Detection of Caries Around Resin-Modified Glass Ionomer and Compomer Restorations Using Four Different Modalities In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- Dyract eXtra Dentsply Refill Compules Shade A2 Lot., 1608001074; Expiry August 2018 (3M ESPE St. Paul MN., USA).
- Ketac Nano 3M Shade A2 Ref. 3304A2 Lot., N733107; Expiry May 2017 (Dentsply DeTrey GmbH, Konstanz, Germany).
- Compoglass F Ivoclar Vivadent Refill: Shade 140/A2 Lot., V19970; Expiry October 2018 (Ivoclar Vivadent AG, Schaan, Liechtenstein).
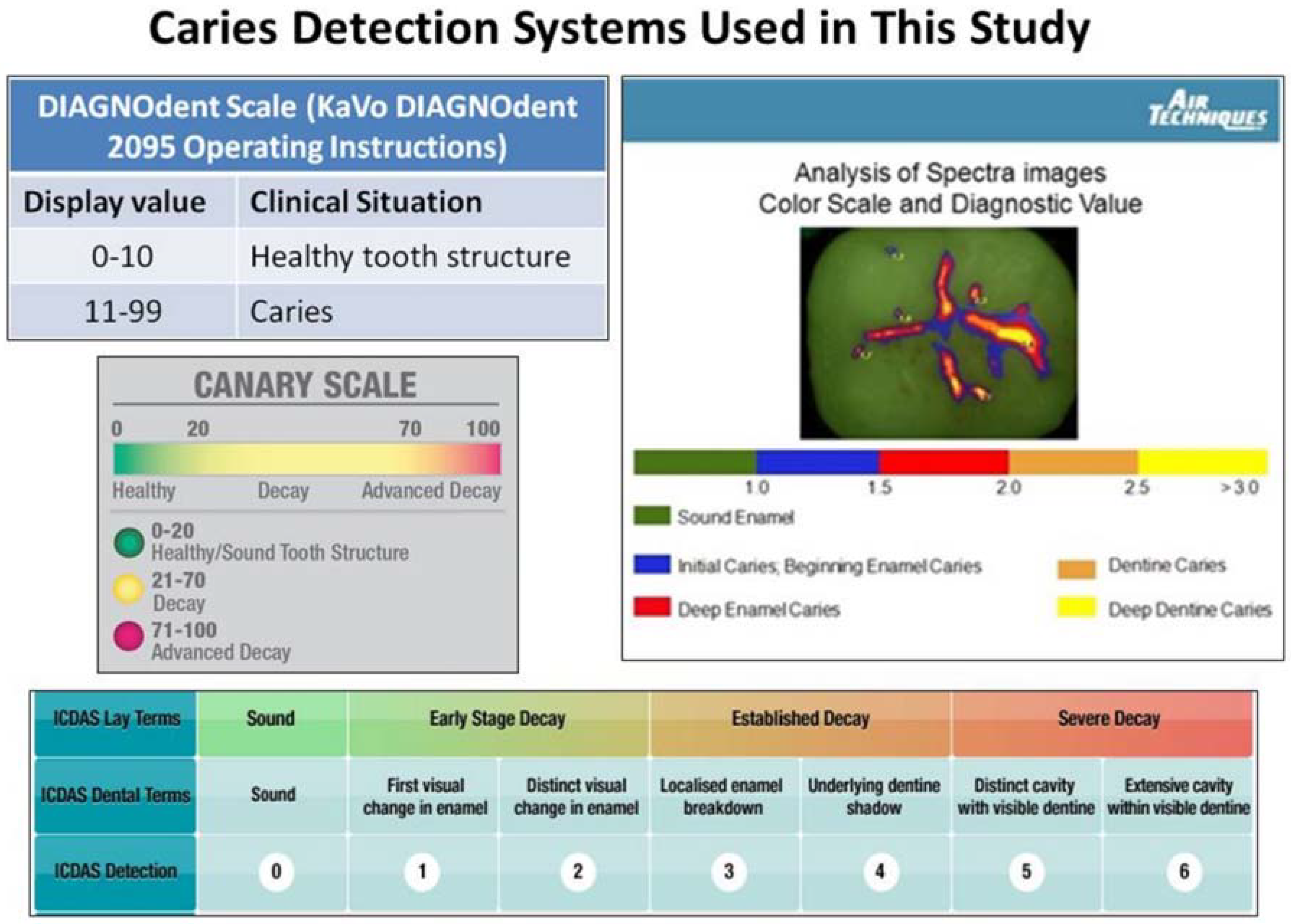
2.2. ICDAS II Visual Examination
- 0
- Sound tooth surface;
- 1
- First visual change in enamel (on a dry tooth surface);
- 2
- Distinct visual change on enamel surface (on a moist and dry tooth surface);
- 3
- Localized enamel breakdown due to caries with no exposed dentin or shadowing beneath the tooth surface;
- 4
- Dark shadow beneath the tooth surface from dentin;
- 5
- Distinct cavity with visible dentin;
- 6
- Extensive distinct cavity with visible dentin and more than half of the surface involved.
2.3. SPECTRA Caries System Examination
2.4. DIAGNODent Examination
2.5. The Canary System Examination
2.6. Blinding of the Participants in This Study
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kopperud, S.E.; Tveit, A.B.; Gaarden, T.; Sandvik, L.; Espelid, I. Longevity of posterior dental restorations and reasons for failure. Eur. J. Oral Sci. 2012, 120, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Hickel, R.; Manhart, J. Longevity of restorations in posterior teeth and reasons for failure. J. Adhes. Dent. 2001, 3, 45–64. [Google Scholar] [PubMed]
- Hara, A.T.; Magalhaes, C.S.; Serra, M.C.; Rodrigues, A.L., Jr. Cariostatic effect of fluoride-containing restorative systems associated with dentifrices on root dentin. J. Dent. 2002, 30, 205–212. [Google Scholar] [CrossRef]
- Serra, M.C.; Cury, J.A. The in vitro effect of glass-ionomer cement restoration on enamel subjected to a demineralization and remineralization model. Quintessence Int. 1992, 23, 143–147. [Google Scholar] [PubMed]
- Tedesco, T.K.; Bonifacio, C.C.; Calvo, A.F.; Gimenez, T.; Braga, M.M.; Raggio, D.P. Caries lesion prevention and arrestment in approximal surfaces in contact with glass ionomer cement restorations—A systematic review and meta-analysis. Int. J. Paediatr. Dent. 2016, 26, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Raggio, D.P.; Tedesco, T.K.; Calvo, A.F.; Braga, M.M. Do glass ionomer cements prevent caries lesions in margins of restorations in primary teeth? A systematic review and meta-analysis. J. Am. Dent. Assoc. 2016, 147, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Mickenautsch, S.; Yengopal, V. Absence of carious lesions at margins of glass-ionomer cement and amalgam restorations: An update of systematic review evidence. BMC Res. Notes 2011, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Yengopal, V.; Mickenautsch, S. Caries-preventive effect of resin-modified glass-ionomer cement (RM-GIC) versus composite resin: A quantitative systematic review. Eur. Arch. Paediatr. Dent. 2011, 12, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Pendrys, D.G. Resin-modified glass-ionomer cement (RM-GIC) may provide greater caries preventive effect compared with composite resin, but high-quality studies are needed. J. Evid.-Based Dent. Pract. 2011, 11, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Kidd, E.A.; Toffenetti, F.; Mjör, I.A. Secondary Caries. Int. Dent. J. 1992, 42, 127–138. [Google Scholar] [PubMed]
- Diniz, M.B.; Eckert, G.J.; González-Cabezas, C.; Cordeiro, R.d.C.L.; Ferreira-Zandona, A.G. Caries Detection around Restorations Using ICDAS and Optical Devices. J. Esthet. Restor. Dent. 2016, 28, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; González-Cabezas, C.; Isaacs, R.L.; Eckert, G.J.; Stookey, G.K. Evaluation of several techniques for the detection of secondary caries adjacent to amalgam restorations. Caries Res. 2004, 38, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Mjör, I. Clinical diagnosis of recurrent caries. J. Am. Dent. Assoc. 2005, 136, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- FDI World Dental Federation. FDI policy statement on Classification of caries lesions of tooth surfaces and caries management systems: Adopted by the FDI General Assembly: 17 September 2011, Mexico City, Mexico. Int. Dent. J. 2013, 63, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.; Johnston, S.; Hewson, N.; Van Dijk, W.; Reich, E.; Eiselé, J.L.; Bourgeois, D. FDI Global Caries Initiative; implementing a paradigm shift in dental practice and the global policy context. Int. Dent. J. 2012, 62, 169–174. [Google Scholar] [CrossRef] [PubMed]
- FDI World Dental Federation. Caries Prevention and Management Chairside Guide. 2017. Available online: http://www.fdiworlddental.org/sites/default/files/media/resources/2017-fdi_cpp-chairside_guide.pdf (accessed on 12 September 2018).
- Ekstrand, K.; Martignon, S.; Ricketts, D.J.; Qvist, V. Detection and activity assessment of primary coronal caries lesions: A methodologic study. Oper. Dent. 2007, 32, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.A.; Hug, I.; Diniz, M.B.; Lussi, A. Performance of fluorescence methods, radiographic examination and ICDAS II on occlusal surfaces in vitro. Caries Res. 2008, 42, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Diniz, M.B.; Rodrigues, J.A.; Hug, I.; Cordeiro Rde, C.; Lussi, A. Reproducibility and accuracy of the ICDAS-II for occlusal caries detection. Community Dent. Oral Epidemiol. 2009, 37, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Jablonski-Momeni, A.; Stachniss, V.; Ricketts, D.N.; Heinzel-Gutenbrunner, M.; Pieper, K. Reproducibility and accuracy of the ICDAS-II for detection of occlusal caries in vitro. Caries Res. 2008, 42, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Bamzahim, M.; Shi, X.Q.; Angmar-Månsson, B. Secondary caries detection by DIAGNOdent and radiography: A comparative in vitro study. Acta Odontol. Scand. 2004, 62, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Lussi, A.; Hellwig, E. Performance of a new laser fluorescence device for the detection of occlusal caries in vitro. J. Dent. 2006, 34, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Spaveras, A.; Karkazi, F.; Antoniadou, M. Caries detection with laser fluorescence devices. Limitations of their use. Stoma Educ. J. 2017, 4, 46–53. [Google Scholar] [CrossRef]
- Neuhaus, K.W.; Rodrigues, J.A.; Seemann, R.; Lussi, A. Detection of proximal secondary caries at cervical class II-amalgam restoration margins in vitro. J. Dent. 2012, 40, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Hitij, T.; Fidler, A. Effect of dental material fluorescence on DIAGNOdent readings. Acta Odontol. Scand. 2008, 66, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Nokhbatolfoghahaie, H.; Alikhasi, M.; Chiniforush, N.; Khoei, F.; Safavi, N.; Zadeh, B.Y. Evaluation of Accuracy of DIAGNOdent in Diagnosis of Primary and Secondary Caries in Comparison to Conventional Methods. J. Lasers Med. Sci. 2013, 4, 149–167. [Google Scholar]
- Rechmann, P.; Charland, D.; Rechmann, B.M.; Featherstone, J.D. Performance of laser fluorescence devices and visual examination for the detection of occlusal caries in permanent molars. J. Biomed. Opt. 2012, 17, 036006. [Google Scholar] [CrossRef] [PubMed]
- Achilleos, E.E.; Rahiotis, C.; Kakaboura, A.; Vougiouklakis, G. Evaluation of a new fluorescence-based device in the detection of incipient occlusal caries lesions. Lasers Med. Sci. 2013, 28, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Gutta, A.; Merdad, H.E. In vitro study of the diagnostic performance of the Spectra Caries Detection Aid. J. Clin. Dent. 2015, 26, 17–22. [Google Scholar]
- Konigm, K.; Flelviming, G.; Hibst, R. Laser-induced autofluorescence spectroscopy of dental caries. Cell Mol. Biol. 1998, 44, 1293–1300. [Google Scholar]
- Graye, M.; Markowitz, K.; Strickland, M.; Guzy, G.; Burke, M.; Houpt, M. In vitro evaluation of the Spectra early caries detection system. J. Clin. Dent. 2012, 23, 1–6. [Google Scholar] [PubMed]
- Melo, M.; Pascual, A.; Camps, I.; Del Campo, A.; Ata-Ali, J. Caries diagnosis using light fluorescence devices in comparison with traditional visual and tactile evaluation: A prospective study in 152 patients. Odontology 2017, 105, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Matos, R.; Novaes, T.F.; Braga, M.M.; Siqueira, W.L.; Duarte, D.A.; Mendes, F.M. Clinical performance of two fluorescence-based methods in detecting occlusal caries lesions in primary teeth. Caries Res. 2011, 45, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, T.; Braga, M.M.; Raggio, D.P.; Deery, C.; Ricketts, D.N.; Mendes, F.M. Fluorescence-based methods for detecting caries lesions: Systematic review, meta-analysis and sources of heterogeneity. PLoS ONE 2013, 8, e60421. [Google Scholar] [CrossRef] [PubMed]
- Jablonski-Momeni, A.; Heinzel-Gutenbrunner, M.; Klein, S.M.C. In vivo performance of the VistaProof fluorescence-based camera for detection of occlusal lesions. Clin. Oral Investig. 2014, 18, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, K.; Rosenfeld, D.; Peikes, D.; Guzy, G.; Rosivack, G. Effect of pit and fissure sealants on caries detection by a fluorescent camera system. J. Dent. 2013, 41, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Silvertown, J.D.; Wong, B.P.; Abrams, S.H.; Sivagurunathan, K.S.; Mathews, S.M.; Amaechi, B.T. Comparison of The Canary System and DIAGNOdent for the in vitro detection of caries under opaque dental sealants. J. Investig. Clin. Dent. 2016. [Google Scholar] [CrossRef] [PubMed]
- Abrams, T.E.; Abrams, S.H.; Sivagurunathan, K.; Silvertown, J.D.; Hellen, W.; Elman, G.I.; Amaechi, B.T. In Vitro Detection of Caries Around Amalgam Restorations Using Four Different Modalities. Open Dent. J. 2017, 11, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Jeon, R.J.; Phan, T.D.T.; Wu, A.; Kulkarni, G.; Abrams, S.H.; Mandelis, A. Photothermal radiometric quantitative detection of the different degrees of demineralization of dental enamel by acid etching. J. Phys. IV Fr. 2005, 125, 721–772. [Google Scholar] [CrossRef]
- Abrams, S.H.; Sivagurunathan, K.; Silvertown, J.D.; Wong, B.; Hellen, A.; Mandelis, A.; Hellen, W.M.P.; Elman, G.I.; Mathew, S.K.; Mensinkai, P.K.; et al. Correlation with Caries Lesion Depth of The Canary System, DIAGNOdent and ICDAS II. Open Dent. J. 2017, 11, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Jeon, R.J.; Matvienko, A.; Mandelis, A.; Abrams, S.H.; Amaechi, B.T.; Kulkarni, G. Detection of interproximal demineralized lesions on human teeth in vitro using frequency-domain infrared photothermal radiometry and modulated luminescence. J. Biomed. Opt. 2007, 12, 034028. [Google Scholar] [CrossRef] [PubMed]
- Silvertown, J.D.; Abrams, S.H.; Sivagurunathana, K.S.; Kennedy, J.; Jeon, J.; Mandelis, A.; Hellen, A.; Hellen, W.; Elman, G.; Ehrlich, R.; et al. Multi-centre clinical evaluation of photothermal radiometry and luminescence correlated with international benchmarks for caries detection. Open Dent. J. 2017, 11, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Matvienko, A.; Jeon, R.J.; Mandelis, A.; Abrams, S.H.; Amaechi, B.T. Photothermal detection of incipient dental caries: Experiment and modeling. Proc. SPIE 2007, 6759. [Google Scholar] [CrossRef]
- Jeon, R.J.; Hellen, A.; Matvienko, A.; Mandelis, A.; Abrams, S.; Amaechi, B.T. Experimental Investigation of Demineralization and Remineralization of Human Teeth Using Infrared Photothermal Radiometry and Modulated Luminescence. Proc. SPIE 2008, 6856. [Google Scholar] [CrossRef]
- Matvienko, A.; Mandelis, A.; Abrams, S. Robust multiparameter method of evaluating the optical and thermal properties of a layered tissue structure using photothermal radiometry. Appl. Opt. 2009, 48, 3192–3203. [Google Scholar] [CrossRef] [PubMed]
- Silvertown, J.D.; Wong, B.P.; Sivagurunathan, K.S.; Abrams, S.H.; Kirkham, J.; Amaechi, B.T. Remineralization of natural early caries lesions in vitro by P11-4 monitored with photothermal radiometry and luminescence. J. Investig. Clin. Dent. 2017. [Google Scholar] [CrossRef] [PubMed]
- Matvienko, A.; Jeon, R.J.; Mandelis, A.; Abrams, S.H.; Amaechi, B.T. Photothermal Detection of Incipient Dental Caries: Experiment and Modeling. Photonics East Proc. SPIE 2007, 6759. [Google Scholar] [CrossRef]
- Hellen, A.; Mandelis, A.; Finer, Y.; Amaechi, B.T. Quantitative remineralization evolution kinetics of artificially demineralized human enamel using photothermal radiometry and modulated luminescence. J. Biophotonics 2011, 4, 788–804. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Ismail, A.I.; Martignon, S.; Ekstrand, K.; Douglas, G.A.; Longbottom, C. ICCMS Guide for Practitioners and Educators. Available online: https://www.iccms-web.com/uploads/asset/59284654c0a6f822230100.pdf (accessed on 12 September 2018).
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2016. [Google Scholar]
- Ferracane, J. Models of Caries Formation around Dental Composite Restorations. J. Dent. Res. 2017, 96, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Boston, D. Initial in vitro evaluation of DIAGNOdent for detecting secondary carious lesions associated with resin composite restorations. Quintessence Int. 2003, 34, 109–116. [Google Scholar] [PubMed]
- Sarrett, D.C. Prediction of clinical outcomes of a restoration based on in vivo marginal quality evaluation. J. Adhes. Dent. 2007, 9 (Suppl. 1), 117–120. [Google Scholar] [PubMed]
- Lino, J.R.; Ramos-Jorge, J.; Coelho, V.S.; Ramos-Jorge, M.L.; Moyses, M.R.; Ribeiro, J.C. Association and comparison between visual inspection and bitewing radiography for the detection of recurrent dental caries under restorations. Int. Dent. J. 2015, 65, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Gostanian, H.V.; Shey, Z.; Kasinathan, C.; Caceda, J.; Janal, M.N. An in vitro evaluation of the effect of sealant characteristics on laser fluorescence for caries detection. Pediatr. Dent. 2006, 28, 445–450. [Google Scholar] [PubMed]
- Hosoya, Y.; Matsuzaka, K.; Inoue, T. Influence of tooth-polishing pastes and sealants on DIAGNOdent values. Quintessence Int. 2004, 35, 605–611. [Google Scholar] [CrossRef]
- Lussi, A.; Reich, E. The influence of toothpastes and prophylaxis pastes on fluorescence measurements for caries detection in vitro. Eur. J. Oral Sci. 2005, 113, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G. New Caries Detection Systems Reliable & Accurate. Clin. Rep. 2012, 5, 1–2. [Google Scholar]
- Liang, R.; Wong, V.; Marcus, M.; Burns, P.; McLaughlin, P. Multimodal imaging system for dental caries detection. Proc. SPIE Lasers Dent. 2007, 6425. [Google Scholar] [CrossRef]
- Hall, A.; Girkin, J.M. A review of potential new diagnostic modalities for caries lesions. J. Dent. Res. 2004, 83, C89–C94. [Google Scholar] [CrossRef] [PubMed]
- Bamzahim, M.; Aljehani, A.; Shi, X.-Q. Clinical performance of DIAGNOdent in the detection of secondary carious lesions. Acta Odontol. Scand. 2005, 63, 26–30. [Google Scholar] [CrossRef] [PubMed]
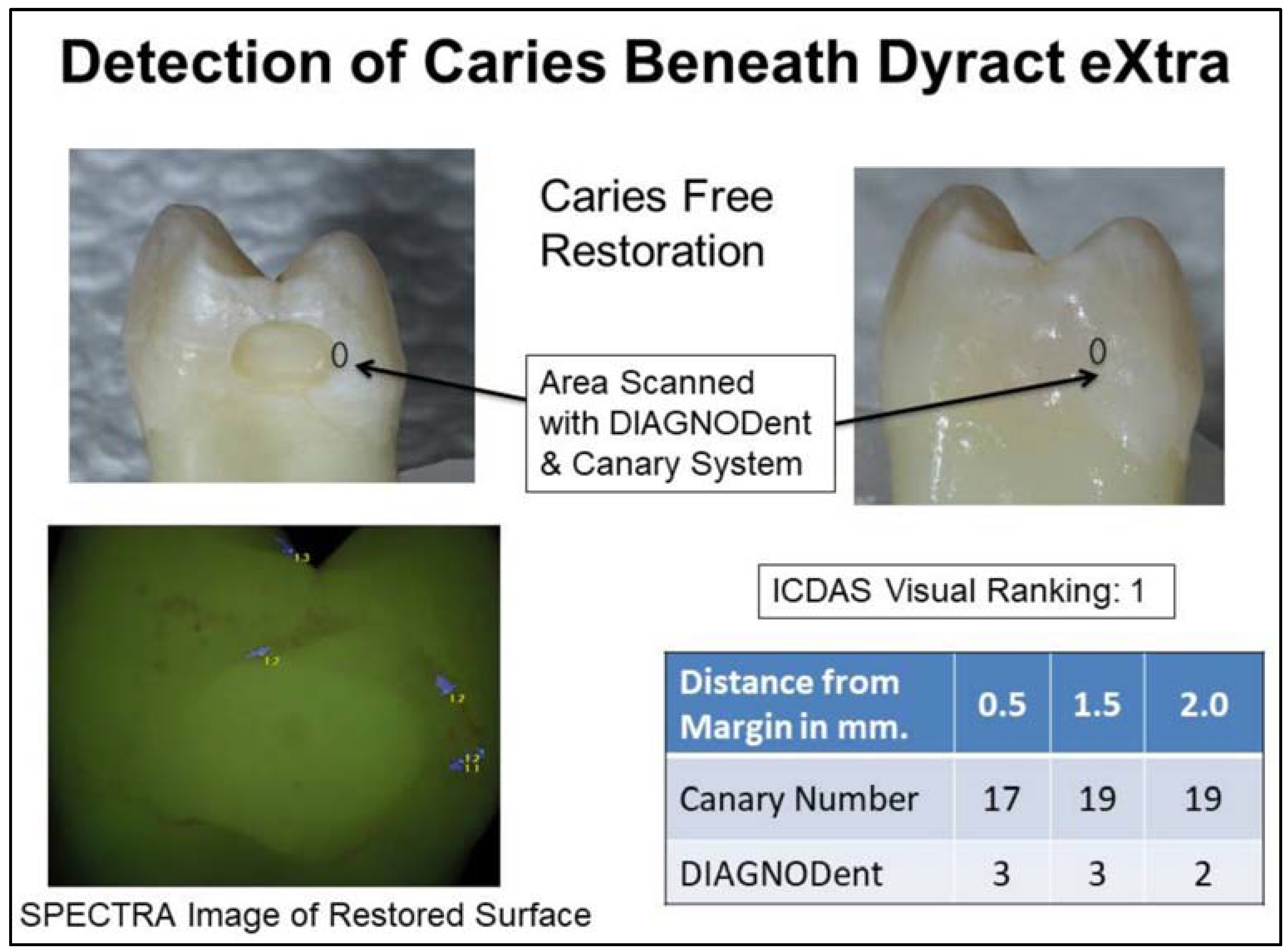

| Distance from the Margins of the Restoration | Canary Number | Peak DIAGNODent | ||||
|---|---|---|---|---|---|---|
| (21–100 Denotes Caries) | (11–99 Denotes Caries) | |||||
| Sound Teeth | Carious Teeth | p-Value 1 | Sound Teeth | Carious Teeth | p-Value 1 | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| By Distance from Margin (All Materials) | ||||||
| At margin | 20.1 (5.7) | 47.7 (19.9) | <0.001 | 17.2 (10.6) | 19.5 (18.7) | 0.414 |
| 0.5 mm | 18.8 (3.3) | 46.8 (18.7) | <0.001 | 10 (7.3) | 11.7 (15.4) | 0.396 |
| 1.5 mm | 19.3 (4.7) | 45 (15.7) | <0.001 | 7.5 (5.9) | 9.4 (7.8) | 0.122 |
| 2 mm | 18.3 (2.6) | 52.2 (19.6) | <0.001 | 5 (3.4) | 8.6 (8.8) | 0.076 |
| Dyract eXtra | ||||||
| At margin | 19.9 (6.3) | 32.6 (12.5) | 0.014 | 20.5 (11.7) | 21 (18.5) | 0.941 |
| 0.5 mm | 17.1 (2.4) | 31.8 (9.4) | <0.001 | 5.1 (1.6) | 9.6 (6.6) | 0.073 |
| 1.5 mm | 17.4 (1.5) | 37.2 (13.4) | 0.003 | 4.9 (1.1) | 7.7 (4.1) | 0.076 |
| 2 mm | 18.6 (1.3) | 40.3 (12.2) | 0.002 | 4.5 (1.6) | 10.8 (13.6) | 0.334 |
| Ketac Nano | ||||||
| At margin | 19.2 (5.8) | 63.2 (14.8) | <0.001 | 11.7 (6.4) | 23.9 (24.9) | 0.211 |
| 0.5 mm | 18.6 (3.7) | 63.7 (13.7) | <0.001 | 11.5 (9.1) | 18.1 (24.4) | 0.496 |
| 1.5 mm | 18.7 (4.1) | 56.7 (16) | <0.001 | 8.9 (7.7) | 15.1 (11.1) | 0.212 |
| 2 mm | 17.8 (3.9) | 68.4 (22.5) | <0.001 | 5.8 (5.2) | 10.7 (6.5) | 0.127 |
| Compoglass F | ||||||
| At margin | 21.4 (5.7) | 47.4 (19) | 0.005 | 19.3 (12) | 12.6 (4.6) | 0.238 |
| 0.5 mm | 21.3 (2.7) | 44.5 (16.1) | <0.001 | 14.8 (6.5) | 6.6 (2.5) | 0.028 |
| 1.5 mm | 22.7 (6.7) | 42.2 (11) | 0.001 | 9.4 (6.9) | 5.8 (2.2) | 0.097 |
| 2 mm | 18.8 (0.5) | 46.8 (9.4) | <0.001 | 4.4 (1) | 4.5 (1.3) | 0.93 |
| Caries Detection System | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|
| ICDAS II | 34.9 (22.4, 49.8) | 52.4 (32.4, 71.7) |
| SPECTRA | 34.9 (22.4, 49.8) | 61.9 (40.9, 79.2) |
| Peak DIAGNODent at margin | 69.8 (54.9, 81.4) | 14.3 (5, 34.6) |
| Peak DIAGNODent at 0.5 mm from margin | 30.2 (18.6, 45.1) | 66.7 (45.4, 82.8) |
| Peak DIAGNODent at 1.5 mm from margin | 19.5 (10.2, 34) | 90.5 (71.1, 97.3) |
| Peak DIAGNODent at 2 mm from margin | 18.8 (8.9, 35.3) | 92.9 (68.5, 98.7) |
| Canary Number at Margin | 97.7 (87.9, 99.61) | 76.2 (54.9, 89.4) |
| Canary Number at 0.5 mm from margin | 90.7 (78.4, 96.3) | 81 (60, 92.3) |
| Canary Number at 1.5 mm from margin | 95.1 (83.9, 98,7) | 71.4 (50, 86.2) |
| Canary Number at 2 mm from margin | 100 (89.3, 100) | 92.9 (68.5, 98.7) |
| Restorative Materials | Canary Number | Peak DIAGNODent |
|---|---|---|
| ICC 1 (95% CI) | ICC 1 (95% CI) | |
| All materials | 0.99 (0.99, 1.0) | 0.98 (0.98, 0.99) |
| Dyract eXtra | 0.98 (0.97, 0.99) | 0.89 (0.78, 0.95) |
| Ketac Nano | 0.99 (0.99, 1.0) | 0.99 (0.98, 1.0) |
| Compglass F | 0.99 (0.97, 0.99) | 0.96 (0.92, 0.98) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abrams, T.; Abrams, S.; Sivagurunathan, K.; Moravan, V.; Hellen, W.; Elman, G.; Amaechi, B.; Mandelis, A. Detection of Caries Around Resin-Modified Glass Ionomer and Compomer Restorations Using Four Different Modalities In Vitro. Dent. J. 2018, 6, 47. https://doi.org/10.3390/dj6030047
Abrams T, Abrams S, Sivagurunathan K, Moravan V, Hellen W, Elman G, Amaechi B, Mandelis A. Detection of Caries Around Resin-Modified Glass Ionomer and Compomer Restorations Using Four Different Modalities In Vitro. Dentistry Journal. 2018; 6(3):47. https://doi.org/10.3390/dj6030047
Chicago/Turabian StyleAbrams, Tamara, Stephen Abrams, Koneswaran Sivagurunathan, Veronika Moravan, Warren Hellen, Gary Elman, Bennett Amaechi, and Andreas Mandelis. 2018. "Detection of Caries Around Resin-Modified Glass Ionomer and Compomer Restorations Using Four Different Modalities In Vitro" Dentistry Journal 6, no. 3: 47. https://doi.org/10.3390/dj6030047
APA StyleAbrams, T., Abrams, S., Sivagurunathan, K., Moravan, V., Hellen, W., Elman, G., Amaechi, B., & Mandelis, A. (2018). Detection of Caries Around Resin-Modified Glass Ionomer and Compomer Restorations Using Four Different Modalities In Vitro. Dentistry Journal, 6(3), 47. https://doi.org/10.3390/dj6030047






