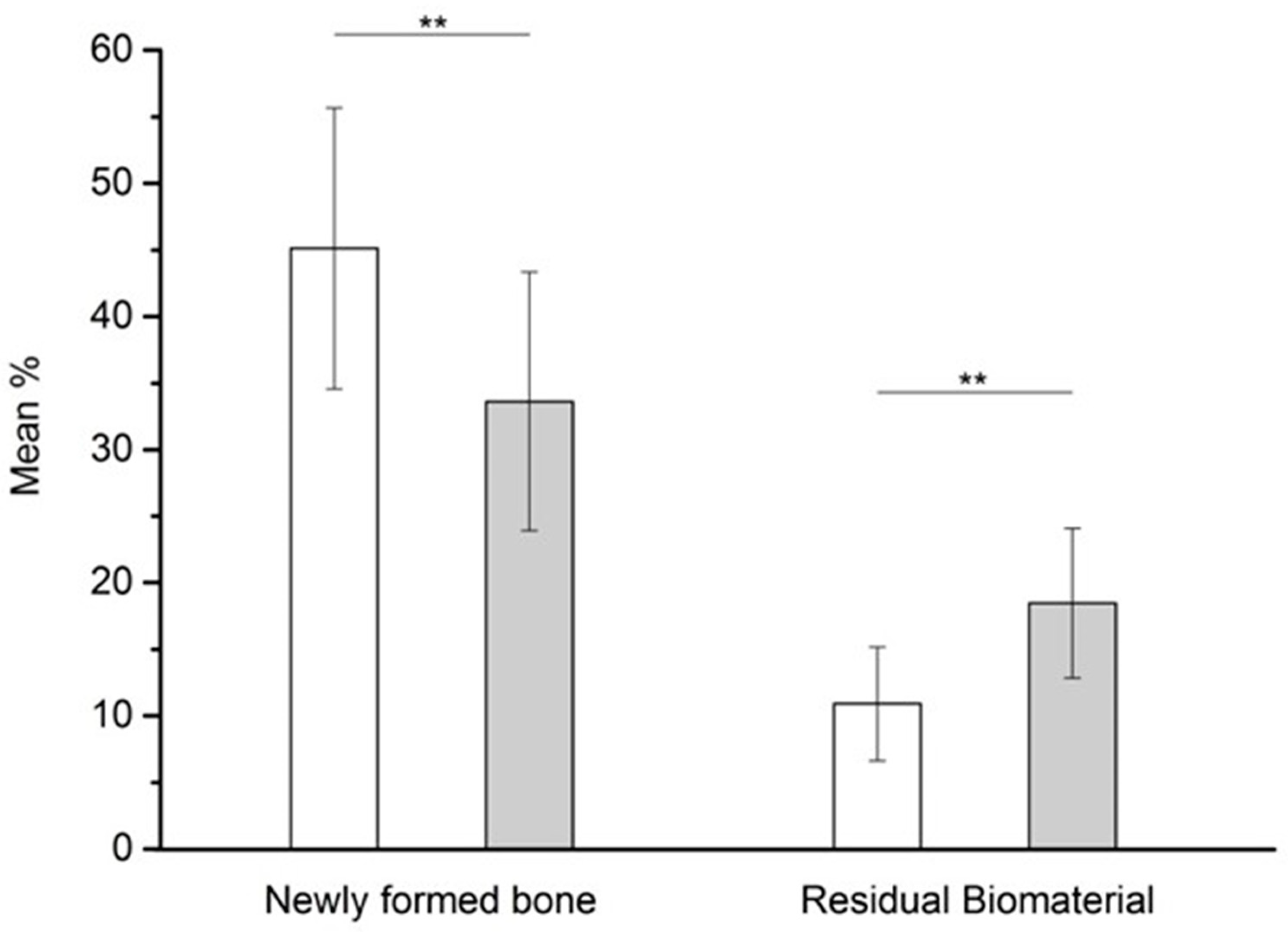
3.5. Histomorphometric Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Araujo, M.G.; Lindhe, J. Ridge alterations following tooth extraction with and without flap elevation: An experimental study in the dog. Clin. Oral Implant. Res. 2009, 20, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Van der Weijden, F.; Dell’Acqua, F.; Slot, D.E. Alveolar bone dimensional changes of post-extraction sockets in humans: A systematic review. J. Clin. Periodontol. 2009, 36, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.A.; Khurshid, Z.; Zafar, M.S.; Najeeb, S. Immediate Implants: Clinical Guidelines for Esthetic Outcomes. Dent. J. 2016, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- De Risi, V.; Clementini, M.; Vittorini, G.; Mannocci, A.; De Sanctis, M. Alveolar ridge preservation techniques: A systematic review and meta-analysis of histological and histomorphometrical data. Clin. Oral Implant. Res. 2015, 26, 50–68. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.M. Maxillary autogenous bone grafting. Oral Maxillofac. Surg. Clin. N. Am. 2011, 23, 229–238. [Google Scholar] [CrossRef]
- Nkenke, E.; Weisbach, V.; Winckler, E.; Kessler, P.; Schultze-Mosgau, S.; Wiltfang, J.; Neukam, F.W. Morbidity of harvesting of bone grafts from the iliac crest for preprosthetic augmentation procedures: A prospective study. Int. J. Oral Maxillofac. Surg. 2004, 33, 157–163. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Felice, P.; Karatzopoulos, G.; Worthington, H.V.; Coulthard, P. Interventions for replacing missing teeth: Horizontal and vertical bone augmentation techniques for dental implant treatment. Cochrane Database Syst. Rev. 2009, 7, CD003607. [Google Scholar] [CrossRef]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef]
- Aerssens, J.; Boonen, S.; Lowet, G.; Dequeker, J. Interspecies differences in bone composition, density, and quality: Potential implications for in vivo bone research. Endocrinology 1998, 139, 663–670. [Google Scholar] [CrossRef]
- Baldini, N.; De Sanctis, M.; Ferrari, M. Deproteinized bovine bone in periodontal and implant surgery. Dent. Mater. 2011, 27, 61–70. [Google Scholar] [CrossRef]
- Jensen, S.S.; Terheyden, H. Bone augmentation procedures in localized defects in the alveolar ridge: Clinical results with different bone grafts and bone-substitute materials. Int. J. Oral. Maxillofac Implant. 2009, 24, 218–236. [Google Scholar]
- Benke, D.; Olah, A.; Mohler, H. Protein-chemical analysis of Bio-Oss bone substitute and evidence on its carbonate content. Biomaterials 2001, 22, 1005–1012. [Google Scholar] [CrossRef]
- Perrotti, V.; Nicholls, B.M.; Horton, M.A.; Piattelli, A. Human osteoclast formation and activity on a xenogenous bone mineral. J. Biomed. Mater. Res. A 2009, 90, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Mordenfeld, A.; Hallman, M.; Johansson, C.B.; Albrektsson, T. Histological and histomorphometrical analyses of biopsies harvested 11 years after maxillary sinus floor augmentation with deproteinized bovine and autogenous bone. Clin. Oral Implant. Res. 2010, 21, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Sartori, S.; Silvestri, M.; Forni, F.; Icaro Cornaglia, A.; Tesei, P.; Cattaneo, V. Ten-year follow-up in a maxillary sinus augmentation using anorganic bovine bone (Bio-Oss). A case report with histomorphometric evaluation. Clin. Oral Implant. Res. 2003, 14, 369–372. [Google Scholar] [CrossRef]
- Traini, T.; Valentini, P.; Iezzi, G.; Piattelli, A. A histologic and histomorphometric evaluation of anorganic bovine bone retrieved 9 years after a sinus augmentation procedure. J. Periodontol. 2007, 78, 955–961. [Google Scholar] [CrossRef]
- Artese, L.; Piattelli, A.; Di Stefano, D.A.; Piccirilli, M.; Pagnutti, S.; D’Alimonte, E.; Perrotti, V. Sinus lift with autologous bone alone or in addition to equine bone: An immunohistochemical study in man. Implant Dent. 2011, 20, 383–388. [Google Scholar] [CrossRef]
- De Angelis, N.; Scivetti, M. Lateral ridge augmentation using an equine flex bone block infused with recombinant human platelet-derived growth factor BB: A clinical and histologic study. Int. J. Periodontics Restor. Dent. 2011, 31, 383–388. [Google Scholar]
- Di Stefano, D.A.; Andreasi Bassi, M.; Cinci, L.; Pieri, L.; Ammirabile, G. Treatment of a bone defect consequent to the removal of a periapical cyst with equine bone and equine membranes: Clinical and histological outcome. Minerva Stomatol. 2012, 61, 477–490. [Google Scholar]
- Di Stefano, D.A.; Artese, L.; Iezzi, G.; Piattelli, A.; Pagnutti, S.; Piccirilli, M.; Perrotti, V. Alveolar ridge regeneration with equine spongy bone: A clinical, histological, and immunohistochemical case series. Clin. Implant Dent. Relat. Res. 2009, 11, 90–100. [Google Scholar] [CrossRef]
- Felice, P.; Piana, L.; Checchi, L.; Corvino, V.; Nannmark, U.; Piattelli, M. Vertical ridge augmentation of an atrophic posterior mandible with an inlay technique and cancellous equine bone block: A case report. Int. J. Periodontics Restor. Dent. 2013, 33, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Ludovichetti, M.; Di Stefano, D.A.; Pagnutti, S.; Vaccari, E.; Ludovichetti, F.S.; Celletti, R. Vertical ridge augmentation using a flexible heterologous cortical bone sheet: Three-year follow-up. Int. J. Periodontics Restor. Dent. 2011, 31, 401–407. [Google Scholar]
- Pistilli, R.; Signorini, L.; Pisacane, A.; Lizio, G.; Felice, P. Case of severe bone atrophy of the posterior maxilla rehabilitated with blocks of equine origin bone: Histological results. Implant Dent. 2013, 22, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Stievano, D.; Di Stefano, A.; Ludovichetti, M.; Pagnutti, S.; Gazzola, F.; Boato, C.; Stellini, E. Maxillary sinus lift through heterologous bone grafts and simultaneous acid-etched implants placement. Five year follow-up. Minerva Chir. 2008, 63, 79–91. [Google Scholar] [PubMed]
- Santini, S.; Barbera, P.; Modena, M.; Schiavon, R.; Bonato, M. Equine-derived bone substitutes in orthopedics and traumatology: Authors’ experience. Minerva Chir. 2011, 66, 63–72. [Google Scholar]
- Green, J.; Schotland, S.; Stauber, D.J.; Kleeman, C.R.; Clemens, T.L. Cell-matrix interaction in bone: Type I collagen modulates signal transduction in osteoblast-like cells. Am. J. Physiol. 1995, 268 Pt 1, C1090–C1103. [Google Scholar] [CrossRef]
- Liu, G.; Hu, Y.Y.; Zhao, J.N.; Wu, S.J.; Xiong, Z.; Lu, R. Effect of type I collagen on the adhesion, proliferation, and osteoblastic gene expression of bone marrow-derived mesenchymal stem cells. Chin. J. Traumatol. 2004, 7, 358–362. [Google Scholar]
- Mizuno, M.; Fujisawa, R.; Kuboki, Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-alpha2beta1 integrin interaction. J. Cell Physiol. 2000, 184, 207–213. [Google Scholar] [CrossRef]
- Regazzoni, C.; Winterhalter, K.H.; Rohrer, L. Type I collagen induces expression of bone morphogenetic protein receptor type II. Biochem. Biophys. Res. Commun. 2001, 283, 316–322. [Google Scholar] [CrossRef]
- Perrotti, V.; Nicholls, B.M.; Piattelli, A. Human osteoclast formation and activity on an equine spongy bone substitute. Clin. Oral Implant. Res. 2009, 20, 17–23. [Google Scholar] [CrossRef]
- Di Stefano, D.A.; Gastaldi, G.; Vinci, R.; Cinci, L.; Pieri, L.; Gherlone, E. Histomorphometric Comparison of Enzyme-Deantigenic Equine Bone and Anorganic Bovine Bone in Sinus Augmentation: A Randomized Clinical Trial with 3-Year Follow-Up. Int. J. Oral Maxillofac. Implant. 2015, 30, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, D.A.; Gastaldi, G.; Vinci, R.; Polizzi, E.M.; Cinci, L.; Pieri, L.; Gherlone, E. Bone Formation Following Sinus Augmentation with an Equine-Derived Bone Graft: A Retrospective Histologic and Histomorphometric Study with 36-Month Follow-up. Int. J. Oral Maxillofac. Implant. 2016, 31, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Piattelli, A.; Degidi, M.; Di Stefano, D.A.; Rubini, C.; Fioroni, M.; Strocchi, R. Microvessel density in alveolar ridge regeneration with autologous and alloplastic bone. Implant Dent. 2002, 11, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Artzi, Z.; Tal, H.; Dayan, D. Porous bovine bone mineral in healing of human extraction sockets: 2. Histochemical observations at 9 months. J. Periodontol. 2001, 72, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Fugazzotto, P.A. GBR using bovine bone matrix and resorbable and nonresorbable membranes. Part 1: Histologic results. Int. J. Periodontics Restor. Dent. 2003, 23, 361–369. [Google Scholar]
- Barone, A.; Todisco, M.; Ludovichetti, M.; Gualini, F.; Aggstaller, H.; Torres-Lagares, D.; Rohrer, M.D.; Prasad, H.S.; Kenealy, J.N. A prospective, randomized, controlled, multicenter evaluation of extraction socket preservation comparing two bovine xenografts: Clinical and histologic outcomes. Int. J. Periodontics Restor. Dent. 2013, 33, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B. Healing of extraction socket grafted with deproteinized bovine bone and acellular dermal matrix: Histomorphometric evaluation. Implant Dent. 2010, 19, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36 (Suppl. 3), S20–S27. [Google Scholar] [CrossRef]
- Chaikof, E.L.; Matthew, H.; Kohn, J.; Mikos, A.G.; Prestwich, G.D.; Yip, C.M. Biomaterials and scaffolds in reparative medicine. Ann. N. Y. Acad. Sci. 2002, 961, 96–105. [Google Scholar] [CrossRef]
- Benlidayi, M.E.; Kurkcu, M.; Oz, I.A.; Sertdemir, Y. Comparison of two different forms of bovine-derived hydroxyapatite in sinus augmentation and simultaneous implant placement: An experimental study. Int. J. Oral Maxillofac. Implant. 2009, 24, 704–711. [Google Scholar]
- Blokhuis, T.J.; Termaat, M.F.; den Boer, F.C.; Patka, P.; Bakker, F.C.; Haarman, H.J. Properties of calcium phosphate ceramics in relation to their in vivo behavior. J. Trauma 2000, 48, 179–186. [Google Scholar] [CrossRef] [PubMed]
| EDEB | ABB | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PT | Gender | Age | Socket | Time | NFB | RB | PT | Gender | Age | Socket | Time | NFB | RB |
| 1 | M | 45 | 15 | 4.6 | 46.92 | 15.15 | 1 | M | 56 | 25 | 3.0 | 42.45 | 12.18 |
| 16 | 42.16 | 16.06 | 2 | M | 45 | 45 | 3.9 | 35.60 | 13.26 | ||||
| 2 | F | 56 | 35 | 3.2 | 44.46 | 8.98 | 46 | 38.01 | 24.05 | ||||
| 36 | 58.43 | 4.54 | 3 | F | 46 | 45 | 5.9 | 42.32 | 23.43 | ||||
| 3 | F | 45 | 27 | 4.3 | 44.28 | 5.74 | 46 | 23.20 | 10.94 | ||||
| 4 | M | 56 | 36 | 6.3 | 41.66 | 7.19 | 47 | 39.11 | 15.81 | ||||
| 5 | M | 57 | 35 | 4.8 | 58.00 | 6.20 | 4 | F | 57 | 26 | 5.0 | 48.22 | 21.14 |
| 36 | 30.11 | 19.44 | 5 | M | 58 | 35 | 5.8 | 46.44 | 19.14 | ||||
| 6 | F | 43 | 35 | 2.7 | 40.61 | 15.15 | 36 | 27.18 | 20.74 | ||||
| 7 | F | 57 | 25 | 4.5 | 27.78 | 13.40 | 37 | 21.69 | 13.29 | ||||
| 8 | F | 56 | 46 | 4.0 | 57.30 | 10.77 | 6 | M | 46 | 15 | 3.4 | 20.67 | 24.40 |
| 47 | 51.60 | 11.54 | 7 | M | 65 | 35 | 3.8 | 19.74 | 23.73 | ||||
| 9 | M | 48 | 45 | 5.4 | 38.45 | 8.32 | 46 | 44.67 | 16.76 | ||||
| 46 | 34.74 | 5.65 | 8 | F | 45 | 25 | 3.6 | 32.39 | 27.04 | ||||
| 10 | M | 65 | 35 | 3.5 | 31.41 | 8.23 | 9 | F | 48 | 35 | 3.8 | 37.77 | 10.91 |
| 11 | F | 56 | 36 | 2.9 | 37.75 | 8.14 | 36 | 44.36 | 13.70 | ||||
| 37 | 31.75 | 16.83 | 10 | M | 65 | 35 | 3.8 | 30.48 | 26.28 | ||||
| 12 | M | 48 | 36 | 6.3 | 61.06 | 7.19 | 36 | 42.96 | 24.50 | ||||
| 37 | 60.97 | 5.67 | 11 | F | 73 | 45 | 6.0 | 45.33 | 27.70 | ||||
| 13 | M | 55 | 36 | 5.1 | 40.82 | 12.27 | 12 | M | 54 | 45 | 4.5 | 33.82 | 16.54 |
| 37 | 58.93 | 10.97 | 46 | 32.85 | 15.76 | ||||||||
| 14 | F | 56 | 35 | 4.8 | 43.51 | 6.44 | 13 | M | 65 | 35 | 3.5 | 20.29 | 26.47 |
| 36 | 38.77 | 6.18 | 14 | M | 55 | 45 | 4.2 | 31.60 | 22.69 | ||||
| 15 | F | 45 | 21 | 3.1 | 42.36 | 10.47 | 46 | 41.15 | 20.67 | ||||
| 16 | M | 46 | 45 | 4.9 | 59.16 | 7.36 | 15 | F | 56 | 36 | 4.3 | 45.79 | 18.31 |
| 46 | 58.32 | 18.56 | 37 | 38.12 | 14.37 | ||||||||
| 47 | 39.17 | 19.66 | 16 | M | 67 | 11 | 4.7 | 38.07 | 17.62 | ||||
| 17 | M | 56 | 35 | 2.9 | 33.36 | 8.10 | 17 | F | 48 | 34 | 2.7 | 24.22 | 18.65 |
| 36 | 49.25 | 11.35 | 35 | 19.84 | 12.33 | ||||||||
| 37 | 62.04 | 16.66 | 18 | M | 56 | 46 | 2.9 | 19.58 | 24.94 | ||||
| 18 | M | 46 | 35 | 5.4 | 57.17 | 15.65 | 47 | 22.85 | 14.40 | ||||
| 36 | 48.44 | 14.79 | 48 | 22.07 | 26.81 | ||||||||
| 37 | 56.42 | 14.59 | 19 | F | 46 | 21 | 5.1 | 20.40 | 20.10 | ||||
| 19 | F | 56 | 22 | 5.2 | 37.57 | 11.12 | 20 | M | 44 | 13 | 4.7 | 29.90 | 13.46 |
| 20 | F | 57 | 45 | 2.8 | 50.58 | 8.25 | 21 | F | 58 | 45 | 5.2 | 41.89 | 11.28 |
| 46 | 32.50 | 13.02 | 46 | 35.68 | 25.52 | ||||||||
| 21 | F | 56 | 36 | 5.7 | 52.06 | 8.40 | 22 | M | 57 | 35 | 3.6 | 49.60 | 9.62 |
| 37 | 29.31 | 5.90 | 36 | 21.11 | 8.87 | ||||||||
| 22 | M | 58 | 36 | 5.4 | 38.51 | 9.10 | 23 | M | 56 | 21 | 3.4 | 46.05 | 10.65 |
| 37 | 37.08 | 13.52 | 24 | F | 55 | 35 | 3.7 | 26.69 | 20.30 | ||||
| 36 | 33.91 | 18.84 | |||||||||||
| Mean | 52.9 | 4.1 | 45.12 | 10.91 | 55.0 | 4.4 | 33.61 | 18.47 | |||||
| SD | 5.9 | 1.2 | 10.54 | 4.27 | 8.1 | 1.2 | 9.71 | 5.62 | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Stefano, D.A.; Zaniol, T.; Cinci, L.; Pieri, L. Chemical, Clinical and Histomorphometric Comparison between Equine Bone Manufactured through Enzymatic Antigen-Elimination and Bovine Bone Made Non-Antigenic Using a High-Temperature Process in Post-Extractive Socket Grafting. A Comparative Retrospective Clinical Study. Dent. J. 2019, 7, 70. https://doi.org/10.3390/dj7030070
Di Stefano DA, Zaniol T, Cinci L, Pieri L. Chemical, Clinical and Histomorphometric Comparison between Equine Bone Manufactured through Enzymatic Antigen-Elimination and Bovine Bone Made Non-Antigenic Using a High-Temperature Process in Post-Extractive Socket Grafting. A Comparative Retrospective Clinical Study. Dentistry Journal. 2019; 7(3):70. https://doi.org/10.3390/dj7030070
Chicago/Turabian StyleDi Stefano, Danilo Alessio, Terry Zaniol, Lorenzo Cinci, and Laura Pieri. 2019. "Chemical, Clinical and Histomorphometric Comparison between Equine Bone Manufactured through Enzymatic Antigen-Elimination and Bovine Bone Made Non-Antigenic Using a High-Temperature Process in Post-Extractive Socket Grafting. A Comparative Retrospective Clinical Study" Dentistry Journal 7, no. 3: 70. https://doi.org/10.3390/dj7030070
APA StyleDi Stefano, D. A., Zaniol, T., Cinci, L., & Pieri, L. (2019). Chemical, Clinical and Histomorphometric Comparison between Equine Bone Manufactured through Enzymatic Antigen-Elimination and Bovine Bone Made Non-Antigenic Using a High-Temperature Process in Post-Extractive Socket Grafting. A Comparative Retrospective Clinical Study. Dentistry Journal, 7(3), 70. https://doi.org/10.3390/dj7030070




