Correlation between Implant Geometry, Bone Density, and the Insertion Torque/Depth Integral: A Study on Bovine Ribs
Abstract
1. Introduction
2. Materials and Methods
Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Javed, F.; Romanos, G.E. The role of primary stability for successful immediate loading of dental implants. A literature review. J. Dent. 2010, 38, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Sakka, S.; Baroudi, K.; Nassani, M.Z. Factors associated with early and late failure of dental implants. J. Investig. Clin. Dent. 2012, 3, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Bergkvist, G.; Koh, K.J.; Sahlholm, S.; Klintstrom, E.; Lindh, C. Bone density at implant sites and its relationship to assessment of bone quality and treatment outcome. Int. J. Oral Maxillofac. Implants 2010, 25, 321–328. [Google Scholar] [PubMed]
- Javed, F.; Ahmed, H.B.; Crespi, R.; Romanos, G.E. Role of primary stability for successful osseointegration of dental implants: Factors of influence and evaluation. Interv. Med. Appl. Sci. 2013, 5, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, H.; Anand, P.S.; Anil, S. Undersized implant site preparation to enhance primary implant stability in poor bone density: A prospective clinical study. J. Oral Maxillofac. Surg. 2011, 69, e506–e512. [Google Scholar] [CrossRef] [PubMed]
- Al-Marshood, M.M.; Junker, R.; Al-Rasheed, A.; Al Farraj Aldosari, A.; Jansen, J.A.; Anil, S. Study of the osseointegration of dental implants placed with an adapted surgical technique. Clin. Oral Implants Res. 2011, 22, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Stocchero, M.; Toia, M.; Cecchinato, D.; Becktor, J.P.; Coelho, P.G.; Jimbo, R. Biomechanical, Biologic, and Clinical Outcomes of Undersized Implant Surgical Preparation: A Systematic Review. Int. J. Oral Maxillofac. Implants 2016, 31, 1247–1263. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.V.; Elias, C.N.; Cavalcanti Lima, J.H. The effects of superficial roughness and design on the primary stability of dental implants. Clin. Implant. Dent. Relat. Res. 2011, 13, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, S.; Laval, J.Y.; Axmann, D.; Weber, H. Influence of implant geometry on primary insertion stability and simulated peri-implant bone loss: An in vitro study using resonance frequency analysis and damping capacity assessment. Int. J. Oral Maxillofac. Implants 2011, 26, 347–355. [Google Scholar] [PubMed]
- Mohlhenrich, S.C.; Heussen, N.; Elvers, D.; Steiner, T.; Holzle, F.; Modabber, A. Compensating for poor primary implant stability in different bone densities by varying implant geometry: A laboratory study. Int J. Oral. Maxillofac. Surg. 2015, 44, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Alkhraisat, M.H.; Pinas, L.; Orive, G. Efficacy of biologically guided implant site preparation to obtain adequate primary implant stability. Ann. Anat. 2015, 199, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, F.; Hasan, I.; Bourauel, C.; Biffar, R.; Mundt, T. Bone stability around dental implants: Treatment related factors. Ann. Anat. 2015, 199, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Szmukler-Moncler, S.; Salama, H.; Reingewirtz, Y.; Dubruille, J.H. Timing of loading and effect of micromotion on bone-dental implant interface: Review of experimental literature. J. Biomed. Mater. Res. 1998, 43, 192–203. [Google Scholar] [CrossRef]
- Trisi, P.; Perfetti, G.; Baldoni, E.; Berardi, D.; Colagiovanni, M.; Scogna, G. Implant micromotion is related to peak insertion torque and bone density. Clin. Oral Implants Res. 2009, 20, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Norton, M.R.; Gamble, C. Bone classification: An objective scale of bone density using the computerized tomography scan. Clin. Oral Implants Res. 2001, 12, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, D.C.; Dial, C.; Mayorga, C. Cone beam CT for pre-surgical assessment of implant sites. J. Calif. Dent. Assoc. 2003, 31, 825–833. [Google Scholar] [PubMed]
- Mah, P.; Reeves, T.E.; McDavid, W.D. Deriving Hounsfield units using grey levels in cone beam computed tomography. Dentomaxillofac. Radiol. 2010, 39, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Watanabe, H.; Honda, E.; Kurabayashi, T. Reliability of voxel values from cone-beam computed tomography for dental use in evaluating bone mineral density. Clin. Oral Implants Res. 2010, 21, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Valiyaparambil, J.V.; Yamany, I.; Ortiz, D.; Shafer, D.M.; Pendrys, D.; Freilich, M.; Mallya, S.M. Bone quality evaluation: Comparison of cone beam computed tomography and subjective surgical assessment. Int. J. Oral Maxillofac. Implants 2012, 27, 1271–1277. [Google Scholar] [PubMed]
- Lekholm, U.; Zarb, G.A. Patient selection and preparation. In Tissue-Integrated Prosthesis: Osseointegration in Clinical Dentistry; Branemark, P.I., Zarb, G.A., Albrektsson, T., Eds.; Quintessence: Chicago, IL, USA, 1985; pp. 199–209. [Google Scholar]
- Misch, C.M. Maxillary autogenous bone grafting. Oral Maxillofac. Surg. Clin. N. Am. 2011, 23, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Trisi, P.; Rao, W. Bone classification: Clinical-histomorphometric comparison. Clin. Oral Implants Res. 1999, 10, 1–7. [Google Scholar] [PubMed]
- Atsumi, M.; Park, S.H.; Wang, H.L. Methods used to assess implant stability: Current status. Int. J. Oral Maxillofac. Implants 2007, 22, 743–754. [Google Scholar] [PubMed]
- Degidi, M.; Daprile, G.; Piattelli, A. Primary stability determination by means of insertion torque and RFA in a sample of 4,135 implants. Clin. Implant. Dent. Relat. Res. 2012, 14, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Sennerby, L.; Meredith, N. Implant stability measurements using resonance frequency analysis: Biological and biomechanical aspects and clinical implications. Periodontol. 2000 2008, 47, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Degidi, M.; Daprile, G.; Piattelli, A.; Iezzi, G. Development of a new implant primary stability parameter: Insertion torque revisited. Clin. Implant. Dent. Relat. Res. 2013, 15, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, S.J.; Cho, I.S.; Kim, S.K.; Kim, T.W. Rotational resistance of surface-treated mini-implants. Angle Orthod. 2009, 79, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Park, K.J.; Kwon, J.Y.; Kim, S.K.; Heo, S.J.; Koak, J.Y.; Lee, J.H.; Lee, S.J.; Kim, T.H.; Kim, M.J. The relationship between implant stability quotient values and implant insertion variables: A clinical study. J. Oral Rehabil. 2012, 39, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Degidi, M.; Daprile, G.; Piattelli, A. Influence of underpreparation on primary stability of implants inserted in poor quality bone sites: An in vitro study. J. Oral. Maxillofac. Surg. 2015, 73, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Shapoff, C.A. Clinical advantages of tapered root form dental implants. Compend. Contin. Educ. Dent. 2002, 23, 42–44, 46, 48, passim. [Google Scholar] [PubMed]
- Pozzi, A.; Mura, P. Immediate Loading of Conical Connection Implants: Up-to-2-Year Retrospective Clinical and Radiologic Study. Int. J. Oral Maxillofac. Implants 2016, 31, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, A.; Tallarico, M.; Moy, P.K. Immediate loading with a novel implant featured by variable-threaded geometry, internal conical connection and platform shifting: Three-year results from a prospective cohort study. Eur. J. Oral. Implantol. 2015, 8, 51–63. [Google Scholar] [PubMed]
- Menicucci, G.; Pachie, E.; Lorenzetti, M.; Migliaretti, G.; Carossa, S. Comparison of primary stability of straight-walled and tapered implants using an insertion torque device. Int. J. Prosthodont. 2012, 25, 465–471. [Google Scholar] [PubMed]
- Kadkhodazadeh, M.; Heidari, B.; Abdi, Z.; Mollaverdi, F.; Amid, R. Radiographic evaluation of marginal bone levels around dental implants with different designs after 1 year. Acta Odontol. Scand. 2013, 71, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, T.; Tanaka, H.; Ayukawa, Y.; Howashi, M.; Masuzaki, T.; Kiyosue, T.; Koyano, K.; Nakamura, S. Primary Stability of a Hybrid Implant Compared with Tapered and Cylindrical Implants in an Ex Vivo Model. Clin. Implant. Dent. Relat. Res. 2015, 17, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.L.; de Castro, D.T.; Shimano, A.C.; Lepri, C.P.; dos Reis, A.C. Analysis of the influence of implant shape on primary stability using the correlation of multiple methods. Clin. Oral Investig. 2015, 19, 1861–1866. [Google Scholar] [CrossRef] [PubMed]
- Waechter, J.; Madruga, M.M.; Carmo Filho, L.C.D.; Leite, F.R.M.; Schinestsck, A.R.; Faot, F. Comparison between tapered and cylindrical implants in the posterior regions of the mandible: A prospective, randomized, split-mouth clinical trial focusing on implant stability changes during early healing. Clin. Implant. Dent. Relat. Res. 2017, 19, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.; Back, T.; Hirsch, J.M. Cutting torque measurements in conjunction with implant placement in grafted and nongrafted maxillas as an objective evaluation of bone density: A possible method for identifying early implant failures? Clin. Implant. Dent. Relat. Res. 2004, 6, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Friberg, B.; Sennerby, L.; Roos, J.; Johansson, P.; Strid, C.G.; Lekholm, U. Evaluation of bone density using cutting resistance measurements and microradiography: An in vitro study in pig ribs. Clin. Oral Implants Res. 1995, 6, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Friberg, B.; Sennerby, L.; Roos, J.; Lekholm, U. Identification of bone quality in conjunction with insertion of titanium implants. A pilot study in jaw autopsy specimens. Clin. Oral Implants Res. 1995, 6, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, D.A.; Arosio, P.; Pagnutti, S. A possible novel objective intraoperative measurement of maxillary bone density. Minerva Stomatol. 2013, 62, 259–265. [Google Scholar] [PubMed]
- Di Stefano, D.A.; Arosio, P.; Piattelli, A.; Perrotti, V.; Iezzi, G. A torque-measuring micromotor provides operator independent measurements marking four different density areas in maxillae. J. Adv. Prosthodont. 2015, 7, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Cappare, P.; Vinci, R.; Di Stefano, D.A.; Traini, T.; Pantaleo, G.; Gherlone, E.F.; Gastaldi, G. Correlation between Initial BIC and the Insertion Torque/Depth Integral Recorded with an Instantaneous Torque-Measuring Implant Motor: An in vivo Study. Clin. Implant. Dent. Relat. Res. 2015, 17 (Suppl. 2), e613–e620. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, G.; Scarano, A.; Di Stefano, D.A.; Arosio, P.; Doi, K.; Ricci, L.; Piattelli, A.; Perrotti, V. Correlation between the bone density recorded by a computerized implant motor and by a histomorphometric analysis: A preliminary in vitro study on bovine ribs. Clin. Implant. Dent. Relat. Res. 2015, 17 (Suppl. 1), e35–e44. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, D.A.; Arosio, P.; Gastaldi, G.; Gherlone, E. The insertion torque-depth curve integral as a measure of implant primary stability: An in vitro study on polyurethane foam blocks. J. Prosthet. Dent. 2018, 120, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, D.A.; Arosio, P. Correlation Between Bone Density and Instantaneous Torque at Implant Site Preparation: A Validation on Polyurethane Foam Blocks of a Device Assessing Density of Jawbones. Int. J. Oral Maxillofac. Implants 2016, 31, e128–e135. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, G.F.A.; Di Stefano, D.; Arosio, P.; Piattelli, A.; Scarano, A.; Perrotti, V. A site-specific intraoperative measurement of bone-to-implant contact during implant insertion: A study on bovine ribs using a computerized implant motor. J. Dent. Sci. 2015, 10, 21–27. [Google Scholar] [CrossRef]
- Andrade, J.M.; Estevez-Perez, M.G. Statistical comparison of the slopes of two regression lines: A tutorial. Anal. Chim. Acta 2014, 838, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.M.; Lee, M.S.; Wang, J.S.; Lin, L.D. The effect of implant design and bone quality on insertion torque, resonance frequency analysis, and insertion energy during implant placement in low or low- to medium-density bone. Int. J. Prosthodont. 2015, 28, 40–47. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, D.; Sennerby, L.; Meredith, N. Influence of implant taper on the primary and secondary stability of osseointegrated titanium implants. Clin. Oral Implants Res. 2004, 15, 474–480. [Google Scholar] [CrossRef] [PubMed]

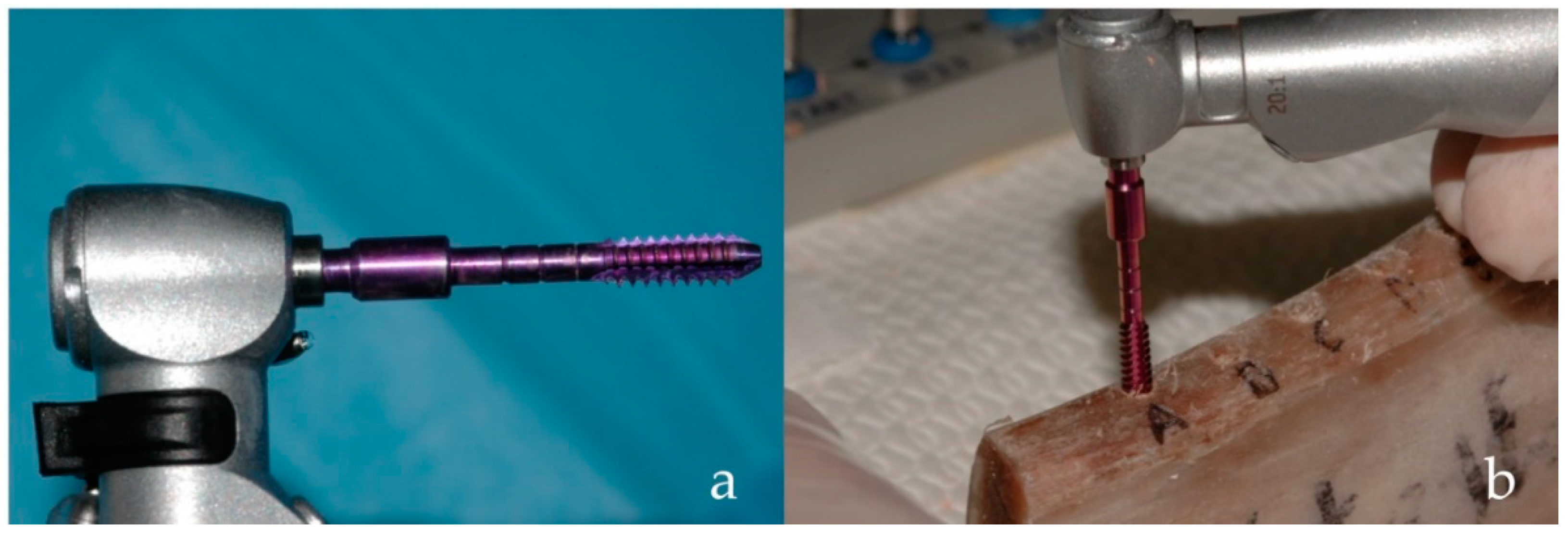
| Implant Characteristics | |||
|---|---|---|---|
| Implant type | C | CT | CM |
| Commercial name | Stone | Tiger Due | Aries Due |
| Head diameter | 4.0 | 4.3 | 4.0 |
| Thread diameter | 4.0 | 4.3 | 3.75 |
| Body diameter | 3.2 | 3.6 | 3.4 |
| Length | 12 | 12 | 12 |
| Thread | Single | Double | Single |
| Thread type | Standard V-thread | Standard V-thread and spiral | Standard V-thread |
| Thread pitch | 0.75 | 0.75 (lead 1.50) | 0.75 |
| Thread depth | 0.4 | 0.4 | 0.175 |
| Threading at neck | Triple threaded, 0.75 mm pitch | Six-fold threaded, 1.50 mm pitch | Triple threaded, 0.75 mm pitch |
| Final drilling sequence | |||
| Final drill | 3 | 3 | 3 |
| Countersink drill | 4 | 4.3 | 4 |
| Measure | Symbol as Provided by the Micromotor | Unit of Measure | Information Provided |
|---|---|---|---|
| Average torque | Cm | Ncm | When recorded at probing, it is a quantity measuring bone density. The mathematical relationship between Cm and density, D, (expressed as g/cm3), when no irrigation is used, is given by the equation D = (Cm + 11.93)/43.97 [46]. |
| Peak torque | Cp | Ncm | When recorded at implant insertion, it is the maximum torque that was exerted by the micromotor during implant placement. In the present work, it has been indicated with the acronym IT (insertion torque). |
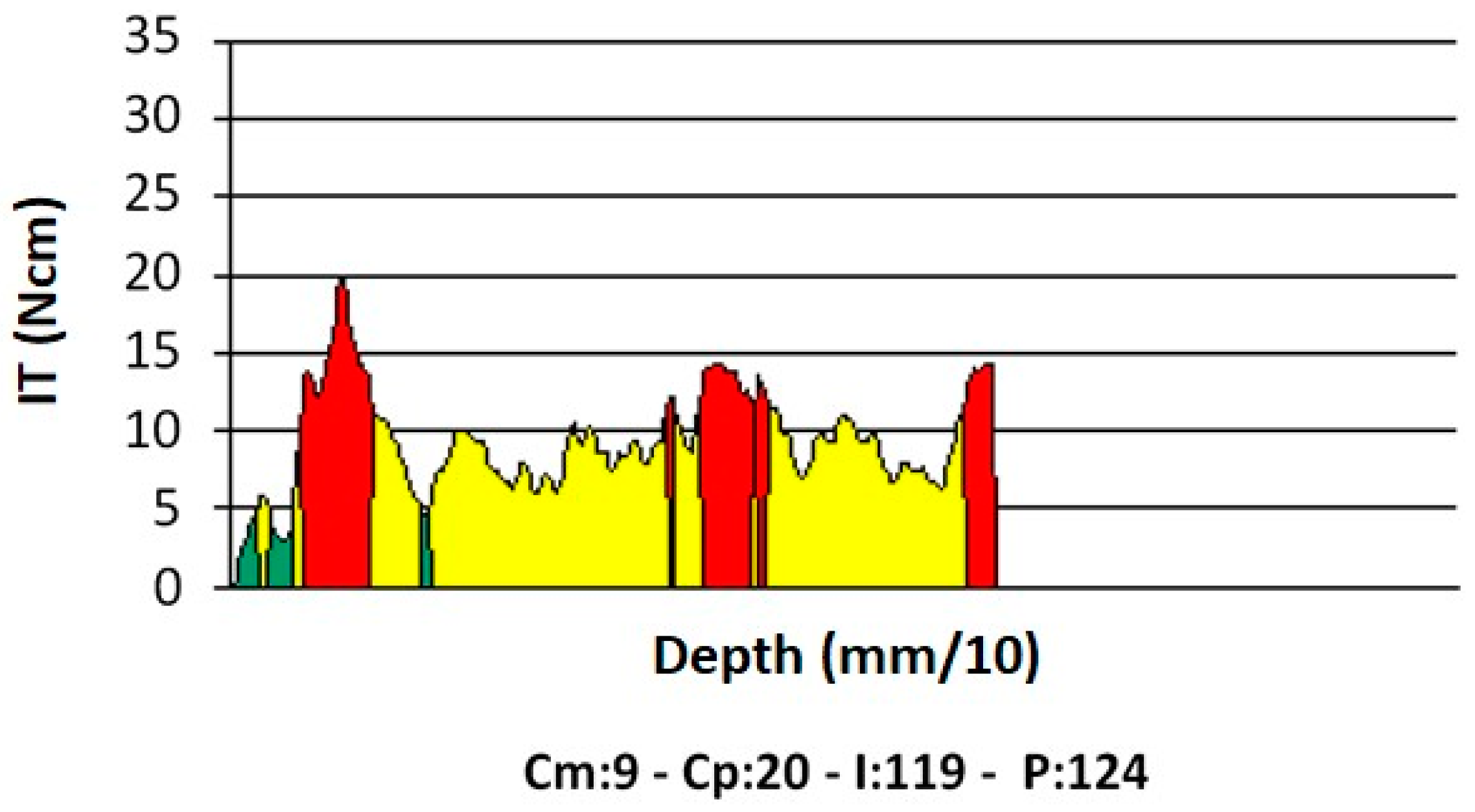
| Integral | I | Ncm2/100 | When measured at implant insertion, it provides the area bounded by the torque–depth curve (Figure 3). If the implant threads are evenly spaced and the rotation speed at insertion is constant (two conditions that are met in this study), the integral (I) is equal to the insertion energy, IE, multiplied by a constant factor. |
| Depth | P | mm/10 | Indicates the depth reached by the probe, when density is being measured, or by the implant when it is being placed. |
| Plot | Implant Type | Parameters of the Regression Line | Pearson’s r | |
|---|---|---|---|---|
| m (slope) | q (intercept) | |||
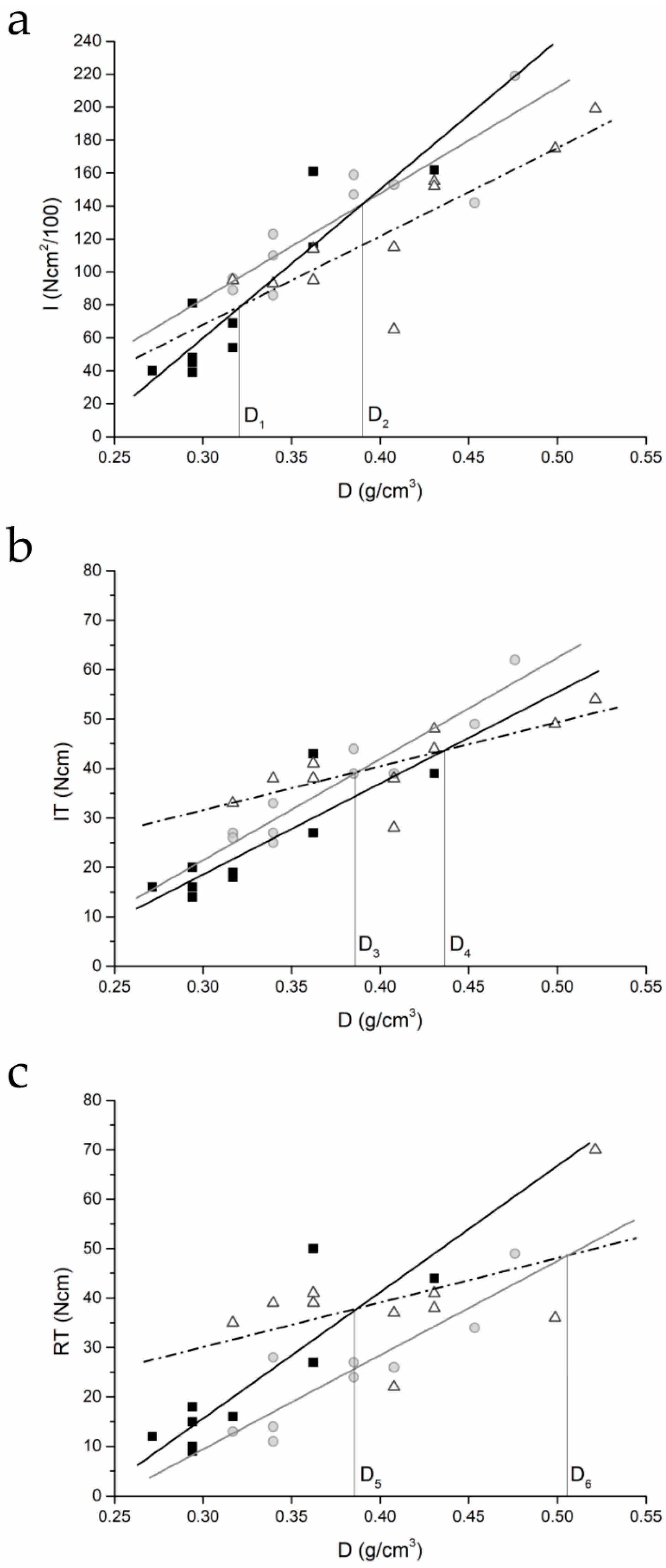
| I–density | C | 904.4 ± 152.4 | −211.3 ± 49.8 | 0.9027 |
| CT | 639.8 ± 122.3 | −108.1 ± 46.5 | 0.8795 | |
| CM | 535.2 ± 125.9 | −92.4 ± 51.9 | 0.8325 | |
| IT–density | C | 184.9 ± 38.1 | −37.0 ± 12.4 | 0.8640 |
| CT | 204.8 ± 23.7 | −39.9 ± 9.0 | 0.9504 | |
| CM | 89.1 ± 27.5 | +4.8 ± 11.4 | 0.7526 | |
| RT–density | C | 255.4 ± 54.3 | −60.9 ± 17.7 | 0.8569 |
| CT | 190.4 ± 33.0 | −47.7 ± 12.5 | 0.8981 | |
| CM | 90.2 ± 55.3 | +3.0 ± 22.8 | 0.4998 | |
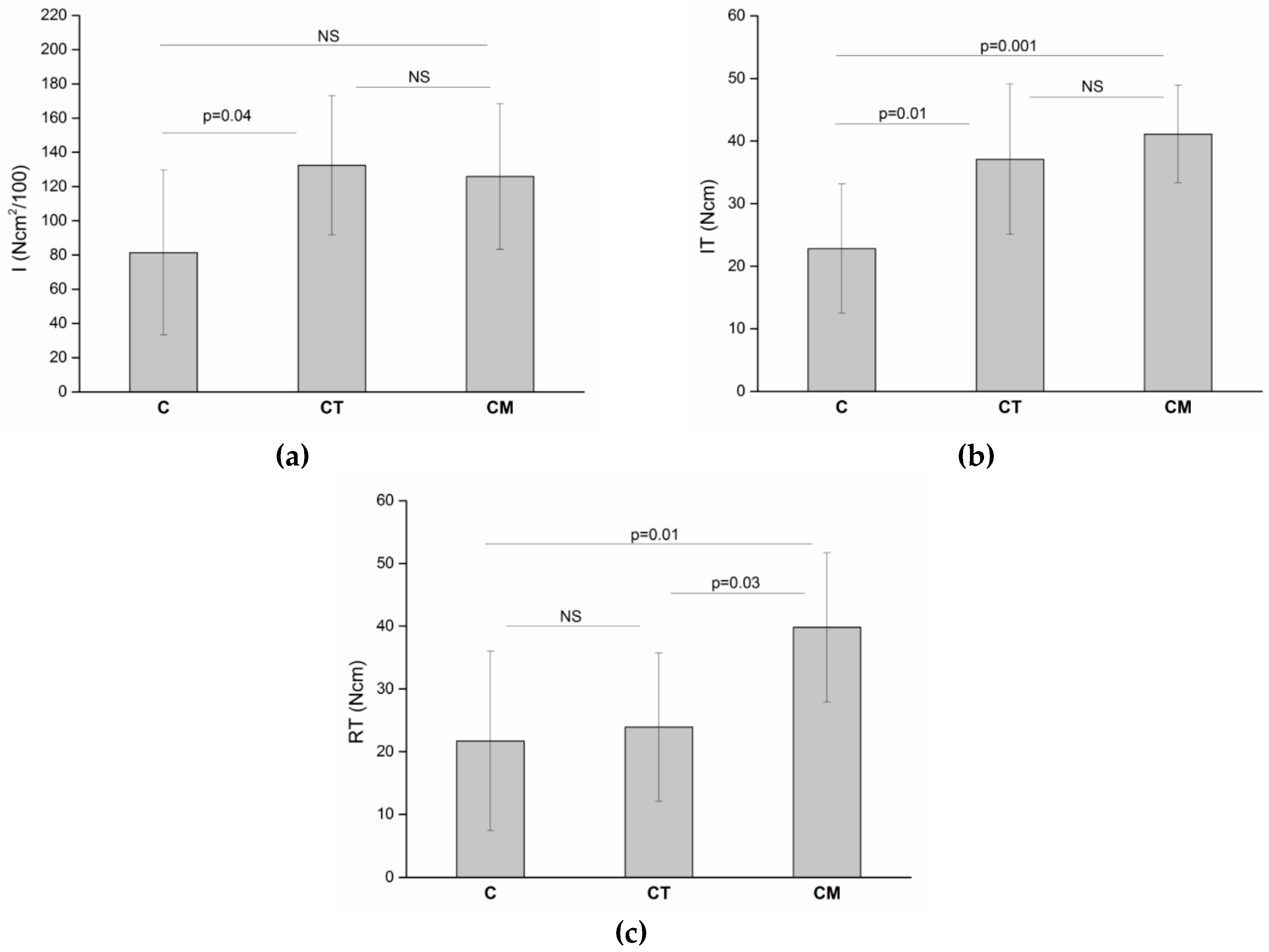
| Implant Type | I | IT | RT |
|---|---|---|---|
| C | 81.4 ± 48.1 | 22.8 ± 10.3 | 21.7 ± 14.3 |
| CT | 132.4 ± 40.7 | 37.1 ± 12.05 | 23.9 ± 11.8 |
| CM | 125.8 ± 42.5 | 41.1 ± 7.8 | 39.8 ± 11.9 |
| P | 0.03 | 0.001 | 0.007 |
| Implant Type | I vs. IT | I vs. RT | IT vs. RT |
|---|---|---|---|
| C | <0.001 * | 0.001 * | 0.30 (NS) |
| CT | 0.003 * | 0.003 * | 0.73 (NS) |
| CM | 0.003 * | 0.005 * | 0.98 (NS) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Stefano, D.A.; Arosio, P.; Perrotti, V.; Iezzi, G.; Scarano, A.; Piattelli, A. Correlation between Implant Geometry, Bone Density, and the Insertion Torque/Depth Integral: A Study on Bovine Ribs. Dent. J. 2019, 7, 25. https://doi.org/10.3390/dj7010025
Di Stefano DA, Arosio P, Perrotti V, Iezzi G, Scarano A, Piattelli A. Correlation between Implant Geometry, Bone Density, and the Insertion Torque/Depth Integral: A Study on Bovine Ribs. Dentistry Journal. 2019; 7(1):25. https://doi.org/10.3390/dj7010025
Chicago/Turabian StyleDi Stefano, Danilo Alessio, Paolo Arosio, Vittoria Perrotti, Giovanna Iezzi, Antonio Scarano, and Adriano Piattelli. 2019. "Correlation between Implant Geometry, Bone Density, and the Insertion Torque/Depth Integral: A Study on Bovine Ribs" Dentistry Journal 7, no. 1: 25. https://doi.org/10.3390/dj7010025
APA StyleDi Stefano, D. A., Arosio, P., Perrotti, V., Iezzi, G., Scarano, A., & Piattelli, A. (2019). Correlation between Implant Geometry, Bone Density, and the Insertion Torque/Depth Integral: A Study on Bovine Ribs. Dentistry Journal, 7(1), 25. https://doi.org/10.3390/dj7010025









