A Review of the Common Models Used in Mechanistic Studies on Demineralization-Remineralization for Cariology Research
Abstract
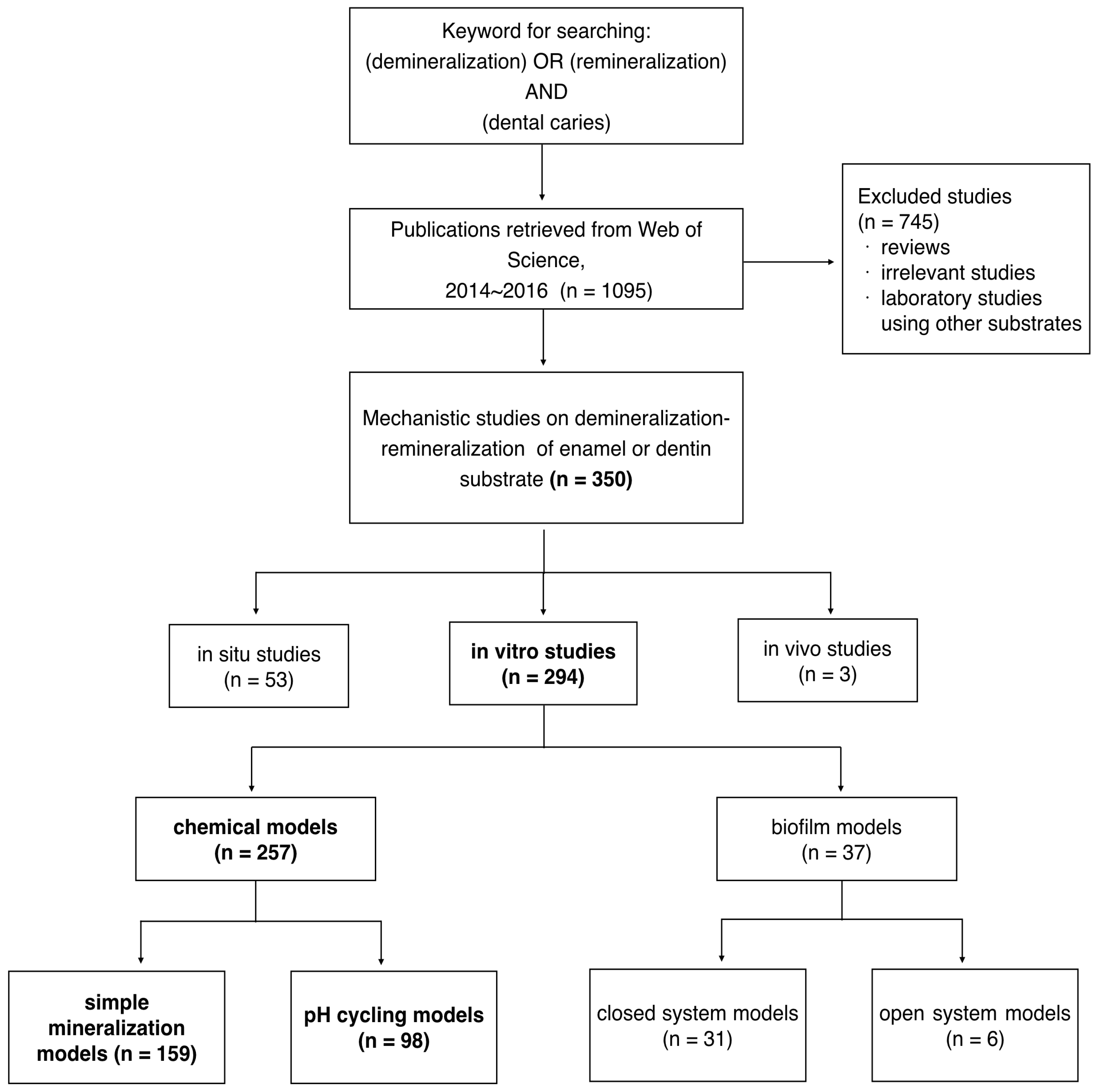
:1. Introduction
2. Types of Mechanistic Studies in Recent Publications
2.1. In Situ Studies
2.2. In Vivo Studies
2.3. In Vitro Studies
3. Demineralization-Remineralization Models Used for In Vitro Studies
3.1. Biofilm Models
3.2. Chemical Models
4. Types of Chemical Models Used in Recent Studies
4.1. Simple Mineralization Models
4.2. PH-Cycling Model
5. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- ten Cate, J.M. Models and role models. Caries Res. 2015, 49, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.A. Encyclopedia of Library and Information Science, 2nd ed.; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- Falagas, M.E.; Pitsouni, E.I.; Malietzis, G.A.; Pappas, G. Comparison of pubmed, scopus, web of science, and google scholar: Strengths and weaknesses. Faseb J. 2008, 22, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.-H.; Kim, H.-Y.; Son, H.-H.; Chang, J. How to design in situ studies: An evaluation of experimental protocols. Restor. Dent. Endod. 2014, 39, 164–171. [Google Scholar] [CrossRef] [PubMed]
- White, D.J. The comparative sensitivity of intra-oral, in vitro, and animal models in the ‘profile’ evaluation of topical fluorides. J. Dent. Res. 1992, 71, 884–894. [Google Scholar] [PubMed]
- Klinge, B.; Jönsson, J. Animal models in oral health sciences. In Handbook of Laboratory Animal Science, Volume II, Third Edition: Animal Models; CRC Press: Boca Raton, FL, USA, 2011; p. 387. [Google Scholar]
- Bowen, W.H. Rodent model in caries research. Odontology 2013, 101, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Yip, H.K.; Cutress, T.W.; Samaranayake, L.P. Artificial mouth model systems and their contribution to caries research: A review. J. Dent. 2003, 31, 161–171. [Google Scholar] [CrossRef]
- Bowen, W.H. Dental caries—Not just holes in teeth! A perspective. Mol. Oral Microbiol. 2016, 31, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Salli, K.M.; Ouwehand, A.C. The use of in vitro model systems to study dental biofilms associated with caries: A short review. J. Oral Microbiol. 2015, 7, 26149. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Eggers, K.; Meyer-Lueckel, H.; Dorfer, C.; Kovalev, A.; Gorb, S.; Paris, S. In vitro induction of residual caries lesions in dentin: Comparative mineral loss and nano-hardness analysis. Caries Res. 2015, 49, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Bowden, G. The role of microbiology in models of dental caries: Reaction paper. Adv. Dent. Res. 1995, 9, 255–269. [Google Scholar] [CrossRef]
- Chu, C.H.; Mei, L.; Seneviratne, C.J.; Lo, E.C.M. Effects of silver diamine fluoride on dentine carious lesions induced by streptococcus mutans and actinomyces naeslundii biofilms. Int. J. Paediatr. Dent. 2012, 22, 2–10. [Google Scholar] [CrossRef] [PubMed]
- McBain, A.J. Chapter 4: In vitro biofilm models: An overview. Adv. Appl. Microbiol. 2009, 69, 99–132. [Google Scholar] [PubMed]
- Mei, M.L.; Li, Q.L.; Chu, C.H.; Lo, E.C.M.; Samaranayake, L.P. Antibacterial effects of silver diamine fluoride on multi-species cariogenic biofilm on caries. Ann. Clin. Microb. Anti. 2013, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Horie, K.; Shimada, Y.; Matin, K.; Ikeda, M.; Sadr, A.; Sumi, Y.; Tagami, J. Monitoring of cariogenic demineralization at the enamel-composite interface using swept-source optical coherence tomography. Dent. Mater. 2016, 32, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shimada, Y.; Matin, K.; Sadr, A.; Sumi, Y.; Tagami, J. Assessment of bacterial demineralization around composite restorations using swept-source optical coherence tomography (SS-OCT). Dent. Mater. 2016, 32, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, H.; Shimada, Y.; Matin, K.; Ikeda, M.; Sadr, A.; Sumi, Y.; Tagami, J. Assessment of cervical demineralization induced by streptococcus mutans using swept-source optical coherence tomography. J. Med. Imaging 2016, 3, 014504. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Diederich, C.; Paris, S. Restoration gaps needed to exceed a threshold size to impede sealed lesion arrest in vitro. J. Dent. 2016, 48, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Kramer, N.; Mohwald, M.; Lucker, S.; Domann, E.; Zorzin, J.I.; Rosentritt, M.; Frankenberger, R. Effect of microparticulate silver addition in dental adhesives on secondary caries in vitro. Clin. Oral Investig. 2015, 19, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T.; Nelis, H.J. In vitro and in vivo model systems to study microbial biofilm formation. J. Microbiol. Meth. 2010, 83, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Skucha-Nowak, M.; Gibas, M.; Tanasiewicz, M.; Twardawa, H.; Szklarski, T. Natural and controlled demineralization for study purposes in minimally invasive dentistry. Adv. Clin. Exp. Med. 2015, 24, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Moron, B.M.; Comar, L.P.; Wiegand, A.; Buchalla, W.; Yu, H.; Buzalaf, M.A.R.; Magalhaes, A.C. Different protocols to produce artificial dentine carious lesions in vitro and in situ: Hardness and mineral content correlation. Caries Res. 2013, 47, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Lynch, R.J.M.; ten Cate, J.M. The effect of lesion characteristics at baseline on subsequent de- and remineralisation behaviour. Caries Res. 2006, 40, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Alsayed, E.Z.; Hariri, I.; Sadr, A.; Nakashima, S.; Bakhsh, T.A.; Shimada, Y.; Sumi, Y.; Tagami, J. Optical coherence tomography for evaluation of enamel and protective coatings. Dent. Mater. J. 2015, 34, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Ozgul, B.M.; Tirali, R.E.; Cehreli, S.B. Effect of biodentine on secondary caries formation: An in vitro study. Am. J. Dent. 2016, 29, 71–74. [Google Scholar] [PubMed]
- Marquezan, M.; Correa, F.N.P.; Sanabe, M.E.; Rodrigues, L.E.; Hebling, J.; Guedes-Pinto, A.C.; Mendes, F.M. Artificial methods of dentine caries induction: A hardness and morphological comparative study. Arch. Oral Biol. 2009, 54, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- ten Cate, J.M.; Duijsters, P.P.E. Alternating demineralization and remineralization of artificial enamel lesions. Caries Res. 1982, 16, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Buzalaf, M.A.R.; Hannas, A.R.; Magalhaes, A.C.; Rios, D.; Honorio, H.M.; Delbem, A.C.B. Ph-cycling models for in vitro evaluation of the efficacy of fluoridated dentifrices for caries control: Strengths and limitations. J. Appl. Oral Sci. 2010, 18, 316–334. [Google Scholar] [CrossRef] [PubMed]
- Zhao, I.S.; Mei, M.L.; Li, Q.L.; Lo, E.C.; Chu, C.H. Arresting simulated dentine caries with adjunctive application of silver nitrate solution and sodium fluoride varnish: An in vitro study. Int. Dent. J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.L.; Lo, E.; Chu, C. Clinical use of silver diamine fluoride in dental treatment. Compend Contin. Educ. Dent. 2016, 37, 93–98. [Google Scholar]
- Lynch, R.J.M.; Mony, U.; ten Cate, J.M. Effect of lesion characteristics and mineralising solution type on enamel remineralisation in vitro. Caries Res. 2007, 41, 257–262. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, O.Y.; Zhao, I.S.; Mei, M.L.; Lo, E.C.-M.; Chu, C.-H. A Review of the Common Models Used in Mechanistic Studies on Demineralization-Remineralization for Cariology Research. Dent. J. 2017, 5, 20. https://doi.org/10.3390/dj5020020
Yu OY, Zhao IS, Mei ML, Lo EC-M, Chu C-H. A Review of the Common Models Used in Mechanistic Studies on Demineralization-Remineralization for Cariology Research. Dentistry Journal. 2017; 5(2):20. https://doi.org/10.3390/dj5020020
Chicago/Turabian StyleYu, Ollie Yiru, Irene Shuping Zhao, May Lei Mei, Edward Chin-Man Lo, and Chun-Hung Chu. 2017. "A Review of the Common Models Used in Mechanistic Studies on Demineralization-Remineralization for Cariology Research" Dentistry Journal 5, no. 2: 20. https://doi.org/10.3390/dj5020020
APA StyleYu, O. Y., Zhao, I. S., Mei, M. L., Lo, E. C.-M., & Chu, C.-H. (2017). A Review of the Common Models Used in Mechanistic Studies on Demineralization-Remineralization for Cariology Research. Dentistry Journal, 5(2), 20. https://doi.org/10.3390/dj5020020





